Abstract
Lactic acid bacteria are gut flora that play key roles in intestinal homeostasis, which may affect a variety of physiological functions. Our preliminary double-blind, placebo-controlled, parallel-group trials have suggested that intake of heat-killed Lactobacillus casei subsp. casei 327 (designated L. K-1) is effective for improving skin conditions. The aim of this study was to confirm the effect of L. K-1 intake in a randomized, double-blind, placebo-controlled, parallel-group study in healthy female volunteers. Sixty-four subjects were allocated to either the placebo food group (group P, n=32) or active food group (group A, n=32), in which subjects consumed lactobacillus K-1 50 mg (approximately 1 × 1011 bacteria) daily for 8 weeks. After excluding subjects who declined to participate (n=1), violated restrictions (n=4), or were judged ineligible by the principal investigators (n=1), data obtained with 58 subjects (30 in group A and 28 in group P) were analyzed for efficacy by comparing differences from pretrial levels between the two groups. When the level of transepidermal water loss (TEWL) was measured at the arm, the level of TEWL at week 4 of the intake period was significantly lower in group A than group P (p=0.021), suggesting an improvement of skin barrier function. Analysis of skin condition questionnaire data revealed a significant reduction in skin flakiness on the face (week 4). No adverse events were associated with intake of the test foods. The safety of L. K-1 was also confirmed in an independent open-label trial in 11 healthy subjects who consumed excessive amounts of L. K-1 250 mg (approximately 5 × 1011 bacteria). Intake of L. K-1 may therefore be beneficial to skin condition improvement.
Keywords: lactic acid bacteria, Lactobacillus casei, transepidermal water loss, skin conditions, clinical study
INTRODUCTION
Lactic acid bacteria, which are Gram-positive, anaerobic, or facultative aerobic cocci or rods, play an important role in intestinal homeostasis through interactions with other intestinal flora and the host [1, 2]. Growing evidence suggests that intake of lactic acid bacteria has potential health benefits [3,4,5]. In particular, lactobacilli have hypocholesterolemic [6], anticancer [7], hypotensive [8], and immunomodulatory activities [9]. Moreover, the health-promoting activities of lactic acid bacteria are species- and strain-specific [10]. Thus, use of a variety of lactic acid bacteria as a dietary supplement has been investigated extensively [11], and interest in the use of lactic acid bacteria for improvement of skin conditions has been growing [12]. Some reports show beneficial effects on atopic dermatitis or allergic rhinitis [13, 14], skin aging [15], and acne [16]. A recent survey suggested an association between abnormal bowel movements and skin problems such as dry skin, and it found that intake of Bifidobacterium-fermented milk and galacto-oligosaccharides led to improved dry skin in healthy Japanese women [17,18,19]. Similarly, it was reported that intake of yogurt prepared using Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus improved skin hydration in females with chronic constipation and dry skin [20].
Lactobacillus casei subsp. casei 327 (designated L. K-1) was isolated from rice [21]. Orally administered L. K-1 remained viable in the intestinal tract, and L. K-1-supplemented fermented milk improved the intestinal environment [21], was effective in improving defecation [22], and suppressed urinary and fecal mutagen levels that had been induced by burned beef consumption [23]. The results of a preliminary double-blind, placebo-controlled, parallel-group study we performed suggested the benefit of heat-killed L. K-1 on dry skin conditions in healthy women (unpublished observation). Consequently, we conducted a double-blind randomized controlled trial to investigate the effects of consuming a dietary supplement containing heat-killed L. K-1 on skin conditions in healthy female volunteers. In addition, an open-label trial of excessive consumption of L. K-1 was performed to confirm its safety.
MATERIALS AND METHODS
Test foods
The dietary supplements used in this study were tablets containing L. casei subsp. casei 327 (designated L. K-1) or a placebo (Kameda Seika Co., Ltd., Niigata, Japan). The compositions of the tablets are shown in Table 1. Each daily dose (0.5 g; 2 tablets) contained 50 mg (approximately 1 × 1011 bacteria) of L. K-1. In Trial 1 (the efficacy examination), subjects consumed two of the designated tablets once a day with a drink for 8 weeks. The dose of L. K-1 was based on our preliminary data, which suggested that the consumption of L. K-1 at a daily dose of 50 mg/day for 6 weeks prevented an increase in transepidermal water loss (TEWL) from dry skin in female subjects. In Trial 2 (the assessment of product safety after excessive consumption), subjects consumed 500 mg of a powdered formulation that contained 250 mg (approximately 5 × 1011 bacteria) of L. K-1 once a day for 4 weeks.
Table 1. Composition of the test tablets.
| Placebo tablet | Active tablet | |
|---|---|---|
| Ingredients | Glucose, starch, calcium stearate, fine granular silica, hydroxypropylcellulose | L. casei subsp. casei 327a, glucose, starch, calcium stearate, fine granular silica, hydroxypropylcellulose |
| Nutritional facts (value for daily dose, 0.5 g) | ||
| Energy (kcal) | 1.8 | 1.8 |
| Protein (g) | 0.00 | 0.03 |
| Fat (g) | 0.01 | 0.01 |
| Carbohydrate (g) | 0.43 | 0.40 |
| Sodium (mg) | 0.10 | 0.32 |
aThe content of L. casei subsp. casei 327 was 50 mg (approximately 1 × 1011 bacteria) per daily dose (0.5 g; two tablets).
Trial 1: efficacy
To explore the efficacy of L. K-1 intake, we conducted a randomized, double-blind, parallel-group, placebo-controlled study, which was supported by funds from Kameda Seika Co.,Ltd., at the Shin-Yokohama Skinlabo Center, Shirayuri Clinic, Medical Corporation Kaiseikai (Yokohama, Japan), supported by funds from Kameda Seika Co., Ltd. The study protocol was approved by the institutional review board of the Aisei Hospital Ueno Clinic (Tokyo, Japan) on September 10, 2015, and conformed to the Principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare, Japan. The study lasted from September 2015 to January 2016 and was registered as UMIN000018976 in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry, Japan.
The study details were disclosed to subjects before their enrollment, and investigators obtained their informed consent. Then, subjects underwent a screening test (lifestyle questionnaire, medical interview, somatometry, physical examination, laboratory tests, and urinalysis). Healthy women aged 20–64 years who had relatively high rates of TEWL in pretrial testing and relatively low stool frequency (approximately 3−5 times a week) were recruited into the study. The exclusion criteria were as follows: (i) regular ingestion of any food or medicine rich in lactic acid bacteria (such as yogurt, beverages, health foods, supplements, or pharmaceuticals that contained lactic acid bacteria); (ii) regular ingestion of any food, medicine, or dietary fiber that affects skin condition or bowel motion; (iii) use of any laxative at the time of the screening test or regular use of such medications; (iv) any gastrointestinal disease or a history of gastrointestinal surgery except appendectomy; (v) any bowel disease that affects bowel movement such as irritable bowel syndrome and ulcerative colitis; (vi) prior cosmetic medical treatments (such as photo facial procedures or injections of Botox, hyaluronic acid, or collagen) at the sites to be examined in this study; (vii) prior cosmetic medical treatments or hormone therapy (including taking oral contraceptives) at sites other than those to be examined in this study in the past one year; (viii) prior beauty skin care treatments such as scrubbing or depilation at the site to be examined in this study in the past one month or intention to receive such during the study period; (ix) skin sunburned at work, recreation, or sports in the past one month or plans that could result in a sunburn during the study period, excluding daily short exposure to sunshine; (x) habitual use of materials (such as nylon towels except soft nylon towels) to wash the body that affect skin conditions at the sites to be examined in this study; (xi) skin damage (such as wounds) or inflammation (such as pimples) that may affect examination in this study; (xii) suffering from asthma or expectation of an asthma attack during this study; (xiii) pollinosis during September to December in the past 3 years or expectation of suffering pollinosis during the study period; (xiv) expectation of developing rough skin, before or after menstruation, at the sites to be examined in this study; (xv) performing shift work or planning to work the night shift during this study; (xvi) plan to travel abroad during this study; (xvii) serious diseases (such as diabetes mellitus or liver, kidney, or heart failure), diseases that affect sex hormone levels, or a case history of such; (xviii) proneness to allergy; (xix) under treatment for or having a history of treatment for any serious disease; (xx) values that are considerably outside screening test reference ranges; (xxi) participation in any other clinical trial or plan to do so after providing informed consent to participate in this study; (xxii) plan to get pregnant or nurse a baby during this study; (xxiii) judgment of unsuitability based on lifestyle questionnaire responses; and (xxiv) judgment of unsuitability by the principal investigator.
Eligible subjects kept a log of their stool status, physical conditions, and usage of medicine starting 2 weeks before pretrial testing (medical interview, measurements of TEWL and stratum corneum hydration, and skin condition questionnaire). Based on the results of the pretrial testing and analysis of log data, 64 of the eligible subjects were randomly and sequentially assigned using random number tables to one of two masked product groups: those receiving L. K-1-containing tablets (group A; n=32) and those receiving placebo tablets (group P; n=32). The required number of subjects was calculated based on the findings of a preliminary clinical trial investigating the effect of L. K-1 intake on TEWL. TTC Co., Ltd. (Tokyo, Japan), performed the allocation, sealed the envelope containing the allocation table, and eventually opened the envelope; thus the subjects, those who recruited the subjects, and the investigators remained blind to all allocation information during the trial. After randomization, it was confirmed that the two groups had similar age distributions.
The subjects ingested the test tablets daily for 8 weeks and recorded their physical conditions and usage of medicine. After the initial intake of the test tablets, clinical surveys (medical interview, somatometry, physical examination, measurements of TEWL and stratum corneum hydration, and skin condition questionnaire) were administered at 4 and 8 weeks. Laboratory tests were also conducted at week 8. During the trial, the principal investigator and assistants instructed the participants (i) to avoid over-exercising, under- and overeating, and lifestyle changes; (ii) not to consume health foods or dietary supplements; (iii) not to change their habit of dietary intake of food fiber, lactic acid bacteria, health foods that contain oligosaccharides, or fermented food; (iv) to refrain from using medicine that improves bowel movement; (v) to avoid excessive sunburns; (vi) to avoid excessive intake of food ingredients that improve skin conditions, such as ceramide, collagen, hyaluronic acid, B vitamins, and isoflavones; (vii) to refrain from changing type and frequency of regularly used skin-care agents; (viii) not to change the type of towel they are using for body washing; (ix) not to get into a hot tub starting one week before the clinical survey; (x) to go to bed by 12:00 a.m. and sleep well on the day before the clinical survey; (xi) to take a bath on the day before but not the day of the clinical survey; and (xii) to undergo the clinical survey at the same time of day (give or take 1 hour). In addition, the subjects were prohibited from (i) foreign travel; (ii) body painting, beauty treatments, or scrubbing the site to be examined; (iii) shaving or hair removal at the site to be examined starting 3 days before testing; (iv) drinking alcohol from the day before testing until the completion of testing; (v) usage of a facial mask from the day before testing until the completion of testing; and (vi) eating or drinking anything except water starting 4 hours before testing until the completion of testing.
The primary outcome measures were TEWL and stratum corneum hydration at the cheek and upper arm, and the secondary outcome was the skin condition questionnaire. Efficacy analysis was performed on data from subjects who completed the study. However, we excluded from the final analysis those (i) who took less than 80% of the expected number of tablets; (ii) who did not keep an adequate log or whose behavior cast doubt on the reliability of their clinical data; (iii) who met the exclusion criteria after enrollment or who did not follow the required restrictions; and (iv) for whom there were justifiable reasons for exclusion. To analyze the safety of consuming the test tablets, we monitored the development of adverse events in subjects who consumed the test tablet at least once.
Trial 2: safety of excessive consumption
To explore the safety of excessive intake of L. K-1, we conducted an open-label study, which was supported by funds from Kameda Seika Co., Ltd., at the Medical Station Clinic (Tokyo, Japan). The study protocol was approved by the institutional review board of the Aisei Hospital Ueno Clinic on April 21, 2016, and conformed to the Principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare, Japan.
Healthy male and female subjects aged 20–64 years old were recruited into the study, were given an explanation of the study details before enrollment, and in turn gave the investigators their informed consent to participate. Then, subjects were screened (by lifestyle questionnaire, medical interview, somatometry, physical examination, laboratory tests, and urinalysis). Eleven eligible subjects (6 males and 5 females) were selected and assigned to treatment with 250 mg of L. K-1 (containing approximately 5 × 1011 bacteria) daily for 4 weeks over the period from April to July 2016. The subjects recorded their physical conditions, usage of medicine, and the status of test food ingestion during the 4-week trial period and the following 2 weeks. Clinical surveys (medical interview, somatometry, physical examination, laboratory test, and urinalysis) were performed on weeks 2 and 4 of the intake period and 2 weeks after the end of the intake period. During the trial, the principal investigator and assistants instructed the participants (i) to avoid over-exercising and under- and overeating; (ii) to consume one packet and enter it into their check sheet every day; (iii) not to change their exercise habits; (iv) not to consume health foods or dietary supplements; and (v) to go to bed by 12:00 a.m. and sleep well on the day before the clinical survey. In addition, the subjects were prohibited from (i) drinking alcohol during the period from the day before testing until the completion of testing; and (ii) eating or drinking anything except water on the day of testing until the completion of testing.
Measurements of TEWL and stratum corneum hydration level
Measurements were taken on the right cheek (an inner position 4 cm from the lower end of the right earlobe) and left upper arm (inner side, 5 cm above the elbow). The skin region of interest was cleansed using a cleansing agent (DRC Cleansing Liquid, DRC Co., Ltd., Osaka, Japan), washed with a cleaner (DRC Face Wash, DRC Co., Ltd.), rinsed with warm water, and wiped. All measurements were made on subjects seated in a room with a constant temperature (21.0 ± 1°C) and humidity (50.0 ± 5%, relative) for 20 min. TEWL was measured using a Tewameter TM#300 (Courage+Khazaka electronic GmbH, Köln, Germany), and stratum corneum hydration was assessed as skin conductance using a Corneometer CM#825 (Courage+Khazaka electronic GmbH). The measurement on the cheek was performed in the lateral decubitus position, and that on the arm was performed in the supine position. Each measurement took 1 min to complete, and a series of 8 values were obtained. The middle 6 TEWL values were used to calculate the mean value, which was expressed as g/hm2. Likewise, the middle 6 corneum hydration values were used to calculate the mean value, which was expressed in arbitrary units (a.u.).
Evaluation of self-described skin conditions
We evaluated self-described skin conditions based on responses to questionnaires filled out before and after test tablet intake (weeks 4 and 8). The items included in the face skin questionnaire were moistness, smoothness, springiness, dullness, texture, flaky skin, wrinkles around the eye, affinity to cosmetics, and pimples, and the items included in the arm skin questionnaire were moistness, smoothness, skin crazing, and flaky skin at the elbow. The condition of the skin was rated on a 7-point scale ranging from 1 (bad condition) to 7 (good condition). The items with a grade of 7 in pretrial testing were excluded from each subject’s evaluation.
Laboratory tests and urinalysis
The following items were qualitatively determined in blood: fasting levels of white and red blood cells, hemoglobin, hematocrit, platelets, total protein, albumin, total bilirubin, alkaline phosphatase, aspartate transaminase, alanine transaminase, lactate dehydrogenase, γ-glutamyltranspeptidase, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, urea nitrogen, creatinine, uric acid, Na, K, Cl, and glucose. The following items were qualitatively determined in urine: levels of protein, glucose, and occult blood.
Statistical analysis
All measured values are expressed as the mean ± standard deviation (SD). Regarding TEWL and stratum corneum hydration values, the changes from the pretrial values were compared statistically between group A and group P. Between-group comparisons were performed using the unpaired Student’s t-test; for skin condition, they were performed with the Mann-Whitney U test. Within-group comparisons were made using the paired Student’s t-test (for laboratory values). Correlation between arm TEWL and age, height, body weight, or body mass index (BMI) was analyzed using Pearson’s product-moment correlation. Values with p<0.05 were considered statistically significant.
RESULTS
Trial 1: efficacy
Subjects
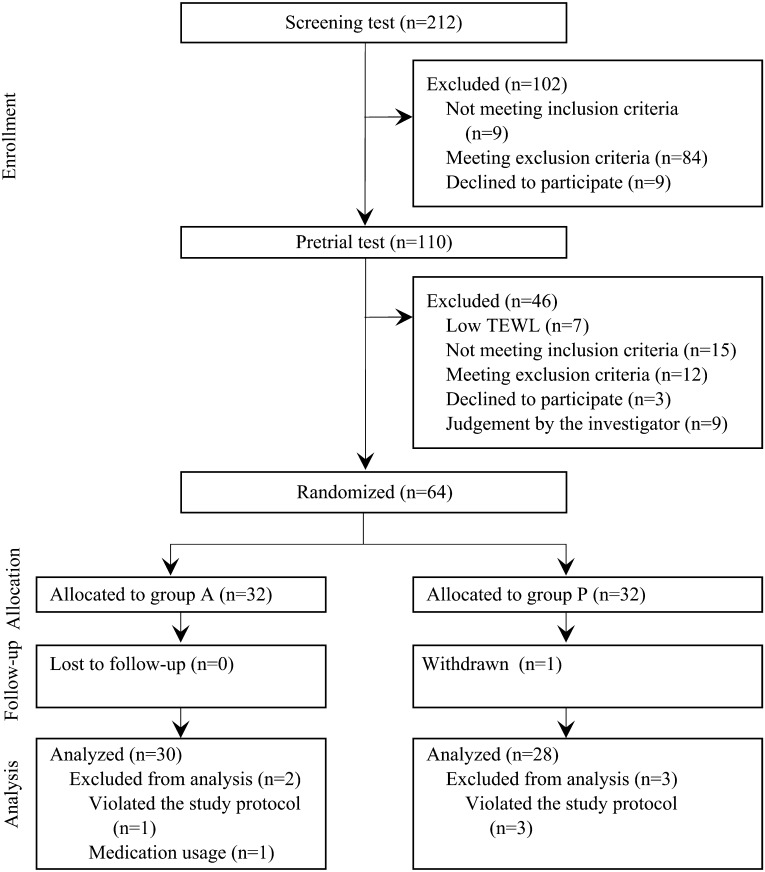
A study outline is shown in Fig. 1. Among the applicants who provided written informed consent (n=212) and completed our screening procedure, 110 subjects were eligible and completed pretrial measurement of TEWL and stratum corneum hydration level. We selected the 64 subjects with the highest TEWL values and allocated them to either the active (L. K-1-containing tablets; group A, n=32) or placebo (group P, n=32) treatment. During the treatment period, we excluded 6 subjects (one in group P who declined further study participation; three in group P and one in group A who violated the study protocol; one in group A who was judged inappropriate for inclusion in the efficacy set by the principal investigators owing to use of antibiotic, anti-inflammatory, and anti-gastric acid medicines). Thus, the data for 58 subjects (30 in group A and 28 in group P) were analyzed. The safety analysis set included all participants (n=32 per group). For analysis of laboratory test values, data for the 1 subject in group P who declined to participate in the study were not considered.
Fig. 1.
Outline of the study.
Subjects in group A consumed L. K-1-containing tablets, and subjects in group P consumed placebo tablets. Exclusion before randomization due to judgment by the investigator (n=9) was mainly based on age, stool frequency, or TEWL value.
The background characteristics for each group are shown in Table 2. There was no significant between-group difference in age, height, body weight, body mass index, TEWL at the cheek or arm, and stratum corneum hydration level at the cheek or arm at the screening test or pretrial test.
Table 2. Background characteristics of the subjects analyzed for efficacy.
| Item | Group | Observed value | p value |
| Number of subjects | A | 30 | NA |
| P | 28 | ||
| Age (years) | A | 41.3 ± 9.0 | 0.766 |
| P | 42.1 ± 11.5 | ||
| Height (cm) | A | 159.27 ± 5.62 | 0.703 |
| P | 158.70 ± 5.69 | ||
| Body weight (kg) | A | 50.50 ± 5.81 | 0.483 |
| P | 49.38 ± 6.20 | ||
| BMI (kg/m2) | A | 19.88 ± 1.85 | 0.537 |
| P | 19.58 ± 1.89 | ||
| TEWL at the cheek (g/hm2) | A | 11.93 ± 2.11 | 0.661 |
| P | 11.71 ± 1.77 | ||
| TEWL at the arm (g/hm2) | A | 9.45 ± 1.02 | 0.061 |
| P | 8.95 ± 0.99 | ||
| Stratum corneum hydration at the cheek (a.u.) | A | 34.31 ± 9.06 | 0.201 |
| P | 30.98 ± 10.47 | ||
| Stratum corneum hydration at the arm (a.u.) | A | 19.69 ± 3.54 | 0.522 |
| P | 20.37 ± 4.44 |
Each value is expressed as the mean ± SD. p values were determined by unpaired Student’s t-tests. NA: not applicable; BMI: body mass index; TEWL: transepidermal water loss.
Effects on TEWL and stratum corneum hydration status
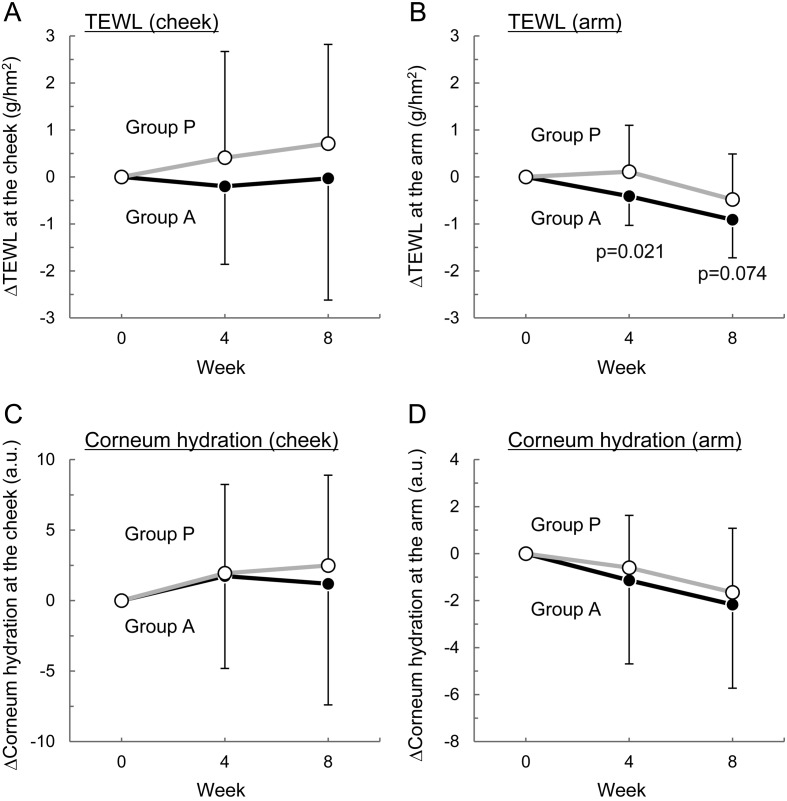
Levels of TEWL and corneum hydration at the cheek and arm before and after test tablets intake are summarized in Table 3, and the changes from the pretrial values are shown in Fig. 2. The cheek TEWL values were lower in group A than in group P throughout the study period, but the differences were not statistically significant (Table 3 and Fig. 2A). The arm TEWL decreased following L. K-1 intake (Table 3), and its change from pretrial values was significantly smaller in group A than in group P (p=0.021) at week 4 (Fig. 2B) but statistically similar between the two groups (p=0.074) at week 8 (Fig. 2B). Since TEWL is a measure of the rate of water loss from the skin, the reduction in TEWL means that L. K-1 intake protects skin from water loss and thereby improves dry skin.
Table 3. TEWL and corneum hydration values before and after L. K-1 intake.
| Item | Group | Pretrial | Week 4 | Week 8 |
|---|---|---|---|---|
| TEWL (g/hm2) | ||||
| Cheek | A | 11.93 ± 2.11 | 11.58 ± 2.30 | 11.90 ± 3.34 |
| P | 11.71 ± 1.77 | 12.12 ± 3.40 | 12.41 ± 3.14 | |
| Arm | A | 9.45 ± 1.02 | 9.05 ± 1.13 | 8.54 ± 1.19 |
| P | 8.95 ± 0.99 | 9.06 ± 1.03 | 8.46 ± 0.87 | |
| Stratum corneum hydration (a.u.) | ||||
| Cheek | A | 34.31 ± 9.06 | 36.50 ± 6.15 | 35.50 ± 7.21 |
| P | 30.98 ± 10.47 | 32.93 ± 9.25 | 33.48 ± 9.89 | |
| Arm | A | 19.69 ± 3.54 | 18.40 ± 3.80 | 17.52 ± 3.45 |
| P | 20.37 ± 4.44 | 19.77 ± 5.02 | 18.71 ± 4.17 | |
Each value represents the mean ± SD. n=30 for group A (except week 4, n=29) and n=28 for group P. TEWL: transepidermal water loss.
Fig. 2.
Time course of the effect of L. K-1 intake on TEWL and stratum corneum hydration at the cheek and arm.
The changes from pretrial values in TEWL (ΔTEWL) at the right cheek (A) and left upper arm (B) and in corneum hydration (Δcorneum hydration) at the right cheek (C) and left upper arm (D) are shown. Each value is expressed as the mean ± SD. p values were calculated between groups using the unpaired Student’s t-test.
We investigated the correlation between arm TEWL and age, height, body weight, or BMI using Pearson’s product-moment correlation. The results are shown in Table 4. While the correlation of arm TEWL in group A (both at weeks 4 and 8) with BMI was higher than with age, height, or body weight, a similar pattern of correlation was observed in group P.
Table 4. Correlation of arm TEWL with age, height, body weight, or BMI.
| Arm TEWL |
||||
|---|---|---|---|---|
| Active |
Placebo |
|||
| Week 4 | Week 8 | Week 4 | Week 8 | |
| Age | 0.254 | 0.128 | 0.108 | 0.101 |
| (0.18) | (0.50) | (0.58) | (0.61) | |
| Height | −0.047 | 0.017 | −0.331 | −0.195 |
| (0.81) | (0.93) | (0.09) | (0.32) | |
| Body weight | 0.143 | 0.266 | 0.113 | −0.013 |
| (0.46) | (0.16) | (0.57) | (0.95) | |
| BMI | 0.229 | 0.325 | 0.377 | 0.117 |
| (0.23) | (0.08) | (0.05) | (0.55) | |
Each value represents Pearson’s product-moment correlation coefficient. p values are shown in parentheses.
Intake of both the experimental and placebo tablets slightly increased the cheek value of stratum corneum hydration and slightly decreased the arm value, and no significant between-group difference was noted at any measurement point (Table 3; Fig. 2C and 2D).
Effect on self-assessed skin condition
We found some differences in survey results between group A and group P (Table 5). Flaky skin on the face was significantly improved in group A after 4 weeks of active tablet intake. However, scores for skin crazing and flaking skin on the elbow were significantly lower in group A than group P.
Table 5. The results of the skin condition questionnaire.
| Position | No. | Item | Group | Week 4 | Week 8 |
|---|---|---|---|---|---|
| Face | Q1 | Moistness | A | (–1, 0, 3) | (–1, 1, 3) |
| P | (–1, 1, 2) | (–1, 1, 3) | |||
| Q2 | Smoothness | A | (–1, 1, 3) | (–1, 1, 4) | |
| P | (–1, 0, 2) | (–1, 1, 3) | |||
| Q3 | Springiness | A | (–1, 1, 3) | (–1, 1, 4) | |
| P | (–1, 1, 3) | (–2, 1, 3) | |||
| Q4 | Dullness | A | (–2, 1, 2) | (–2, 1, 2) | |
| P | (–1, 0, 2) | (–1, 1, 3) | |||
| Q5 | Texture | A | (–1, 1, 3) | (–1, 1, 4) | |
| P | (0, 1, 2) | (0, 1, 4) | |||
| Q6 | Flaky skin | A | (–1, 1, 3)** | (–2, 1, 4) | |
| P | (–2, 0, 2) | (–1, 0, 3) | |||
| Q7 | Wrinkles around the eye | A | (–1, 1, 2) | (–2, 1, 3) | |
| P | (–1, 1, 2) | (–1, 1, 3) | |||
| Q8 | Affinity to cosmetics | A | (–1, 1, 3) | (–1, 1, 4) | |
| P | (–1, 1, 3) | (–1, 1, 4) | |||
| Q9 | Pimples | A | (–1, 1, 3) | (–1, 1, 3) | |
| P | (–2, 0, 2) | (–2, 0, 3) | |||
| Arm | Q10 | Moistness | A | (–1, 1, 2) | (–1, 1, 3) |
| P | (–1, 1, 2) | (–1, 1, 3) | |||
| Q11 | Smoothness | A | (–1, 1, 2) | (–1, 1, 3) | |
| P | (–1, 1, 2) | (–1, 1, 3) | |||
| Q12 | Skin crazing at the elbow | A | (–1, 1, 3) | (–1, 1, 2)** | |
| P | (0, 1, 3) | (0, 1, 3) | |||
| Q13 | Flaky skin at the elbow | A | (–1, 0, 4) | (–1, 1, 3)* | |
| P | (–1, 1, 3) | (–1, 1, 3) | |||
The data are 2.5th percentile, the median, and 97.5th percentile of the change from the pretrial value graded on a 7-point scale ranging from 1 (bad condition) to 7 (good condition). n=30 for group A and n=28 (except Q9, n=27) for group P. *p<0.05 compared with group P by Mann-Whitney U test. **p<0.01 compared with group P by Mann-Whitney U test.
Adverse events
A total of 26 mild adverse events (14 in group A and 12 in group P) occurred in trial 1. The principal investigator judged that none of the mild adverse events were related to intake of either tablet. Regarding laboratory tests results, slight deviations from the reference values were observed in 10 subjects in group A and 9 subjects in group P, but none of the differences were judged to be of any clinical significance.
Trial 2: safety of excessive L. K-1 consumption
All of the enrolled subjects who completed the study were included in the safety analysis set. The clinical and demographic characteristics of the subjects were as follows: age, 39.27 ± 13.62 years; height, 164.49 ± 7.79 cm; body weight, 54.90 ± 9.50 kg; BMI, 20.15 ± 2.12 kg/m2; systolic blood pressure, 107.5 ± 10.1 mmHg; diastolic blood pressure, 64.6 ± 5.0 mmHg; and heart rate, 69.2 ± 8.9 bpm.
During this study, 6 mild adverse events (headache, n=1; cold, n=1; cold symptoms, n=3; and shoulder muscle stiffness, n=1) were observed in 2 subjects and judged by the principal investigators to be unrelated to the test treatment.
There were several slight but significant changes (from pretrial values) in parameters in tests for hematology and blood biochemistry (Table 6) as well as somatometry. These included changes in white blood cell count, hematocrit, total bilirubin, total protein, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and chloride (Table 6), as well as blood pressure (the pretrial, week 2, week 4, and posttrial values were 107.5 ± 10.1, 101.8 ± 8.2, 104.3 ± 10.5, and 101.6 ± 10.0 mmHg, respectively, for systolic blood pressure and 64.6 ± 5.0, 62.4 ± 6.4, 61.0 ± 7.2, and 59.5 ± 6.9 mmHg, respectively, for diastolic blood pressure; significant changes are underlined). None of these changes were of any clinical significance.
Table 6. Effects of excessive intake of L. K-1 on hematology and blood chemistry parameters.
| Item | Pretrial | Week 2 | Week 4 | Posttrial |
|---|---|---|---|---|
| White blood cell (number/μl) | 4,581.8 ± 995.8 | 5,181.8 ± 984.7* | 5,018.2 ± 965.2 | 5,145.5 ± 773.8* |
| Red blood cell (number × 104 μl) | 446.5 ± 48.9 | 452.5 ± 50.1 | 449.4 ± 50.9 | 445.1 ± 53.3 |
| Hemoglobin (g/dl) | 13.50 ± 1.33 | 13.67 ± 1.41 | 13.35 ± 1.36 | 13.40 ± 1.35 |
| Hematocrit (%) | 42.01 ± 3.46 | 43.52 ± 4.09* | 42.85 ± 3.92* | 42.65 ± 4.46 |
| Platelet (number × 104 μl) | 22.87 ± 4.73 | 23.08 ± 4.36 | 23.64 ± 4.16 | 22.39 ± 4.17 |
| AST (U/l) | 19.4 ± 3.5 | 17.5 ± 3.0 | 18.7 ± 2.8 | 18.9 ± 3.1 |
| ALT (U/l) | 14.4 ± 5.1 | 13.1 ± 4.7 | 13.1 ± 3.6 | 13.5 ± 4.8 |
| LDH (U/l) | 167.6 ± 25.8 | 166.6 ± 17.8 | 170.5 ± 23.7 | 169.2 ± 22.8 |
| ALP (U/l) | 183.3 ± 41.1 | 203.0 ± 60.1 | 198.5 ± 82.2 | 188.1 ± 40.5 |
| γ-GTP (U/l) | 17.8 ± 7.6 | 17.4 ± 6.9 | 17.0 ± 6.2 | 17.1 ± 7.6 |
| Total bilirubin (mg/dl) | 0.79 ± 0.18 | 0.63 ± 0.18* | 0.65 ± 0.18 | 0.72 ± 0.38 |
| Albumin (g/dl) | 4.55 ± 0.25 | 4.53 ± 0.22 | 4.45 ± 0.24 | 4.44 ± 0.16 |
| Total protein (g/dl) | 7.21 ± 0.35 | 7.12 ± 0.33 | 7.11 ± 0.42 | 7.03 ± 0.33* |
| Blood urea nitrogen (mg/dl) | 13.18 ± 3.18 | 13.23 ± 3.91 | 13.65 ± 4.21 | 13.08 ± 3.79 |
| Creatinine (mg/dl) | 0.780 ± 0.137 | 0.791 ± 0.128 | 0.798 ± 0.132 | 0.781 ± 0.134 |
| Uric acid (mg/dl) | 4.86 ± 1.24 | 5.05 ± 1.40 | 5.31 ± 1.37 | 4.98 ± 1.29 |
| Total cholesterol (mg/dl) | 195.0 ± 28.1 | 181.7 ± 20.8 | 181.6 ± 27.1 | 183.3 ± 21.8 |
| LDL-C (mg/dl) | 115.7 ± 21.4 | 102.8 ± 17.9* | 101.6 ± 24.7* | 102.5 ± 15.4* |
| HDL-C (mg/dl) | 67.9 ± 12.7 | 61.5 ± 10.4 | 61.8 ± 11.1* | 62.5 ± 12.6 |
| Triglycerides (mg/dl) | 57.2 ± 19.9 | 74.0 ± 38.7 | 81.8 ± 87.4 | 79.1 ± 49.5 |
| Glucose (mg/dl) | 83.1 ± 6.8 | 81.9 ± 8.0 | 81.1 ± 11.8 | 81.3 ± 6.0 |
| Na (mEq/l) | 141.1 ± 0.9 | 141.5 ± 1.4 | 142.0 ± 1.5 | 141.6 ± 1.7 |
| K (mEq/l) | 4.53 ± 0.45 | 4.54 ± 0.45 | 4.58 ± 0.42 | 4.41 ± 0.41 |
| Cl (mEq/l) | 104.8 ± 1.4 | 104.9 ± 1.4 | 103.6 ± 1.7* | 104.8 ± 1.8 |
Each value is the mean ± SD of 11 subjects. *p<0.05 by paired Student’s t-test compared with the respective pretrial values. AST: aspartate transaminase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; ALP: alkaline phosphatase; γ-GTP: γ-glutamyltranspeptidase; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol
DISCUSSION
The present randomized, double-blind, placebo-controlled, parallel-group study was conducted to determine whether oral intake of heat-killed L. casei subsp. casei 327 (L. K-1) is an effective way to improve skin condition in female volunteers. Subjects ingested either a placebo or heat-killed L. K-1 (50 mg, approximately 1 × 1011 bacteria per day) for 8 weeks. Intake of L. K-1 was found to reduce TEWL, a physical measurement of the rate of water loss from the skin, at the arm, but not at the cheek. This may be partly due to differential use of facial skin cosmetics or flu masks, which may bias the measurement of skin hydration levels. Thus, usage of cosmetics and masks should be carefully managed in future studies. L. K-1 intake did not significantly improve stratum corneum hydration level (another skin hydration parameter employed in this study). While TEWL is a measure of transpiration and skin barrier function [24], corneum hydration level measures the reactive capacitance of the skin, with the stratum corneum acting as a dielectric membrane [25]. Since they are different measures of dry skin, the results using these measures may not be consistent. However, multiple approaches to analyze skin hydration conditions are needed to validate current results. Analysis of the skin condition questionnaire data found a significant reduction of skin flakiness on the face (week 4) in group A, but skin flakiness and crazing at the elbow significantly increased (week 8). This inconsistency also emphasizes the need for multiple approaches. Another limitation of this study is the period of the survey, which was conducted from September to January. Skin moisture may vary depending on environmental changes [26]. It may be necessary to conduct a trial in different seasons to extend our results.
The gut microbiome consists of approximately 100 trillion microbial cells of a variety of species, and crosstalk between gut microorganisms and the host plays a pivotal role in human health [27]. Diet and the gut flora composition can affect the condition of the skin. As mentioned in the Introduction, growing evidence suggests that intake of potentially probiotic lactic acid bacteria can improve skin health [17,18,19,20]. The basis of such activities is believed to be the restriction of unfavorable intestinal bacterial population, which produce phenols that may disturb skin conditions [18]. In contrast to these studies, our study used heat-killed lactobacillus cells as a dietary supplement. Whether or not the benefits of the preparation are mediated through a similar mechanism should be studied in future studies.
Regarding the safety of L. K-1 intake, no clinically significant adverse events developed during the intake period of Trial 1. Moreover, Trial 2 demonstrated that excessive amounts of L. K-1 (250 mg, approximately 5 × 1011 bacteria) for 4 weeks were safe to consume. Thus, L. K-1 is safe, and its intake can be used to improve skin health.
Acknowledgments
TTC Co., Ltd. (Tokyo, Japan), supported the work of this clinical trial as a contract research organization.
References
- 1.Hooper LV, Gordon JI. 2001. Commensal host-bacterial relationships in the gut. Science 292: 1115–1118. [DOI] [PubMed] [Google Scholar]
- 2.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. 2015. Role of the normal gut microbiota. World J Gastroenterol 21: 8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinto E, Jiménez P, Caro I, Tejero J, Mateo J, Girbés T. 2014. Probiotic lactic acid bacteria: a review. Food Nutr Sci 5: 1765–1775. [Google Scholar]
- 4.Dicks LM, Botes M. 2010. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes 1: 11–29. [DOI] [PubMed] [Google Scholar]
- 5.Wedajo B. 2015. Lactic acid bacteria: benefits, selection criteria and probiotic potential in fermented food. J Prob Health 3: 129. [Google Scholar]
- 6.Jones ML, Martoni CJ, Prakash S. 2012. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr 66: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 7.Redman MG, Ward EJ, Phillips RS. 2014. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol 25: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 8.Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. 2005. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr 24: 257–265. [DOI] [PubMed] [Google Scholar]
- 9.Wells JM. 2011. Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact 10Suppl 1: S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. 2014. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr 54: 175–189. [DOI] [PubMed] [Google Scholar]
- 11.Ljungh A, Wadström T. 2006. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7: 73–89. [PubMed] [Google Scholar]
- 12.Jeong JH, Lee CY, Chung DK. 2016. Probiotic lactic acid bacteria and skin health. Crit Rev Food Sci Nutr 56: 2331–2337. [DOI] [PubMed] [Google Scholar]
- 13.Żukiewicz-Sobczak W, Wróblewska P, Adamczuk P, Silny W. 2014. Probiotic lactic acid bacteria and their potential in the prevention and treatment of allergic diseases. Cent Eur J Immunol 39: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroi M, Uchi S, Nakamura K, Sato S, Shimizu N, Fujii M, Kumagai T, Saito M, Uchiyama K, Watanabe T, Yamaguchi H, Yamamoto T, Takeuchi S, Furue M. 2011. Beneficial effect of a diet containing heat-killed Lactobacillus paracasei K71 on adult type atopic dermatitis. J Dermatol 38: 131–139. [DOI] [PubMed] [Google Scholar]
- 15.Lee DE, Huh CS, Ra J, Choi ID, Jeong JW, Kim SH, Ryu JH, Seo YK, Koh JS, Lee JH, Sim JH, Ahn YT. 2015. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: a randomized, double blind, placebo-controlled study. J Microbiol Biotechnol 25: 2160–2168. [DOI] [PubMed] [Google Scholar]
- 16.Bowe WP, Logan AC. 2011. Acne vulgaris, probiotics and the gut-brain-skin axis—back to the future? Gut Pathog 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki K, Masuoka N, Kano M, Iizuka R. 2014. Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef Microbes 5: 121–128. [DOI] [PubMed] [Google Scholar]
- 18.Kano M, Masuoka N, Kaga C, Sugimoto S, Iizuka R, Manabe K, Sone T, Oeda K, Nonaka C, Miyazaki K, Ishikawa F. 2013. Consecutive intake of fermented milk containing Bifidobacterium breve strain Yakult and galactooligosaccharides benefits skin condition in healthy adult women. Biosci Microbiota Food Health 32: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori N, Kano M, Masuoka N, Konno T, Suzuki Y, Miyazaki K, Ueki Y. 2016. Effect of probiotic and prebiotic fermented milk on skin and intestinal conditions in healthy young female students. Biosci Microbiota Food Health 35: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isawa K, Noma T, Yamamoto M, Kimura K, Ito H, Taketomo N, Numano K, Kawashima M. 2008. Verifying the ability of yogurt prepared with LB81 lactic acid bacteria to improve skin function. J Intestinal Microbiol 22: 1–5. [Google Scholar]
- 21.Kumagai T, Seno K, Kawamura H, Watanabe T, Okada S. 2004. Effect of Lactobacillus casei subsp. casei 327 on the growth of bifidobacteria and its survival in the intestine. Food Sci Technol Res 10: 143–146. [Google Scholar]
- 22.Seno K, Kumagai T, Watanabe T, Okada S. 2000. Effect of administration of fermented milk using plant origin lactic acid bacteria on defecation. Nippon Shokuhin Kagaku Kogaku Kaishi 47: 555–559 (in Japanese). [Google Scholar]
- 23.Kumagai T, Kawamura H, Watanabe T, Okada S. 2002. Suppression effect of plant origin lactic acid bacteria on urinary and fecal mutagenicity arising from eating burned beef. Nippon Shokuhin Kagaku Kogaku Kaishi 49: 484–490 (in Japanese). [Google Scholar]
- 24.Lehman PA, Franz TJ. 2012. Assessing the bioequivalence of topical retinoid products by pharmacodynamic assay. Skin Pharmacol Physiol 25: 269–280. [DOI] [PubMed] [Google Scholar]
- 25.Clarys P, Clijsen R, Barel AO. 2011. Influence of probe application pressure on in vitro and in vivo capacitance (Corneometer CM 825®) and conductance (Skicon 200 EX®) measurements. Skin Res Technol 17: 445–450. [DOI] [PubMed] [Google Scholar]
- 26.Misery L, Myon E, Martin N, Consoli S, Boussetta S, Nocera T, Taieb C. 2007. Sensitive skin: psychological effects and seasonal changes. J Eur Acad Dermatol Venereol 21: 620–628. [DOI] [PubMed] [Google Scholar]
- 27.Shreiner AB, Kao JY, Young VB. 2015. The gut microbiome in health and in disease. Curr Opin Gastroenterol 31: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]