Abstract
Due to the enduring organ shortage, living donor liver transplantation has been a valuable treatment strategy for advanced liver disease patients for over 20 years. A variety of reviews have summarized the extensive data now available on medical and psychosocial risks to living donors in the aftermath of donation. However, evidence on donor medical and psychosocial outcomes beyond the first year postdonation has not been synthesized in any previous review. The evidence base on such “long-term” outcomes has been growing in recent years. A review of this evidence would therefore be timely and could serve as an important resource to assist transplant centers in their efforts to fully educate prospective donors and gain informed consent, as well as develop appropriate postdonation clinical care and surveillance plans. We reviewed recent literature on long-term donor outcomes, considering (a) medical outcomes, including mortality risk, rates of complications, abnormalities detected in laboratory testing, and the progress of liver regeneration; and (b) donorreported psychosocial outcomes reflecting physical, emotional, and interpersonal/socioeconomic wellbeing, as well as overall health-related quality of life. We summarize limitations and gaps in available evidence, and we provide recommendations for future research and clinical care activities focused on long-term outcomes in liver donors.
Introduction
Living liver donation presents significantly greater risks to donors than kidney donation, the most prevalent type of living organ donation. A sizable research literature documents the likelihood of medical and psychosocial decrements perioperatively and during the remainder of the first year after liver donation. However, whereas living kidney donor outcomes have been charted even decades postdonation, evidence on long-term liver donor risk and safety issues is very slim and has appeared in the literature only within the past 5–6 years. Living donor liver transplantation (LDLT) has been utilized as a treatment strategy for advanced liver disease patients for over 20 years. Thus, the opportunity—and obligation—now exist to examine long-term outcomes in liver donors with the same rigor and intensity as in kidney donors.
Moreover, in contrast to numerous reviews of liver donors’ short-term outcomes (1–9), there has been no review or synthesis of even the limited data available on long-term outcomes. Absent such a synthesis, transplant centers are likely to struggle to include adequate longterm risk information in the education and informed consent process for prospective donors. There is evidence that liver donors want more such information. For example, Castedal et al (10) report that, while 85–94% of liver donors were highly satisfied with predonation information about perioperative risks and short-term complications, only 53% felt well-informed about potential long-term complications and 47% reported that they had little to no information about long-term issues. Furthermore, studies of donors predonation and in the early years postdonation note that up to 50% worry about permanent and long-term health effects of donation (11–13).
Our goal, therefore, was to review recent literature on long-term medical and psychosocial outcomes in liver donors and, for this purpose, we defined “long-term” as beyond the first year postdonation. Although even 2 or 3 years postdonation may seem relatively short-term, the dearth of literature beyond 1 year and the lack of previous integrative reviews make this a meaningful starting point. Because long-term evidence must be considered in context, we briefly summarize findings on short-term outcomes. We then review long-term findings, focusing on studies published in 2010 (when longterm studies first appeared) or later. We conclude with recommendations regarding a research and clinical care agenda for considering long-term outcomes in the future.
Summary of Short-Term Outcomes: The Focus on Safety
LDLT helps to address the critical and growing gap between need versus availability of organs. Despite the organ shortage, however, it is well-recognized that living donor safety comes first. Highly publicized cases of liver donor deaths have sharpened this focus even further. As summarized in recent reviews (2,6,7,9), major efforts to minimize donor risks have been undertaken through refinement of surgical techniques and development of international medical and ethical guidelines for donor selection and management (14–16). In the United States, safety concerns have prompted the development of policies and practice requirements focused on donor informed consent, medical and psychosocial evaluation, and postdonation clinical surveillance and reporting (2,17).
The drive to maximize safety has also spurred a significant research literature. Table 1 summarizes key findings. Estimates of donor mortality and complication rates have converged across studies (3,6,9,18–20), and an international survey reported rates of near-miss events (19). Consistent findings of persistently low platelet counts have been interpreted as reflecting mild portal hypertension contributing to splenomegaly; portal hypertension may be related to reduction in overall hepatic mass (9). However, the speed and degree of liver regeneration have been judged as within safe limits (21).
Table 1.
Key findings on donor outcomes in the short term (approximately first year) after living liver donation
| Medical outcomes |
|
| Psychosocial outcomes |
|
LDLT, living donor liver transplantation.
With regard to psychosocial outcomes, reviews document consistent findings, as noted in Table 1 (1,5,8). In general, donors do not regret having donated. Generic, non-donation-specific health-related quality of life (HRQOL) in donors is high. However, when they are asked more specifically about psychosocial outcomes that they perceive are linked to the donation experience, many report at least some physical, emotional, and social/socioeconomic difficulties. The fact that such problems are reported even by donors with high general HRQOL may be explained by an insensitivity of generic HRQOL measures to the specific donation-related outcomes that donors experience (1,5).
Long-Term Outcomes
Medical outcomes
The major areas considered to date include mortality, complications, liver remnant regeneration, and laboratory test findings. The eight relevant studies are summarized in terms of methodologies and domains assessed in Table 2.
Table 2.
Medical outcomes studies of donors assessed in the long term after living liver donation
| First author, year, country, reference | Donor cohort sample size and graft types | Number of study sites | Study design | Enrollment rate | Loss to follow-up (longitudinal studies only) | Years since donation | Long-term (≥1 year postdonation) medical outcomes |
|---|---|---|---|---|---|---|---|
| Muzaale, 2012, USA (18) | 4,111 (996 LLS, 2742 RL, 359 LL) | Not applicable; national cohort of all donors | Donor and matched comparison groups (national samples of living kidney donors and healthy participants from NHANES III) retrospective review of prospectively recorded data | 100% of eligible donors included | No known loss to follow-up; ascertainment utilized multiple databases | Median, 7.6 year Interquartile range, 4.2–10.1 years |
Mortality |
| Adcock, 2010, Canada (23) | 202 (all RL) | 1 | Single group, retrospective review of prospectively recorded data | 100% of eligible donors included | 7% | Mean, 2.8 year, SD, 1.7 Range, 1.0–7.0 years |
Complications by type and Clavien grade |
| Abecassis, 2012, USA (20) | 740 (707 RL, 33 LL) | 9 | Single group, composed of “retrospective” subgroup (enrolled postdonation with retrospective chart review plus prospective data collection thereafter, n = 396) and “prospective” subgroup (enrolled at donation and followed, n = 344) | Not reported | Not reported | Retrospective subgroup: Median, 3.4 years Range, 0.0–10.4 years Prospective subgroup: Median, 1.8 years Range, 0.0–6.9 years |
Complications by type and Clavien grade |
| Castedal, 2010, Sweden (10) | Subset of 24 of 34 enrolled (13 LLS, 11 RL). Subset included donors with known predonation liver volumes | 1 | Single group, cross-sectional follow-up, with comparison to predonation medical records data | For 34 enrolled: 94% of all donors; 97% of those contacted. (Subset of 24 is 69% of donors contacted.) | For 34 enrolled: Mean, 6.0 years Range, 1.0–12.0 years (specific data for n = 24 not provided) |
Liver remnant regeneration based on volumetric analysis of MRI scans | |
| Klink, 2014, Germany (25) | 47 (24 LLS, 18 RL, 5 LL) | 1 | Single group, retrospective review of prospectively recorded data | Not reported | Not reported | Mean, 1.9 years Range, 0.08–7.0 years |
Liver remnant regeneration based on volumetric analysis of MRI or CT scans |
| Murad, 2016, USA (26) |
|
1 | Single group, cross-sectional follow-up, with comparison to predonation medical records data | For 68 enrolled: 70% of eligible donors. Subset of 45 is 46% of eligible donors. Subset of 60 is 62%of eligible donors. | N = 40: Median 6.0 years Range, 1.7–10.9 years N = 60: Median 5.5 year Range 1.5–10.9 years |
Liver remnant regeneration based on volumetric analysis of MRI scans (postdonation) and CT scans (predonation) Liver function and other laboratory tests | |
| Trotter, 2011, USA (28) | 487 (all adult to adult; all RL or LL; exact n’s and/or %s not reported) | 9 | Single group, including donors enrolled postdonation with retrospective chart review plus prospective data collection thereafter, and donors enrolled at donation and followed. | Not reported | Not reported | Range, 0.0–4.0 years | Liver function and other laboratory tests |
| Lei, 2013, China (29) | 300 (7 LLS, 251 RL, 42 LL) | 1 | Single group, retrospective review of prospectively recorded data | 84% | Not reported | Mean 3.7 years Range 0.2–10.8 years |
Liver function and other laboratory tests |
CT, computed tomography; LLS, left lateral segment; RL, right lobe; LL, left lobe; NHANES, National Health and Nutrition Examination Survey.
Mortality
Muzaale et al (18), in the first comprehensive analysis of long-term mortality risk, found that cumulative mortality in a U.S. national cohort of living liver donors was similar to that in national samples of living kidney donors and healthy community residents at 2 years postdonation (0.3%, 0.2%, 0.3% for the three groups, respectively), 5 years (0.4%, 0.4%, 0.4%), 9 years (0.9%, 1.0%, 0.8%), and 11 years (1.2%, 1.2%, 1.4%). Risk did not vary by type of graft donated. These findings suggest no decrease in longevity in the first decade after liver donation.
It has been argued, however, that this conclusion may be premature (22). For example, because living liver donors are highly screened before donation and thus likely to be healthier than the community resident group, their mortality rates would be expected to be lower than this comparison group. The lack of between-group differences could therefore indicate poorer donor outcomes. Muzaale et al note that kidney donors may comprise the more appropriate comparator because they are also highly screened. However, kidney and liver donors differ in other ways, and they suggest that an ideal comparator might be individuals approved for liver donation but who do not donate for reasons unrelated to health. However, a representative sample of such individuals would be difficult to identify. Despite limitations, the Muzaale et al data serve as the current standard for estimating longterm donor mortality (22).
Complications
The distribution of complications beyond the first year postdonation has been examined in two reports, both of which focused on right lobe donors and found complication rates of ≈40% in the first year postdonation (20,23). Adcock et al (23) found rates of 1.3% in the second year, 1.0% in the third through fifth years, and 0% in donors with >5 years of follow-up in their single-site study. There were no donor deaths and only three complications beyond 1 year postdonation: two at Clavien Grades I to II (keloid; small bowel obstruction), and one at Grade III (incisional hernia).
In the Adult to Adult Living Donor Liver Transplantation Cohort Study (A2ALL), involving a cohort from nine sites with up to ≈10 years of follow-up, 6% of donors first experienced complications at ≥1 year postdonation (20). Hernia, bowel obstruction, and psychological complications were the most common long-term complications and developed even 5 years or more postdonation. Moreover, these same types of complications took longer to resolve. Thus, while 95% of all complications resolved within 1 year of onset, only 75% of hernias and 42% of psychological complications resolved within 1 year. Two of three deaths in the cohort occurred >1 year postdonation and were due to psychological complications (one drug overdose; one suicide). Although potential risk factors for complications were examined, these analyses did not distinguish between short- and longterm complications. Nevertheless, for hernia (in which over half of cases developed in the long-term years), significant risk factors included male gender, older age, and higher BMI at donation.
Despite the importance of the A2ALL findings, these data have limitations. For example, few A2ALL donors had follow-up >6 years (24); it is unknown whether additional complications may develop later. In addition, after the first year or so postdonation, many donors may have sought care at facilities other than the transplant center. Thus, long-term complication rates may have been underestimated. While Adcock et al had high follow-up rates, thus reducing the ascertainment issues, their total follow-up period was shorter (7-year maximum), with few donors having >5 years of follow-up.
Liver remnant regeneration
Three single-site reports compare predonation MRI or computed tomography scans with postdonation scans from up to 12 years postdonation in order to examine liver volume regeneration (10,25,26). One report included serial assessments postdonation, and findings show that volume regeneration continues beyond the first year postdonation (25); this report showed that ultimately the regenerated liver volumes in the vast majority of assessments were indistinguishable from preoperative volumes. The two additional long-term cross-sectional follow-up studies also found little to no pre- versus postdonation mean differences in total volumes (10,26). No differences were noted in late-term total volume as a function of type of graft donated (10,25). However, samples in the studies were relatively small (Table 2), limiting any ability to identify graft-related differences.
Aside from volume, an unanswered question concerns whether component parts of the liver regenerate in the same proportions present before resection (27). This issue may be particularly pertinent for interpreting other health-related changes observed in donors and, therefore, for gauging long-term risks associated with donation, as discussed further below.
Laboratory tests
Beyond the first year postdonation several reports have shown that, while liver function and other laboratory tests are largely normal in liver donors, mean platelet counts in donor samples assessed either cross-sectionally or monitored longitudinally are lower than predonation levels (26,28,29). Approximately 10% of A2ALL donors were found to have platelet counts <150 × 1000/mm3 at 2–3 years postdonation (28); other studies’ findings are similar across follow-up periods of up to 11 years (26,29).
We noted above that speculation about the etiology of the low platelet count centers around inadequate liver regeneration, leading to elevated portal pressure that contributes to splenomegaly (9,28,29). Yet the long-term studies (10,25,26) suggest that regeneration, as measured by volume, would likely have been complete by the longterm time points when platelet counts were assessed. Not only does this emphasize the need to better understand the composition of the regenerated liver (and, namely, the nature of the regeneration of the portal venous complex) (27), but also it is unknown whether any increase in portal pressure (and whether any increase is sustained) increases risk for future hepatic fibrosis (9,28).
Psychosocial outcomes
Donation-related outcomes
Eleven recent studies (10,26,30–38) focused on donors’ long-term perceptions of how donation affected their well-being. Although additional reports have included some donors who were >1 year postdonation, their samples were primarily donors who were early postdonation and/or no specific long-term outcomes were presented. Table 3 summarizes the methodologies of the 11 studies and domains assessed. Generally donors were 4–7 years postdonation at assessment.
Table 3.
Psychosocial outcomes studies of donors assessed in the long term after living liver donation1
| First author, year, country, reference | Donor cohort sample size and graft types | Study design2,3 | Response rate | Years since donation | Outcome measures | Donor-reported outcomes in four domains
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Overall views about donation | Physical symptoms and health concerns | Emotional well-being | Interpersonal and socioeconomic concerns | ||||||
| Castedal, 2010, Sweden (10) | 34 (23 LLS; 11 RL) | Single group, cross-sectional | 94% of all donors; 97% of those contacted | Median, 6.0 years Range, 1–12 years |
Items created by the authors |
|
|
|
|
| Noma, 2011, Japan (30) | 30 (graft type not reported; all adult to adult) | Single group, follow-up | 55% | Mean, 4.3 years, SD, 0.5 Range, 3–5 years |
Validated scales: State-Trait Anxiety Inventory Beck Depression Inventory |
|
|||
| Sotiropoulos, 2011, Germany (31) | 83 (all RL) | Single group, cross-sectional | 75% of those alive; 99% of those able to be contacted | Median, 5.7 years Range, 3.8–10.7 years |
Items created by the authors |
|
|
|
|
| Imamura, 2013, Japan (32) | 47 (29 RL, 18 extended LL) | Single group, cross-sectional | 53% | Mean, 7.2 years Range, 2.9–14.0 years |
Items created by the authors |
|
|
||
| Fournier, 2013, France (33) | 26 (graft type not reported; 24 adult to pediatric, 2 adult to adult) | Single group, cross-sectional | 71% | Mean, 2.0 years Range, 1.2–4.7 years |
Open-ended qualitative items created by the authors |
|
|
|
|
| Fukuda, 2014, Japan (34) | 81 (≈68% LLS; 29% hyper-reduced LLS; ≈2% RL; ≈11% LL; exact n’s and/or %s not reported) | Single group, cross-sectional | 81% | Mean 3.8 years for larger group of 100 in study Range, 2.2–6.0 years |
Items created by the authors |
|
|
|
|
| Kroencke, 2014, Germany (35) | 40 (31 with final assessment) (19 LLS, 19 RL; 2 LL) | Donor and nondonor groups, prospective | 78–100% (postdonation; varies by measure) | All were 2 years at final postdonation assessment | Items created by the authors Validated scale: Hospital Anxiety and Depression Scale |
|
|
||
| Dew, 2016, USA (36) | 517 (all adult to adult; all RL or LL; exact n’s and/or %s not reported) | Single group, cross-sectional | 66% of eligible donors, 71% of those located | Mean 5.8 years SD 1.9 years Range 3–10 years |
Items from previous studies: Simmons’ health worries items and items on views about donation; Holtzman’s items on socio-economic concerns Validated scales: Checklist of Donation-Related Physical Symptoms; Better Person Scale; Post-traumatic Growth Inventory-Short Form |
|
|
|
|
| DiMartini, 2016, USA (37) | 271 (139 with final assessment) (228 RL, 43 LLS or LL, exact n’s and/or %s not reported) | Single group, prospective | 91% of eligible donors | All were 2 years at final postdonation assessment | Items from previous studies: Simmons’ items about interpersonal relationships; Holtzman’s and Smith’s items about socioeconomic concerns |
|
|||
| Humphreville, 2016, USA (38) | 107 (≈85% RL; remainder were LLS; exact n’s and/or %s not reported) | Single group, cross-sectional | 84% | Median 6.9-years mean 7.7-years, SD 3.4-years Range 2–15.7 |
Items created by the authors |
|
|
|
|
| Murad, 2016, USA (26) | 68 (all RL or LL; exact n’s and/or %s not reported) | Single group, cross-sectional | 70% of eligible donors | Median 5.5 years Range, 1.5–10.9 years |
Items created by the authors |
|
|
|
|
LLS, left lateral segment; RL, right lobe; LL, left lobe.
Some studies also assessed generic health-related quality of life; these findings are summarized in Figure 1.
All studies were single-site except Dew et al (36) and DiMartini et al (37), which each included nine sites. Both reports describe data from the Adult to Adult Living Donor Transplantation Cohort study but assess independent cohorts.
Only Kroencke et al (35) included a comparison group in their analyses, consisting of 37 individuals approved as donors but who did not donate. Fournier et al (33) also included individuals who did not donate but did not perform formal comparisons with donors. Imamura et al (32) included additional donors but they were not all beyond 1 year postdonation and have thus been excluded.
Overall views about donation
Similar to findings for donors assessed earlier postdonation, the long-term studies find that most donors (76%–92%) express positive overall views about having donated (10,31,33,36,38). Most have no regrets (96%–100%) (10,33,34), and were comfortable with and/or would make the same decision to donate again (85–100%) (10,26,31,33,36,38).
Physical health symptoms and health concerns
In most studies, well over half of all donors (up to 75%) report ongoing symptoms and/or donation-related health problems (10,31,33–36,38). Studies are consistent in the types of problems reported: the most common are gastrointestinal issues (including heartburn, nausea, fat and food intolerances, chronic diarrhea); general abdominal discomfort and muscle weakness leading to daily activity impairments; incisional pain and discomfort; and incisional hernias (10,31,34–36,38). One report on the impact of incisional scaring (32) found that up to 62% of donors had enduring pain, numbness, or discomfort, with 30% reporting that these issues interfered with activities of daily living.
Regarding general concerns, although most donors (97%) report that they are almost or fully recovered from surgery, up to one quarter judge their health to be worse than before donation or are worried about their current health (34,36,38). From 31% to 44% worry about their future health (33,34,36).
Emotional distress and well-being
Rates of donorreported clinically significant depressive symptoms range from 4% to 22% (10,31,35,38), with as many as half of the cases having no predonation depression history (38). As noted earlier, in the A2ALL cohort, two deaths beyond 1 year resulted from psychological complications (20).
Two reports examined change in average depression and anxiety levels from predonation to >1 year postdonation (30,35). In both studies, depression levels were low, stable over time, and were similar to comparison groups and/or normative levels. However, anxiety levels significantly decreased, and were similar to or better than comparison groups’ levels at final assessment.
In terms of donation-related psychological benefits, from 14% to 31% of donors report improved self-esteem (31,38) and 65% reported feeling general benefit (26). A2ALL study donors were, on average, at least as likely as previously studied living donor cohorts to feel that they were “better persons” for having donated, and they reported levels of personal psychological growth from the donation experience similar to growth levels experienced by populations experiencing other types of life stressors (36).
Interpersonal and socioeconomic concerns
Concerning interpersonal issues, donor relationships with family or the recipient are only rarely worse postdonation (0–7%), with 26% to 56% of donors reporting improved relationships (26,37). Two studies examined interpersonal concerns related to surgical scarring and body image, with one noting that one quarter of donors experienced worsened body image (26), and the other noting discomfort in about two thirds of donors in exposing the scar in daily life (e.g. in public spas) (32).
Among socioeconomic issues, studies find a wide range of employment rates postdonation (48–95%) (10,26,34,38), but donors generally do not attribute any unemployment or career path changes to donation (10,33,34,36–38). From 55% to 65% of donors incurred out-of-pocket costs (26,36,37) and 15–37% report financial strains due to donation (33,36,37). Castedal et al (10) noted that 32% received only partial, if any, reimbursement for lost income. Problems obtaining or keeping insurances (health and/or life) are reported by 3% to 11% of donors (26,31,36,37).
Risk factors for poor psychosocial outcomes
Risk factors have received limited consideration. A key difficulty is that psychosocial outcomes span multiple domains, making it difficult to arrive at either an overall picture of donors with better versus poorer outcomes, or who is at risk for poorer outcomes. Furthermore, most samples are small, precluding multivariable risk factor analysis. In the largest cohort to date, Dew et al (36) used a multivariable exploratory technique to identify distinct donor groups based on their responses across psychosocial domains. They identified one donor group (15% of the sample) with high psychological benefit from donation and low levels of physical health problems or socioeconomic problems. In the remaining four identified groups, donors showed less favorable profiles. For example, one group (31% of the sample) included donors who were the most likely to have physical health and socioeconomic problems and who showed the lowest psychological benefit. From a large set of potential risk factors encompassing donor demographics, clinical characteristics, and recipient vital status, only three factors emerged as important: men, non-Hispanic white donors, and younger donors were most likely to fall into the groups that had the less-than-optimal combinations of psychological benefit versus physical and socioeconomic problems.
Generic HRQOL outcomes
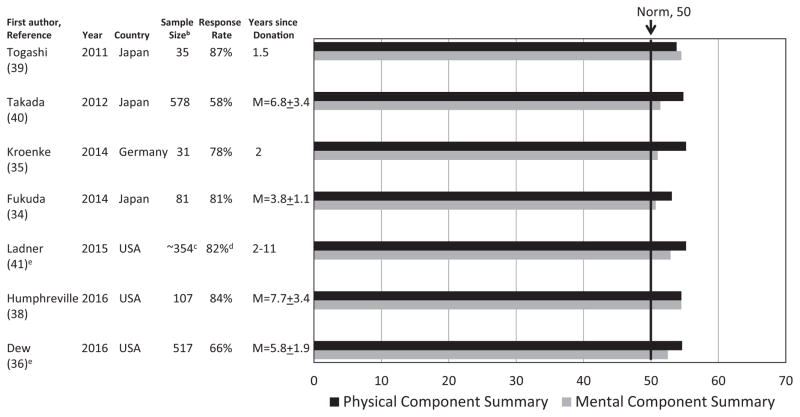
Eight publications, reflecting seven independent studies, assessed donors’ long-term non-donation-specific HRQOL (30,34–36,38–41). All used the Short Form-36 (SF-36). Additional study characteristics are included in Figure 1.
Figure 1. Average SF-36 Physical Component Summary and Mental Component Summary scores in seven recent studies of long-term HRQOL in living liver donorsa.
HRQOL, health-related quality of life.
aStandard deviation or standard error bars for these averages are not displayed because this information is not provided in all reports. In an additional report on a subset of donors from the Takada et al (40) report, Noma et al (30) found similarly high average HRQOL levels with another generic measure (World Health Organization QOL Assessment, Brief Version). bTogashi et al (39) and Ladner et al (41) do not report sample size by graft type donated; for Takada et al (40), there were 367 left lobe and 211 right lobe donors. For all remaining studies, see Table 3 for information on sample distribution by graft type donated. cNumber of donors with any SF-36 follow- up data between 3 months and 11 years postdonation. The number of donors with data specifically between 2 and 11 years postdonation is not reported. dResponse rate pertains to the 354 donors with any data postdonation. Response rate for individuals with data between 2 and 11 years postdonation is not reported. eBoth reports describe findings from the Adult to Adult Living Donor Transplantation Cohort study. Ladner et al (41) describe research conducted during the first period of funding; Dew et al (36) describe research conducted during the second period of funding.
Average HRQOL levels
Figure 1 displays findings for donors’ average scores on the two key SF-36 summary variables. Relative to country-specific norms, these reports consistently show that general HRQOL is, on average, high in the long-term and exceeds normative levels.
Two reports also compared their samples’ average HRQOL levels to other community-based comparison populations (35,36) and found few to no clinically meaningful differences. Two studies reporting prospective data (35,39) noted that SF-36 Physical Component Summary (PCS) scores declined in the early months postdonation, but scores then rebounded. Mental Component Summary (MCS) scores were stable over time in both studies.
Risk factors for poor HRQOL
Studies have not identified any demographic or clinical risk factors associated with lower long-term PCS scores (34,35,39,40). In contrast, lower long-term MCS scores are associated with more predonation concerns about donation (34), a longer postdonation hospitalization (36), and more long-term medical comorbidities in the donor (40). Only two studies considered differences by type of graft donated, with one reporting poorer HRQOL in right or left lobe donors versus left lateral segment donors (35), and the second study comparing right and left lobe donors and observing no differences (40).
Conclusions and Recommendations
There is growing international consensus that the longterm impact of living liver donation demands greater attention in both research and clinical arenas (5,8,14–16). There have been calls for Western countries to increase the proportion of liver transplants from living donors in order to reduce the organ shortage (42,43). This, and the fact that LDLT is already prevalent in Asia and the Middle East because it is the culturally accepted transplant option (44), together make data collection on long-term outcomes imperative if we are to ethically and responsibly gain donors’ informed consent and develop appropriate postdonation clinical surveillance plans.
Several conclusions are suggested by the long-term outcomes research to date. First, long-term mortality and risks of most types of complications appear low, particularly relative to short-term risks. Unlike growing evidence of increased risk for end-stage renal disease in kidney donors (45), long-term complications resulting in advanced liver disease in liver donors have not been reported, although lack of reporting does not mean that such disease has never occurred. However, the absence of evidence may also reflect the fact that the liver regenerates. Liver regeneration appears complete by several years postdonation, based on volumetric analyses. Donors show some enduring laboratory test abnormalities, principally low platelet counts. They show high general HRQOL and do not regret donation. But donor reports of enduring donation-related physical symptoms, health concerns, psychological distress, and financial burdens are all too common.
Perhaps the most significant gap in the long-term outcomes literature is the lack of data beyond the first decade postdonation. Other priority areas for research, as well as clinical activities that might promote optimal longterm outcomes, are shown in Table 4. An important limitation in existing research is that data emanate from a small pool of mostly single-site studies. These studies often rely on small samples, and uniformly fail to consider whether power is sufficient to examine effects of interest. Furthermore, psychosocial studies are predominantly cross-sectional, response rates range widely and, while generic HRQOL has been assessed with validated scales, most studies of donation-specific psychosocial concerns rely on unvalidated measures despite the availability of more robust scales to measure the concepts of interest. Detailed examinations of risk factors for poor medical or psychosocial outcomes are rare. In particular, unlike kidney donors, the liver donor population is very heterogeneous in terms of specific graft type donated, and it is known that right lobe donors face higher risks than other liver donors in the short-term. It is important to delineate whether this is also the case in the long-term. Yet type of graft donated is not consistently reported or considered in analyses of medical or psychosocial outcomes. Furthermore, mechanisms accounting for long-term laboratory test abnormalities and donor-reported difficulties remain poorly understood. With respect to the latter, since only one report adopted a qualitative approach to patient assessment (but did not always distinguish between actual donors and donor candidates who did not donate) (33), the question arises as to whether the full range of donor concerns have been adequately captured in the long-term literature to date.
Table 4.
Long-term outcomes in living liver donors: Recommendations for a research and clinical agenda for the future
| Area | Recommendations | |
|---|---|---|
| Research | Duration of follow-up: | Extend analyses of medical and psychosocial outcomes beyond the first decade postdonation. |
| Study samples: | Identify and assess suitable comparison groups of individuals with similar health status as living liver donors before donation; consider whether individuals undergoing other types of abdominal surgeries might be enrolled for comparison purposes, including, if feasible, individuals undergoing liver resection for benign disease. | |
| Study sites: | Examine long-term mortality in national cohorts beyond the United States, including cohorts that represent the different major areas of the world where LDLT is performed. For other long-term medical and psychosocial outcomes, expand the limited range of countries and areas of the world that have provided data to date. Design studies that directly compare long-term medical and psychosocial outcomes across sites in different countries and consider whether meta-analyses could be performed to examine differences across sites, countries, or geographical regions. |
|
| Assessments: | Utilize research methodologies (e.g. qualitative approaches) to uncover any additional areas of donor psychosocial concerns inadequately assessed or overlooked in quantitative investigations. In quantitative investigations of psychosocial outcomes, utilize established, validated measures rather than items with unknown performance characteristics. |
|
| Risk factors and mechanistic factors: | Enroll samples of sufficient size so that analyzes are powered to detect clinically significant differences between donors depending on graft donated or on other risk factors. Calculate and report power to detect effects as part of the study design. Move beyond cross-sectional/retrospective studies to perform prospective data collection with serial measurements to determine trajectories of onset and change in medical and psychosocial outcomes. Expand research comparing medical and psychosocial outcomes in donors as a function of graft type (e.g. left lateral segment vs. left lobe vs. right lobe donation). Perform long-term mechanistic studies in order to better understand pathways leading to and influencing observed clinical abnormalities and psychosocial difficulties. Examine whether medical outcomes in the long term are related to or predict the degree of psychosocial difficulties expressed by living donors, including impact on overall HRQOL. Identify predonation and early postdonation risk factors and potential biomarkers for long-term development or persistence of medical complications, abnormalities in liver regeneration, and abnormalities detected in laboratory testing. Identify predonation and early postdonation risk factors for impaired long-term psychosocial outcomes in the domains of physical, emotional, and interpersonal/socioeconomic well-being. Assess donor perceptions of gaps in predonation education and informed consent based on their experiences in the long term since donation. |
|
| Clinical | Predonation | Incorporate information on long-term medical and psychosocial outcomes into predonation educational and informed consent discussions with donors. |
| Donation and early postdonation | Develop preventive interventions or alternative surgical techniques to avoid development of common long-term medical complications such as incisional hernias. Provide postdonation education that includes strategies to prevent or manage the most common long-term complications and donor-reported symptoms (e.g. gastrointestinal problems and associated limitations in daily life). Augment efforts to assist donors to identify additional financial resources as needed to address unexpected financial burdens arising from donation or its aftermath. |
|
| Long-term postdonation | Provide routine clinical follow-up care to donors well beyond the first year postdonation and include assessment not only of medical but also psychosocial parameters. Provide heightened clinical surveillance into the long term for individuals with short-term complications and abnormal laboratory findings. |
LDLT, living donor liver transplantation; HRQOL, health-related quality of life.
These types of limitations in the research base hamper transplant centers’ abilities to educate and inform potential living donors during the evaluation process. Postdonation, international guidelines call for clinical follow-up by transplant centers for at least 2 years, preferably longer (15), or even recommend lifelong follow-up (16). In the United States, the Scientific Registry of Transplant Recipients is conducting a feasibility study to explore whether a living kidney and liver donor registry could be formed to collect long-term follow-up information (46). Although difficulties in following donors for either research or clinical surveillance and care have been discussed in many forums (14,24), such information is critical for understanding—and potentially intervening upon—the risks and potential sequelae of living liver donation. These activities will help to ensure that donor safety continues to come first.
Acknowledgments
Preparation of this article was supported in part by National Institute of Diabetes & Digestive & Kidney Diseases grants U01-DK62467 and U01- DK85587.
Abbreviations
- A2ALL
Adult to Adult Living Donor Liver Transplantation Cohort Study
- HRQOL
healthrelated quality of life
- LDLT
living donor liver transplantation
- MCS
Mental Component Summary
- PCS
Physical Component Summary
- SF-36
Short-Form-36
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Dew MA, Zuckoff A, DiMartini AF, DeVito Dabbs AJ, et al. Prevention of poor psychosocial outcomes in living organ donors: From description to theory-driven intervention development and initial feasibility testing. Prog Transplant. 2012;22:280–292. doi: 10.7182/pit2012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaPointe Rudow D, Warburton KM. Selection and postoperative care of the living donor. Med Clin N Am. 2016;100:599–611. doi: 10.1016/j.mcna.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Middleton PF, Duffield M, Lynch SV, et al. Living donor liver transplantation–adult donor outcomes: A systematic review. Liver Transpl. 2006;12:24–30. doi: 10.1002/lt.20663. [DOI] [PubMed] [Google Scholar]
- 4.Nadalin S, Capobianco I, Panaro F, et al. Living donor transplantation in Europe. Hepatobiliary Surg Nutr. 2016;5:159–175. doi: 10.3978/j.issn.2304-3881.2015.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh ND, Ladner D, Abecassis M, Butt Z. Quality of life for donors after living donor liver transplantation: A review of the literature. Liver Transpl. 2010;16:1352–1358. doi: 10.1002/lt.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quintini C, Hashimoto K, Diago T, Miller C. Is there an advantage of living over deceased donation in liver transplantation? Transplant Int. 2013;26:11–19. doi: 10.1111/j.1432-2277.2012.01550.x. [DOI] [PubMed] [Google Scholar]
- 7.Song GW, Lee SG. Living donor liver transplantation. Curr Opin Organ Transplant. 2014;19:217–222. doi: 10.1097/MOT.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 8.Thys K, Schwering KL, Siebelink M, et al. Psychosocial impact of pediatric living-donor kidney and liver transplantation on recipients, donors, and the family: A systematic review. Transplant Int. 2015;28:270–280. doi: 10.1111/tri.12481. [DOI] [PubMed] [Google Scholar]
- 9.Trotter JF. Challenges in living donor liver transplantation. Clin Liver Dis. 2014;18:651–660. doi: 10.1016/j.cld.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Castedal M, Andersson M, Polanska-Tamborek D, Friman S, Olausson M, Fehrman-Ekholm I. Long-term follow-up of living liver donors. Transplant Proc. 2010;42:4449–4454. doi: 10.1016/j.transproceed.2010.09.114. [DOI] [PubMed] [Google Scholar]
- 11.DiMartini A, Cruz RJ, Dew MA, et al. Motives and decision making of potential living liver donors: Comparisons between gender, relationships and ambivalence. Am J Transplant. 2012;12:136–151. doi: 10.1111/j.1600-6143.2011.03805.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishizaki M, Kaibori M, Matsui K, Kwon AH. Change in donor quality of life after living donor liver transplantation surgery: A single-institution experience. Transplant Proc. 2012;44:344–346. doi: 10.1016/j.transproceed.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Trotter JF, Talamantes M, McClure M, et al. Right hepatic lobe donation for living donor liver transplantation: Impact on donor quality of life. Liver Transpl. 2001;7:485–493. doi: 10.1053/jlts.2001.24646. [DOI] [PubMed] [Google Scholar]
- 14.Barr ML, Belghiti J, Villamil FG, et al. A report of the Vancouver Forum on the care of the live organ donor: Lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81:1373–1385. doi: 10.1097/01.tp.0000216825.56841.cd. [DOI] [PubMed] [Google Scholar]
- 15.Miller CM, Durand F, Heimbach JK, et al. The international liver transplant society guidelines on living liver donation. Transplantation. 2016;100:1238–1243. doi: 10.1097/TP.0000000000001247. [DOI] [PubMed] [Google Scholar]
- 16.Manas D, Burnapp L, Andrews PA. Summary of the British transplantation society UK guidelines for living donor liver transplantation. Transplantation. 2016;100:1184–1190. doi: 10.1097/TP.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 17.Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) Policy, Chapter 14: Living Donation, with modifications approved. 2016 Apr 14; [cited 2016 May 30]. Available from https://optn.transplant.hrsa.gov/governance/policies/
- 18.Muzaale AD, Dagher NN, Montgomery RA, Taranto SE, McBride MA, Segev DL. Estimates of early death, acute liver failure, and long-term mortality among live liver donors. Gastroenterology. 2012;142:273–280. doi: 10.1053/j.gastro.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Cheah YL, Simpson MA, Pomposelli JJ, Pomfret EA. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: A world-wide survey. Liver Transpl. 2013;19:499–506. doi: 10.1002/lt.23575. [DOI] [PubMed] [Google Scholar]
- 20.Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy—a comprehensive report. Am J Transplant. 2012;12:1208–1217. doi: 10.1111/j.1600-6143.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olthoff KM, Emond JC, Shearon TH, et al. Liver regeneration after living donor transplantation: Adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015;21:79–88. doi: 10.1002/lt.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trotter JF, Everhart JE. Outcomes among living liver donors. Gastroenterology. 2012;142:207–210. doi: 10.1053/j.gastro.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Adcock L, Macleod C, Dubay D, et al. Adult living liver donors have excellent long-term medical outcomes: The University of Toronto liver transplant experience. Am J Transplant. 2010;10:364–371. doi: 10.1111/j.1600-6143.2009.02950.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown RS, Jr, Smith AR, Dew MA, Gillespie BW, Hill-Callahan P, Ladner DP. Predictors of donor follow-up after living donor liver transplantation. Liver Transpl. 2014;20:967–976. doi: 10.1002/lt.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klink T, Simon P, Knopp C, et al. Liver remnant regeneration in donors after living donor liver transplantation: Long-term follow-up using CT andMR imaging. Fortschr Röntgenstr. 2014;186:598–605. doi: 10.1055/s-0033-1355894. [DOI] [PubMed] [Google Scholar]
- 26.Murad SD, Fidler JL, Poterucha JJ, et al. Longterm clinical and radiological follow-up of living liver donors. Liver Transpl. 2016;22:934–942. doi: 10.1002/lt.24442. [DOI] [PubMed] [Google Scholar]
- 27.Millis J. Small-for-size donor syndrome? Liver Transpl. 2011;17:355–356. doi: 10.1002/lt.22277. [DOI] [PubMed] [Google Scholar]
- 28.Trotter JF, Gillespie BW, Terrault NA, et al. Laboratory test results after living liver donation in the Adult-to-Adult living donor liver transplantation cohort study. Liver Transpl. 2011;17:409–417. doi: 10.1002/lt.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei J, Yan L, Wang W. Donor safety in living donor liver transplantation: A single-center analysis of 300 cases. PLoS ONE. 2013;8:e61769. doi: 10.1371/journal.pone.0061769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noma S, Hayashi A, Uehara M, Uemoto S, Murai T. Comparison between psychosocial long-term outcomes of recipients and donors after adult-to-adult living donor liver transplantation. Clin Transplant. 2011;25:714–720. doi: 10.1111/j.1399-0012.2010.01337.x. [DOI] [PubMed] [Google Scholar]
- 31.Sotiropoulos GC, Radtke A, Molmenti EP, et al. Long-term follow-up after right hepatectomy for adult living donation and attitudes toward the procedure. Ann Surg. 2011;254:694–700. doi: 10.1097/SLA.0b013e31823594ae. [DOI] [PubMed] [Google Scholar]
- 32.Imamura H, Soyama A, Takatsuki M, et al. Self-assessment of postoperative scars in living liver donors. Clin Transplant. 2013;27:E605–E610. doi: 10.1111/ctr.12226. [DOI] [PubMed] [Google Scholar]
- 33.Fournier V, Foureur N, Rari E. The ethics of living donation for liver transplant: Beyond donor autonomy. Med Health Care Philos. 2013;16:45–54. doi: 10.1007/s11019-012-9430-8. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda A, Sakamoto S, Shigeta T, et al. Clinical outcomes and evaluation of the quality of life of living donors for pediatric liver transplantation: A single-center analysis of 100 donors. Transplant Proc. 2014;46:1371–1376. doi: 10.1016/j.transproceed.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 35.Kroencke S, Nashan B, Fischer L, Erim Y, Schulz KH. Donor quality of life up to two years after living donor liver transplantation: A prospective study. Transplantation. 2014;97:582–589. doi: 10.1097/01.TP.0000438206.04348.b2. [DOI] [PubMed] [Google Scholar]
- 36.Dew MA, DiMartini AF, Ladner DP, et al. Psychosocial outcomes 3 to 10 years after donation in the Adult to Adult Living Donor Liver Transplantation Cohort Study. Transplantation. 2016;100:1257–1269. doi: 10.1097/TP.0000000000001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiMartini A, Dew MA, Liu Q, et al. Social and financial outcomes of living liver donation: A prospective investigation within the Adult-to-Adult Living Liver Cohort Study-2 (A2ALL-2) Am J Transplant. 2016 doi: 10.1111/ajt.14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphreville VR, Radosevich DM, Humar A, et al. Longterm health-related quality of life after living liver donation. Liver Transpl. 2016;22:53–62. doi: 10.1002/lt.24304. [DOI] [PubMed] [Google Scholar]
- 39.Togashi J, Sugawara Y, Tamura S, et al. Donor quality of life after living donor liver transplantation: A prospective study. J Hepato-biliary-pancreatic Sci. 2011;18:263–267. doi: 10.1007/s00534-010-0340-y. [DOI] [PubMed] [Google Scholar]
- 40.Takada Y, Suzukamo Y, Oike F, et al. Long-term quality of life of donors after living donor liver transplantation. Liver Transpl. 2012;18:1343–1352. doi: 10.1002/lt.23509. [DOI] [PubMed] [Google Scholar]
- 41.Ladner DP, Dew MA, Forney S, et al. Long-term quality of life after liver donation in the adult to adult living donor liver transplantation cohort study (A2ALL) J Hepatol. 2015;62:346–353. doi: 10.1016/j.jhep.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy GA, Selzner N, Grant DR. Fostering liver living donor liver transplantation. Curr Opin Organ Transplant. 2016;21:224–230. doi: 10.1097/MOT.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 43.Salvalaggio PR, Seda Neto J, Alves JA, et al. Consensus, dilemmas, and challenges in living donor liver transplantation in Latin America. Transplantation. 2016;100:1161–1164. doi: 10.1097/TP.0000000000001180. [DOI] [PubMed] [Google Scholar]
- 44.Chen CL, Kabiling CS, Concejero AM. Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol. 2013;10:746–751. doi: 10.1038/nrgastro.2013.194. [DOI] [PubMed] [Google Scholar]
- 45.Grams ME, Sang Y, Levey AS, et al. Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med. 2016;374:411–421. doi: 10.1056/NEJMoa1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Advisory Committee on Organ Transplantation, U.S. Department of Health and Human Services (HHS) Virtual Meeting. Washington, D.C: 2015. Nov 17, [cited 2016 May 30] Available from http://www.organdonor.gov/legislation/26meetingsummary.pdf. [Google Scholar]