Abstract
Many probiotic lactobacilli and their extracellular polysaccharides (EPS) have beneficial immunological properties. However, it is unclear how they elicit the host immune response. We thus investigated the immunological properties of UV-killed Lactobacillus delbrueckii TU-1 and L. plantarum KM-9 cells as well as their extracellular polysaccharides (EPSs). High-performance liquid chromatography and ion exchange chromatography analyses showed that their EPSs differ in sugar composition and sugar fractionation. The immunological properties were evaluated in a semi-intestinal model using a Transwell co-culture system that employed human intestinal epithelial (Caco-2) cells on the apical side and murine macrophage (RAW264.7) cells on the basolateral side. The UV-killed cells and EPSs were added to the apical side to allow direct contact with Caco-2 cells and incubated for 6 hr. After incubation, the amounts of tumor necrosis factor-α and several cytokines released by RAW264.7 or Caco-2 cells were quantified by cytotoxic activity on L929 cells (murine fibrosarcoma cell line) and quantitative reverse-transcriptase PCR. We found that the UV-killed cells and their EPSs had immunological effects on RAW264.7 cells via Caco-2 cells. The RAW264.7 cells showed different cytokine production profiles when treated with UV-killed cells and EPSs. The UV-killed cells and EPSs promoted a Th1-type cellular response. Furthermore, we found that the UV-killed cells sent positive signals through Toll-like receptor (TLR) 2. Meanwhile, neither EPS sent a positive signal through TLR4 and TLR2. This evidence suggests that both UV-killed cells of the lactobacillus strains and their EPSs trigger a Th1-type immune response in a human host, with the former triggering the response via the TLRs expressed on its epithelium and the latter employing a mechanism yet to be determined, possibly involving a novel receptor that is designed to recognize specific patterns of repeating sugar in the EPSs.
Keywords: Lactobacillus delbrueckii, L. plantarum, extracellular polysaccharides, Transwell co-culture, Toll-like receptors
INTRODUCTION
It is well known that many lactic acid bacteria have beneficial effects on the immune system of their hosts and are therefore used as probiotics [1]. These probiotic effects have been attributed not only to the bacterial cells but also to their extracellular polysaccharides (EPSs) [2]. For example, both in vitro and in vivo experiments [3, 4] demonstrated that Lactobacillus plantarum N14 (LP14) cells and their EPSs strongly induce the expression of Th1-type cytokine genes. Meanwhile, we previously showed through an in vitro study using the Transwell co-culture system [5] that EPSs produced by different strains of L. delbrueckii show different cytokine production profiles. Among the EPSs, those produced by L. delbrueckii TU-1 markedly increased tumor necrosis factor-α production and induced excessive Th1-type cytokine (IL-12, IL-15, and IL-18) mRNA expression in murine macrophage (RAW264.7) cells [5]. However, it has not been determined whether the cells of L. delbrueckii TU-1 have the same immunomodulatory properties as their EPSs.
Pattern recognition receptors within the host intestine, such as those of the membrane-bound Toll-like receptor (TLR) family, recognize components of bacterial cells and signal the presence of intestinal bacteria to the host immune system [6]. Murofushi et al. [7] reported that heat-killed cells of LP14 and its EPS exerted marked immunomodulatory activity through TLR2 and TLR4, which recognize peptidoglycan and lipopolysaccharides, respectively. Moreover, Balachandran et al. [8] reported that human monocyte cells are capable of recognizing a polysaccharide produced by Spirulina platensis through TLR2 but not one produced through TLR4. Thus, we hypothesized that the immune response caused by the EPSs, as well as the cells of L. plantarum and L. delbrueckii strains, are mediated by TLR2, TLR4, or both. This study was designed to evaluate the immunological properties of UV-killed cells of L. delbrueckii TU-1 and a wild-type (WT) strain of L. plantarum and their EPSs in a semi-intestinal model using a Transwell co-culture system. We also aimed to gain insight into the mechanisms involved in the immunological activities of the UV-killed cells and their EPSs by studying the roles of TLR4 and TLR2.
MATERIALS AND METHODS
Bacterial strains
The bacterial strains L. delbrueckii TU-1 and L. plantarum subsp. plantarum KM-9 were used in this study. These strains were isolated in our laboratory from a commercial yogurt and a pickled turnip, respectively, and their taxonomic identities were confirmed by species-specific PCR assays, as described previously [9, 10].
Preparation of EPSs
L. delbrueckii TU-1 and L. plantarum subsp. plantarum KM-9 were cultured anaerobically at 37°C for 24 hr in whey medium containing 0.5% (wt/vol) yeast extract (Becton, Dickinson and Company, Sparks, MD, USA) and 10% (wt/vol) whey powder (Shizen Kenkou Co., Ltd., Kyoto, Japan) that had been hydrolyzed with proteinase K (Wako Pure Chemical Industries, Osaka, Japan) for 7 hr at 55°C before use. Purified EPS was prepared by following the methodology of Nagai et al. [11]. Briefly, bacterial cells and precipitates were removed by centrifugation (14,000 × g, 20 min, 4°C). Crude EPS was precipitated from the supernatant by the addition of 1.5 vol of cold ethanol and collected by centrifugation (14,000 × g, 20 min, 4°C). The crude EPS was dissolved in distilled water and insoluble material was removed by centrifugation (14,000 × g, 20 min, 4°C). The crude EPS was purified by additional precipitation with 1.5 vol of cold ethanol. The precipitated EPSs were treated with 10% (wt/vol) trichloroacetic acid at 4°C, and the denatured proteins were removed by centrifugation (14,000 × g, 20 min, 4°C). Partially purified EPS was obtained by dialysis of the supernatant containing the crude EPS against distilled water at 4°C for two days, followed by lyophilization. The crude EPS was dissolved in 50 mM Tris-HCl buffer (pH 8.0) containing 1 mM MgCl and treated with 2 µg/ml DNase (Roche Applied Science, Basel, Switzerland) and 2 µg/ml RNase (Wako) at 37 \°C for 6 hr. The proteins in the crude EPS were digested with 0.2 mg/ml of proteinase K for 16 hr at 37 ^°C. The reaction was stopped by heating at 80 ^°C for 10 min. The EPSs were then applied to centrifugal concentrators (Amicon Ultra 0.5 ml 10 K, Merck Millipore, Darmstadt, Germany) and centrifuged at 14,000 × g for 30 min. After centrifugation, sufficient distilled water was added to the filter unit until the final volume was 500 µl. The EPSs in the above solution were subjected to an additional precipitation with 1.5 vol of cold ethanol to obtain purified EPS. The total carbohydrate concentration of the purified EPS was then determined using the phenol-H2SO4 reaction with glucose as a standard [12]. The whey medium used for the EPS preparations might contain some sugar species [13] that might have been carried over to our EPS preparations, affecting the subsequent immunological assays. Therefore, we subjected the whey medium to the same EPSs purification procedure to prepare a ‘whey sample’ for use as a control.
Preparation of UV-killed cells
L. delbrueckii TU-1 and L. plantarum subsp. plantarum KM-9 were cultured anaerobically at 37°C for 24 hr in MRS broth. After cultivation, the bacterial cells were collected by centrifugation (5,000 × g, 10 min, 4°C), washed three times with sterile phosphate-buffered saline (PBS; 0.8% NaCl, 0.02% KH2PO4, and 0.115% Na2HPO4, pH 7.4), and resuspended in PBS. Bacterial cells of each strain were then killed under ultraviolet light (UV) for 10–12 hr. After the UV treatment, cell death was confirmed using a conventional plate count method. Bacterial cell suspensions of the strains were then adjusted to a concentration of 1.0 × 108 cells/ml, which was determined using direct microscopic counting.
Cell culture
Cells from the human intestinal epithelial cell line Caco-2 were cultured in Dulbecco’s Modified Eagle Medium (DMEM, glutamine, high glucose; Wako) supplemented with 1% MEM non-essential amino acid solution (NEAA; Gibco BRL, Grand Island, NY, USA), 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS; Daiichi Kagaku, Tokyo, Japan). Cells from the murine macrophage cell line RAW264.7 were cultured in DMEM (glutamine, low glucose) supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% heat-inactivated FBS. Human intestinal epithelial (Caco-2) cells from passages 48–62, and RAW264.7 cells from passages 10–20, were used. Cells from the murine fibrosarcoma cell line L929 were cultured in Eagle’s Minimum Essential Medium (MEM; Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cell cultures were incubated in a humidified 5% CO2 incubator at 37°C.
Transepithelial electrical resistance measurement
The integrity of the Caco-2 monolayer was determined by measuring the transepithelial electrical resistance (TER). Tight junctions serve as barriers to paracellular diffusion, and TER reflects the tightness of the junctions between the epithelial cells. To measure the TER value, Caco-2 cells were grown in Transwelll inserts (1.12 cm, 0.4 µm pore size; Costar, Corning, Cambridge, MA, USA) with polycarbonate membranes. Caco-2 cells were seeded at a density of 3.0 × 105 cells/well. The medium was changed every three days. The monolayer cells were gently rinsed with Hank’s Balanced Salts Solution (HBSS; 137 mM NaCl, 5.36 mM KCl, 1.67 mM CaCl2, 1 mM MgCl2, 1.03 mM MgSO4, 0.44 mM KH2PO4, and 0.34 mM Na2HPO4, pH 7.4) and then equilibrated in the same solution for 30 min in a humidified 5% CO2 incubator at 37°C (apical side, 200 µl; basolateral side, 800 µl). The integrity of the cell monolayer was evaluated by measuring the TER using Millicelln ERS equipment (Merck Millipore). The cell monolayer was used when the TER value was >400 Ω cm2.
Caco-2 / RAW264.7 co-culture system
A Caco-2 / RAW264.7 co-culture system was used as described by Tanoue et al. [14]. In brief, Caco-2 cells were seeded at 3.0 × 105 cells/well onto Transwell insert plates (1.12 cm2, 0.4 µm pore size; Costar, Corning). The cells, which were fully differentiated (TER value > 400 Ω cm2), were subjected to the following experiment: RAW264.7 cells were seeded at 2.1 × 105 cells/well in 24-well tissue culture plates and incubated overnight to fully adhere to the well. After all the medium had been replaced with RPMI 1640 (Gibco BRL), the Transwell insert plates with Caco-2 cells were added into multiple-plate wells preloaded with RAW264.7 cells. Then, 1.0 × 108 UV-killed cells/ml, 100 µg/ml of EPSs, and the whey sample were applied to the apical sides of individual wells and incubated at 37°C for 6 hr. During incubation, the TER value of the upper chamber filter was measured using a Millicell ERS apparatus. Lipopolysaccharide (LPS) samples (from Escherichia coli O127, Wako) of 5 ng/ml and 10 ng/ml were added to the basolateral side for only the positive control after 3 hr of incubation. A negative control of the co-culture system was not exposed to UV-killed cells or EPSs. After incubation, all culture supernatants from the basolateral sides were collected for tumor necrosis factor (TNF)-α measurement. The treated Caco-2 cells and RAW264.7 cells were then harvested to isolate total RNA for the real-time PCR assays described below.
Tumor necrosis factor-α measurement
Amounts of TNF-α in the culture medium were quantified as described by Takada et al. [15] by measuring cytotoxic activity on L929 cells using murine TNF-α as the standard. Briefly, L929 cells were plated in 96-well microplates containing MEM supplemented with 10% heat-inactivated FBS and cultured for 3 hr. The medium was replaced with 50 µl of fresh RPMI 1640 Medium (supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 g/ml streptomycin) containing 400 µg/ml actinomycin D and 50 µl of each sample. The cells were cultured for 20 hr at 37°C under an atmosphere containing 5% CO2. After the medium was removed, each cell lysate was stained with 0.1% crystal violet in ethanol/formaldehyde for 15 min at room temperature. Then, the cells were washed with water and dried. Each cell lysate was dissolved in 100 µl of ethanol:PBS (1:1, v/v). The absorbance of the stained solutions was measured using a microplate reader. The concentration of TNF-α was calculated using a standard curve.
RNA isolation and quantitative polymerase chain reaction
Total RNA was isolated from Caco-2 cells and RAW264.7 cells using Sepasol®-RNA I Super (Nacalai Tesque, Kyoto, Japan), according to the manufacturer’s protocols. Total RNA was transcribed into cDNA in a 20 µl reaction mixture containing 10 µl RNA solution, 4.2 µl diethyl pyrocarbonate-treated water, 2.0 µl 10 × reverse transcription buffer, 0.8 µl 25 × dNTPs, 2.0 µl oligo p(dT) primer, and 1.0 µl reverse transcriptase (50 U) using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Warrington, UK). Real-time PCR was performed using a Thermal Cycler d\Dice Real Time System (Takara BIO Inc., Ohtsu, Japan) with Premix Ex TaqTM (Takara) and a TaqMan® Gene Expression Assay (Applied Biosystems) for mouse and human cytokines, according to the manufacturer’s protocol. The sequences of the PCR primers [16,17,18,19] used in this study are shown in Table 1. In this study, mouse β-actin and human peptidylprolyl isomerase A (PPIA) were used as endogenous control genes for the RAW264.7 cells and Caco-2 cells, respectively. The gene expression levels for each individual sample were normalized to endogenous gene expression, as determined by the relative quantification method (ΔΔCT) [20].
Table 1. Sequences of the primers used in RT-PCR analysis.
| Cytokines | Primer | Sequence |
|---|---|---|
| Mouse β-actin | Sense primer | TGTGATGGTGGGAATGGGTCAG |
| Antisense primer | TTTGATGTCACGCACGATTTCC | |
| Mouse IL-1α | Sense primer | AAGTTTGTCATGAATGATTCCCTC |
| Antisense primer | GTCTCACTACCTGTGATGAGT | |
| Mouse IL-10 | Sense primer | GTGAAGACTTTCTTTCAAACAAAG |
| Antisense primer | CTGCTCCACTGCCTTGCTCTTATT | |
| Mouse IL-12 | Sense primer | CGTGCTCATGGCTGGTGCAAAG |
| Antisense primer | CTTCATCTGCAAGTTCTTGGGC | |
| Mouse IL-18 | Sense primer | ATGGTACAACCGCAGTAATACGG |
| Antisense primer | AGTGAACATTACAGATTTATCCC | |
| Mouse IFN-γ | Sense primer | TACTGCCACGGCACAGTCATTGAA |
| Antisense primer | GCAGCGACTCCTTTTCCGCTTCCT | |
| Mouse IL-15 | Sense primer | TTCTCTTCTTCATCCTCCCCCT |
| Antisense primer | ATGAAGAGGCAGTGCTTTGA | |
| Human PPIA | Sense primer | AATGCTGGACCCAACAC |
| Antisense primer | TCCACAATATTCATGCCTT | |
| Human IL-12 | Sense primer | TTGTGGCTACCCTGGTCCT |
| Antisense primer | AGAGTTTGTCTGGCCTTCTGG | |
| Human IL-8 | Sense primer | TGGCTCTCTTGGCAGCCTTC |
| Antisense primer | TCTCCACAACCCTCTGCACC | |
| Human TGF-β1 | Sense primer | GCTGCTGTGGCTACTGGTGC |
| Antisense primer | CATAGATTTCGTTGTGGGTTTC |
Sugar component analysis of EPSs
EPS samples were hydrolyzed in 1 N H2SO4 at 90°C for 4 hr. Each hydrolysate was neutralized with BaCO3, and the precipitate was removed by filtration using a 0.45-µm filter unit (Merck Millipore). The sugar composition of each EPS was determined using high-performance liquid chromatography (HPLC) with a Rezex RCM-Monosaccharide column (7.8 × 300 mm; Shimadzu, Kyoto, Japan). Monosaccharides were detected using an evaporative light-scattering detector (ELSD; Shimadzu). Isocratic elution was conducted with distilled water at a flow rate of 0.5 ml/min at 70°C. The molar ratio of monosaccharides in the sample was estimated using a standard curve. Partially purified EPSs were fractionated using anion exchange chromatography with DEAE Sepharose Fast Fow (GE Healthcare UK Ltd., Amersham Place, UK). Elution was conducted with a linear gradient from 0 to 0.3 M NaCl in Tris-HCl buffer (pH 8.8). The eluate was photometrically monitored for protein at 280 nm using a microplate reader and at 490 nm for neutral sugars using the phenol-H2SO4 reaction [12].
Antibody treatment
For blocking experiments, cultured Caco-2 cells were pretreated for 30 min with anti-TLR2 (Hycult Biotech, the Netherlands) antibodies, anti-TLR4 (Hycult Biotech) antibodies, or an isotype-matched control IgG1 (Santa Cruz Biotechnology, Dallas, TX, USA) (anti-TLR2, 10 µg/ml; anti-TLR4, 10 µg/ml; and IgG1, 10 µg/ml), and then the Transwell inserts containing Caco-2 cells were washed with PBS three times. EPSs (100 µg/ml) and the UV-killed cells (1.0 × 108 cells/ml) were applied into the apical side and incubated at 37°C for 6 hr. LPS (10 ng/ml), a TLR4 agonist, and 50 µg/ml of the TLR2 agonist Pam3CSK4 (Novus Biologicals, Littleton, CO, USA) were added to the basolateral side only for the positive control group after 3 hr of the incubation. For the negative control, the co-culture system was not exposed to the EPSs or the UV-killed cells. After incubation, all culture supernatants from the basolateral side were collected for nitric oxide (NO) measurement by using the Griess reaction [21]. In order to examine the inhibition of TLR2 and TLR4 expression, the treated Caco-2 cells were then harvested to isolate total RNA for quantitative RT-PCR (qPCR) of IL-8 mRNA expression [22, 23].
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test for co-culture data and the Tukey-Kramer test for antibody data, respectively. Asterisks (*, **) are used to indicate statistical differences, with significance levels of p<0.05 and p<0.01, respectively.
RESULTS
TNF-α production by RAW264.7 cells in a co-culture system
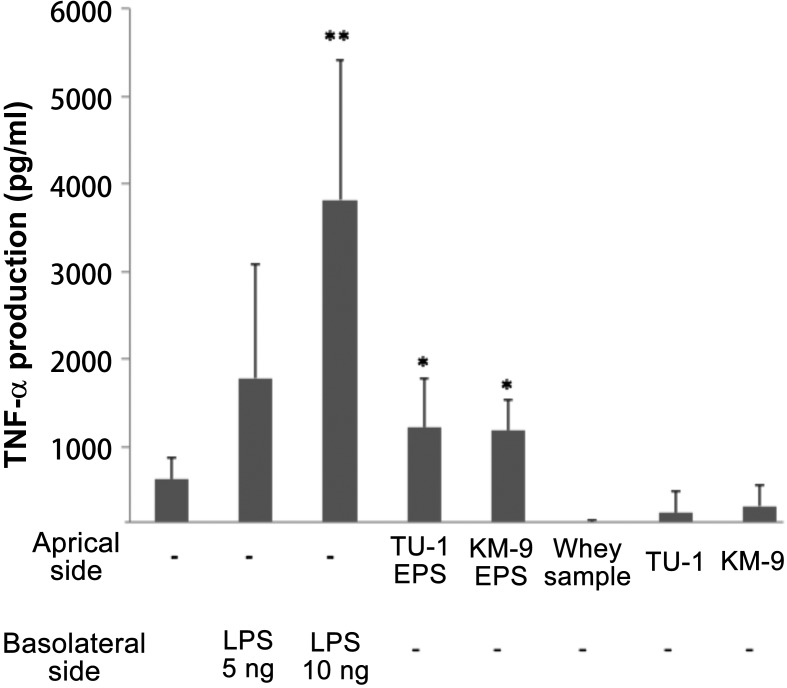
LPS (10 ng/ml) treatment from the basolateral side showed approximately five times higher TNF-α production than the control (Fig. 1). Caco-2 cells treated with EPSs from L. delbrueckii TU-1 or L. plantarum KM-9 showed approximately twice the TNF-α production of the control. Meanwhile, Caco-2 cells treated with UV-killed L. delbrueckii TU-1 and L. plantarum KM-9 cells showed the same TNF-α production level as the control. It should be noted that the TER value of the Caco-2 cells showed no change compared with that of the control after the addition of UV-killed cells or EPSs (data not shown).
Fig. 1.
Effects of EPSs and UV-killed cells on the production of TNF-α in the Caco-2/RAW264.7 co-culture model.
LPS-stimulated cultures were used as a positive control. Values represent the means ± standard error (SE; n=3). Significant differences were observed between the sample-treated group and the control group (**p<0.01; *p<0.05).
Cytokine expression by RAW264.7 cells in the co-culture system
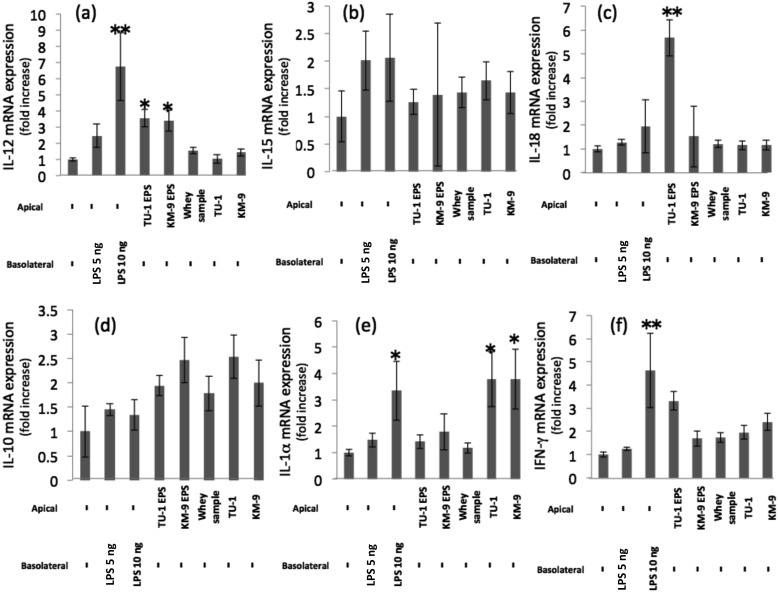
The expression of cytokine mRNA in RAW264.7 cells was examined using a qPCR method. IL-12 mRNA expression was enhanced approximately five-fold, compared with the control after LPS treatment (10 ng/ml) from the basolateral side (Fig. 2a). Treatment with L. delbrueckii TU-1 or L. plantarum KM-9 EPS resulted in upregulated mRNA expression compared with the control, whereas the treatment with UV-killed L. delbrueckii TU-1 or L. plantarum KM-9 cells resulated in no change. IL-18 mRNA expression was upregulated after treatment with the EPS of L. delbrueckii TU-1 (Fig. 2c). IL-1α mRNA expression was upregulated after treatment with UV-killed L. delbrueckii TU-1 or L. plantarum KM-9 cells (Fig. 2e). No significant changes in IL-15, IL-10, or IFN-γ mRNA expression compared with the control were seen after treatment with EPS or UV-killed cells (Fig. 2b, 2d, and 2f).
Fig. 2.
Effects of EPSs and UV-killed cells on cytokines (IL-12, IL-15, IL-18, IL-10, IL-1α, and IFN-γ) production in the Caco-2/RAW264.7 co-culture model.
Values represent the mean ± SE (n=3). Significant differences were observed between the sample-treated group and the control group (**p<0.01; *p<0.05). (a) IL-12, (b) IL-15, (c) IL-18, (d) IL-10, (e) IL-1-α, (f) IFN-γ.
Cytokine expression by Caco-2 cells in the co-culture system
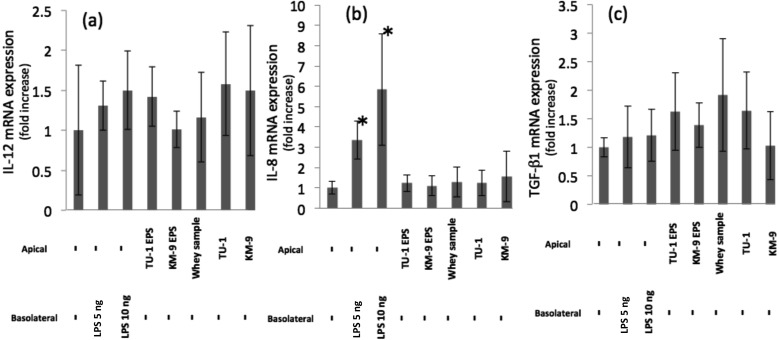
The expression of cytokine mRNA in Caco-2 cells was examined using the qPCR method. IL-12, IL-8, and TGFβ-1 mRNA levels showed no significant changes compared with the control after treatment with UV-killed cells or EPSs (Fig. 3a, 3b, and 3c).
Fig. 3.
Effects of EPSs and UV-killed cells on the production of IL-12, IL-8, and TGF-β1 in the Caco-2/RAW264.7 co-culture model.
Expression of mRNA in the Caco-2 cells was measured by quantitative RT-PCR, as described in the materials and methods section. Significant differences were observed between the sample-treated group and the control group (**p<0.01; *p<0.05). (a) IL-12, (b) IL-8, (c) TGF- β1.
Antibody treatment
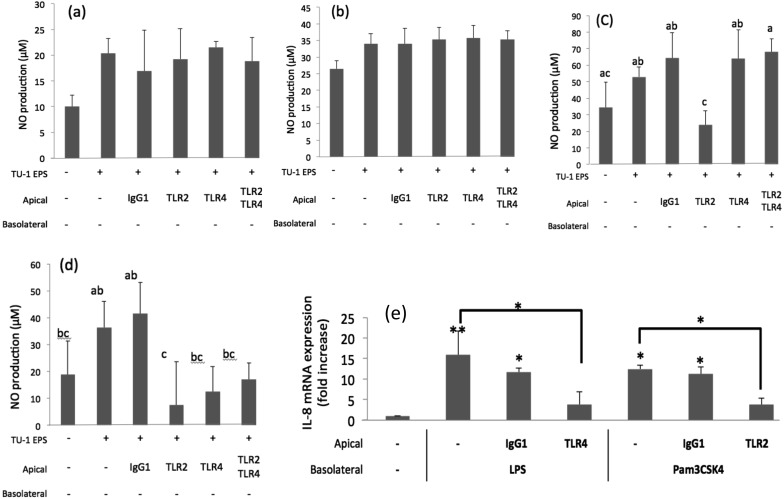
We evaluated whether anti-TLR2 or anti-TLR4 antibodies modified the effect of the EPSs or UV-killed cells in the co-culture system. No effect was observed on the production of NO when the EPS of L. delbrueckii TU-1 (Fig. 4a) or L. plantarum KM-9 (Fig. 4b) was applied to Caco-2 cells that had been treated with anti-TLR2 antibodies, anti-TLR4 antibodies, or both antibodies. As shown in Fig. 4c and 4d, UV-killed L. delbrueckii TU-1 or L. plantarum KM-9 cells increased NO production in control cells normally expressing TLR2 and TLR4. Meanwhile, UV-killed L. delbrueckii TU-1 or L. plantarum KM-9 cells decreased NO production in Caco-2 cells that had been treated with anti-TLR2 antibodies. Anti-TLR4 antibodies completely abolished the capacity of LPS to reduce the levels of IL-8 mRNA; similarly, anti-TLR2 antibodies completely abolished the capacity of Pam3CSK4 to reduce the levels of IL-8 mRNA (Fig. 4e).
Fig. 4.
Effects of Caco-2 cell pretreatment with anti-TLR antibodies on nitric oxide (NO) production in RAW264.7 cells.
Expression of the IL-8 mRNA in RAW264.7 cells was measured by quantitative RT-PCR as described in the materials and methods section. Values represent the mean ± SE (n = 3). Significant differences were observed between the sample-treated group and the control group (**p<0.01; *p<0.05). NO production after treatment with (a) the EPS of L. delbrueckii TU-1, (b) the EPS of L. plantarum KM-9, (c) UV-killed L. delbrueckii TU-1cells, (d) UV-killed L. plantarum KM-9 cells, (e) IL-8 mRNA expression after treatment with LPS and Pam3Csk4. Different letters (a, b and c) indicate significant differences between groups (p<0.05).
Sugar component analysis by HPLC
The monosaccharide composition of each EPS was determined using HPLC. The EPSs of L. delbrueckii TU-1 contained glucose (Glc) and galactose (Gal) at a ratio of 1:2.76. The EPSs of L. plantarum KM-9 contained Glc and Gal at a ratio of 1:1.14.
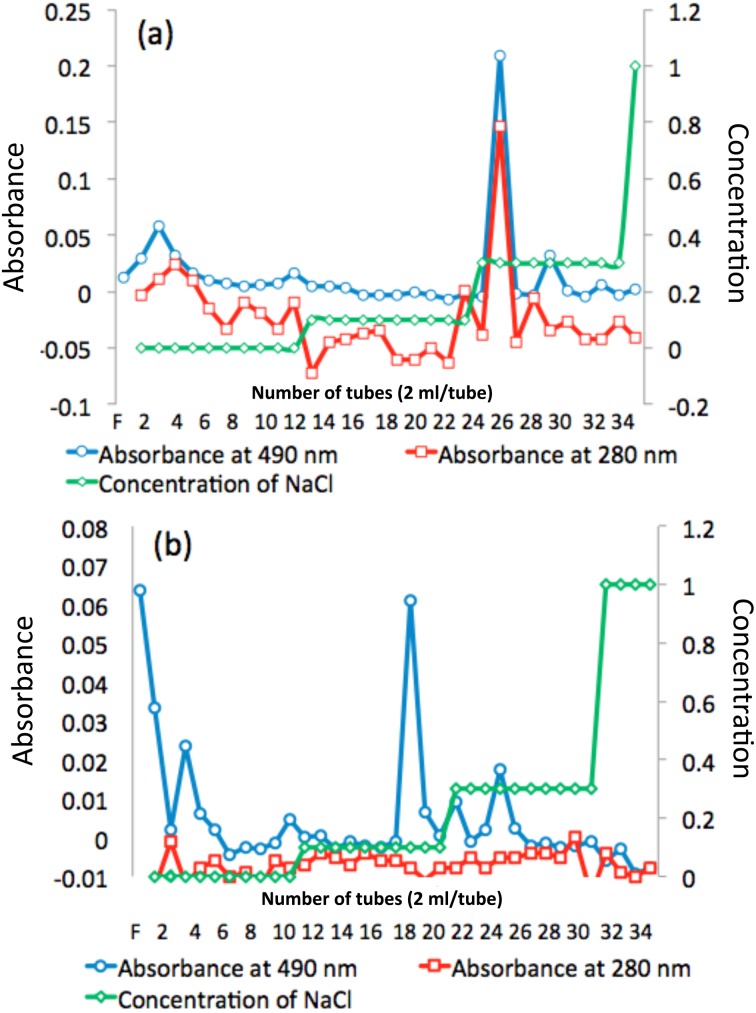
EPS fractionation
As shown in Fig. 5a, the EPS of L. delbrueckii TU-1 was fractionated into one peak (fractions 21 to 23). As shown in Fig. 5b, the EPS of L. plantarum KM-9 was fractionated into three peaks (fractions 2 to 5, fractions 17 to 19, and fractions 22 to 25).
Fig. 5.
Fractionation of extracellular polysaccharides (EPSs) by anion-exchange chromatography.
(a) the EPS of L. delbrueckii TU-1 and (b) the EPS of L. plantarum KM-9. The eluate was monitored for neutral sugars at 490 nm and for proteins at 280 nm using the phenol-H2SO4 method. The dashed green line indicates the concentration of NaCl.
DISCUSSION
We have demonstrated that UV-killed L. delbrueckii TU-1, UV-killed L. plantarum KM-9 cells, and their EPSs have appreciable immunological effects on mouse macrophages (RAW264.7 cells) via Caco-2 cells. Our results also show that UV-killed L. delbrueckii TU-1 and L. plantarum KM-9 cells and their EPSs upregulate the expression of proinflammatory cytokine (TNF-α, IL-12, IL-18, and IL-1α) mRNAs. However, the UV-killed cells and the EPSs induced different cytokine production profiles in RAW264.7 cells, despite being derived from the same strain. It was reported elsewhere [24] that naive T cells need IL-12 to differentiate into Th1 cells, which then produce cytokines (IL-12, IL-18, and IFN-γ), further promoting differentiation of Th1 cells. Meanwhile, IL-18 is well known to enhance Th1-type cellular responses [25], where IL-18 produced by activated macrophage cells, for example, may act synergistically with IFN-γ and IL-12 from T cells and induce natural killer (NK) cell activity [26]. Meanwhile, IL-1α activates Th1 cells to active NK cells [27]. It was thus suggested that, in the present study, UV-killed L. delbrueckii TU-1 and L. plantarum KM-9 cells and their EPSs caused a Th1-type cellular response. In fact, LPS applied to the basolateral side not only enhanced TNF-α production but also upregulated IL-8 mRNA expression in Caco-2 cells. Although IL-8 is known to be secreted excessively by a variety of cells, such as intestinal epithelial cells, at sites of inflammation [28], the levels of IL-8 mRNA expression in Caco-2 cells triggered by the UV-killed cells and EPSs remained comparable to those of their controls. The evidence suggests that UV-killed L. delbrueckii TU-1 and L. plantarum KM-9 cells and their EPSs exert a Th1-type cellular response, which is different from the corresponding proinflammatory response exerted by LPS. The EPS-producing probiotic bacterial strains or their purified EPSs are thus considered to be potentially beneficial biologics against microbial (i.e., influenza, norovirus) infections. IL-12, IL-8, and TGF-β1 mRNA expression in Caco-2 cells was not affected by the presence of EPSs or UV-killed cells. It is thus suggested that some of the signals that are not tested in this study were released from the Caco-2 cells to act on the RAW264.7 cells, which then upregulated cytokine gene expression. Further studies are necessary to evaluate this possibility and identify the unknown signal or signals.
In the present study, EPSs appeared to have stronger immunological properties than UV-killed cells. The TNF-α production was increased in a dose-dependent manner, with the highest production observed at 100 µg/ml LPS (data not shown). Our calculations suggest that both strains produce only 2 µg/ml EPSs at a concentration of 1.0 × 108 cells/ml, which was the same bacterial quantity as that applied to the apical side of the insert in this study. It should be pointed out that Caco-2 cells were exposed to isolated EPSs at approximately 50 times the amount of EPS that 1.0 × 108 cells of both strains could actually produce. This, in turn, suggests that the immunological properties of the isolated EPSs and the UV-killed cells were overestimated and underestimated, respectively, in the present experimental format.
Our studies revealed that the UV-killed L. delbrueckii TU-1 and L. plantarum KM-9 cells enhance NO production through the TLR2. TLR2 is known to recognize peptidoglycan and lipoteichoic acids, which are present in the cell membrane of Gram-positive bacteria [29]. Several previous researchers have reported that TLR2 plays an important role in the recognition of probiotic bacteria. For example, the strong Th1 skewing effect of L. acidophilus X37 was reduced by blocking TLR2 in dendritic cells [30]. In addition, L. fermentum YIT0159 and lipoteichoic acids purified from L. fermentum reduced levels of TNF-α in splenocytes from TLR2−/− mice compared with WT mice [31]. These findings suggest that lipoteichoic acids, at least that from some Lactobacillus strains, isa potent TLR2 ligand and a key molecule responsible for immunostimulation by these bacteria. Similarly, we demonstrated here that TLR2 plays an important role in the immunological properties of UV-killed L. delbrueckii TU-1 and L. plantarum KM-9 cells because NO production was abolished when anti-TLR2 antibodies were used. This suggests that the surface components of UV-killed L. delbrueckii TU-1 and L. plantarum KM-9 cells are recognized by TLR2.
Neither the EPS of L. delbrueckii TU-1 nor the EPS of L. plantarum KM-9 seemed to have an effect on macrophages which have been investigated in this study through TLR4 and TLR2. Makino et al. [32] reported that EPS-dependent stimulation of NK cell activity was abrogated in myeloid differentiation factor 88 (MyD88)-knockout (KO) mice. MyD88 is an important adapter molecule in TLR signal transduction pathways, and MyD88-KO mice are unresponsive to ligands of TLR2, TLR4, TLR5, TLR7, and TLR9 [33]. On the other hand, Gorska et al. [34] reported that polysaccharides of L. rhamnosus LOCK0900 were not recognized by TLR2 or TLR4. These findings provide evidence that the physical and chemical properties of the EPSs used in this study strongly influence the way that host cells recognize them. In this connection, our HPLC analysis and ion exchange chromatography analyses revealed that the EPS of L. delbrueckii TU-1 and the EPS of L. plantarum KM-9 are composed of glucose and galactose, but that the molar ratios of the two saccharides are quite different. The evidence suggests that the EPS of L. delbrueckii TU-1 and the EPS of L. plantarum KM-9 are composed of different repeating sugar units with different chemical properties, which in turn elicit different immunological responses from the host cells. Nagai et al. [11] found that the EPS produced by L. delbrueckii OLL1073R-1 was fractionated into neutral EPS (NPS) and acidic EPS (APS) by anion exchange chromatography, and that oral administration of APS, but not NPS, resulted in recovery of the survival rate of influenza virus-infected mice. It should be noted that we prepared our EPS samples thoroughly following the methodology described by Nagai et al. [11] and thus assumed that their purities as EPSs were appropriate enough for subsequent in vitro experiments, although it is possible that they contained other concomitants. If this was the case, the observed immune-modulating properties of our EPS preparation might be ascribed to the concomitants. Based on the findings and speculation above, it will be necessary to conduct more comprehensive immunochemical characterization (purity of the EPSs, their sugar composition, molecular mass, presence of charged substituents). We may then evaluate a possible link between the configurations of EPSs and their immune-regulating properties.
In conclusion, both UV-killed cells of the lactobacillus strains and their EPSs may trigger a Th1-type immune response in a host, with the former being via the TLRs expressed on their epithelium and the latter employing a mechanism yet to be determined, possibly with a novel receptor that is designed to recognize specific patterns of repeating sugar in the EPSs, which will be an interesting topic for future research.
Acknowledgments
This work was supported by Special Coordination Funds for Promoting Science and Technology, the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program (Innovative BioProduction Kobe), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
REFERENCES
- 1.Perdigón G, Fuller R, Raya R. 2001. Lactic acid bacteria and their effect on the immune system. Curr Issues Intest Microbiol 2: 27–42. [PubMed] [Google Scholar]
- 2.Naidu AS, Bidlack WR, Clemens RA. 1999. Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr 39: 13–126. [DOI] [PubMed] [Google Scholar]
- 3.Nagata Y, Yoshida M, Kitazawa H, Araki E, Gomyo T. 2010. Improvements in seasonal allergic disease with Lactobacillus plantarum No. 14. Biosci Biotechnol Biochem 74: 1869–1877. [DOI] [PubMed] [Google Scholar]
- 4.Hashiguchi K, Nagata Y, Yoshida M, Murohushi Y, Kitazawa H. 2011. Chemical and immunological characterization of extracellular polysaccharides produced by Lactobacillus plantarum No. 14. Nihon Nyusankin Gakkaishi 22: 100–105. [Google Scholar]
- 5.Kishimoto M, Nomoto R, Osawa R. 2015. In vitro evaluation of immunological properties of extracellular polysaccharides produced by Lactobacillus delbrueckii strains. Biosci Microbiota Food Health 34: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philpott DJ, Girardin SE. 2004. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 41: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 7.Murofushi Y, Villena J, Morie K, Kanmani P, Tohno M, Shimazu T, Aso H, Suda Y, Hashiguchi K, Saito T, Kitazawa H. 2015. The toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14. Mol Immunol 64: 63–75. [DOI] [PubMed] [Google Scholar]
- 8.Balachandran P, Pugh ND, Ma G, Pasco DS. 2006. Toll-like receptor 2-dependent activation of monocytes by Spirulina polysaccharide and its immune enhancing action in mice. Int Immunopharmacol 6: 1808–1814. [DOI] [PubMed] [Google Scholar]
- 9.Tilsala-Timisjärvi A, Alatossava T. 1997. Development of oligonucleotide primers from the 16S-23S rRNA intergenic sequences for identifying different dairy and probiotic lactic acid bacteria by PCR. Int J Food Microbiol 35: 49–56. [DOI] [PubMed] [Google Scholar]
- 10.Osawa R, Kuroiso K, Goto S, Shimizu A. 2000. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl Environ Microbiol 66: 3093–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagai T, Makino S, Ikegami S, Itoh H, Yamada H. 2011. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int Immunopharmacol 11: 2246–2250. [DOI] [PubMed] [Google Scholar]
- 12.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356. [Google Scholar]
- 13.Mehra R, Barile D, Marotta M, Lebrilla CB, Chu C, German JB. 2014. Novel high-molecular weight fucosylated milk oligosaccharides identified in dairy streams. PLoS One 9: e96040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanoue T, Nishitani Y, Kanazawa K, Hashimoto T, Mizuno M. 2008. In vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264.7 cells. Biochem Biophys Res Commun 374: 565–569. [DOI] [PubMed] [Google Scholar]
- 15.Takada K, Ohno N, Yadomae T. 1994. Binding of lysozyme to lipopolysaccharide suppresses tumor necrosis factor production in vivo. Infect Immun 62: 1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura-Uemura J, Kitazawa H, Kawai Y, Itoh T. 2003. Functional alteration of murine macrophages stimulated with extracellular polysaccharides from Lactobacillus delbrueckii ssp. Bulgaricus OLL1073R-1. Food Microbiol 20: 267–273. [Google Scholar]
- 17.Nishimura H, Fujimoto A, Tamura N, Yajima T, Wajjwalku W, Yoshikai Y. 2005. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J 19: 19–28. [DOI] [PubMed] [Google Scholar]
- 18.Zenhom M, Hyder A, de Vrese M, Heller KJ, Roeder T, Schrezenmeir J. 2011. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J Nutr 141: 971–977. [DOI] [PubMed] [Google Scholar]
- 19.Bahrami B, Macfarlane S, Macfarlane GT. 2011. Induction of cytokine formation by human intestinal bacteria in gut epithelial cell lines. J Appl Microbiol 110: 353–363. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 21.Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. 1988. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 27: 8706–8711. [DOI] [PubMed] [Google Scholar]
- 22.Henrick BM, Nag K, Yao XD, Drannik AG, Aldrovandi GM, Rosenthal KL. 2012. Milk matters: soluble Toll-like receptor 2 (sTLR2) in breast milk significantly inhibits HIV-1 infection and inflammation. PLoS One 7: e40138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun 69: 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockinger B, Veldhoen M. 2007. Differentiation and function of Th17 T cells. Curr Opin Immunol 19: 281–286. [DOI] [PubMed] [Google Scholar]
- 25.Kohno K, Kurimoto M. 1998. Interleukin 18, a cytokine which resembles IL-1 structurally and IL-12 functionally but exerts its effect independently of both. Clin Immunol Immunopathol 86: 11–15. [DOI] [PubMed] [Google Scholar]
- 26.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378: 88–91. [DOI] [PubMed] [Google Scholar]
- 27.Madrigal-Estebas L, Doherty DG, O’Donoghue DP, Feighery C, O’Farrelly C. 2002. Differential expression and upregulation of interleukin-1alpha, interleukin-1beta and interleukin-6 by freshly isolated human small intestinal epithelial cells. Mediators Inflamm 11: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struyf S, Gouwy M, Dillen C, Proost P, Opdenakker G, Van Damme J. 2005. Chemokines synergize in the recruitment of circulating neutrophils into inflamed tissue. Eur J Immunol 35: 1583–1591. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Akira S. 2004. Microbial recognition by Toll-like receptors. J Dermatol Sci 34: 73–82. [DOI] [PubMed] [Google Scholar]
- 30.Zeuthen LH, Fink LN, Frøkiaer H. 2008. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 124: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y. 2003. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol 10: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makino S, Sato A, Goto A, Nakamura M, Ogawa M, Chiba Y, Hemmi J, Kano H, Takeda K, Okumura K, Asami Y. 2016. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J Dairy Sci 99: 915–923. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill LA, Bowie AG. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364. [DOI] [PubMed] [Google Scholar]
- 34.Górska S, Schwarzer M, Jachymek W, Srutkova D, Brzozowska E, Kozakova H, Gamian A. 2014. Distinct immunomodulation of bone marrow-derived dendritic cell responses to Lactobacillus plantarum WCFS1 by two different polysaccharides isolated from Lactobacillus rhamnosus LOCK 0900. Appl Environ Microbiol 80: 6506–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]