Abstract
The endothelial surface layer (ESL) consists of the endothelial cell (EC) glycocalyx and adsorbed proteins, and forms a barrier between blood and the EC. Enzymatic shedding of the ESL in response to cytokines may expose receptors for leukocyte (WBC) adhesion and increase vascular permeability. Thus, intravital microscopy was used to explore stabilization of the ESL with low molecular weight heparin (LMWH) to mitigate structural changes with inflammation. Following bolus infusions (i.v.) of LMWH (0.12–1.6 mg/kg), shedding of glycans in response to 10−7M fMLP was measured by loss of fluorescently labeled lectins bound to the EC and WBC-EC adhesion was monitored in post-capillary venules of rat mesentery. During a 30 min exposure to fMLP, a 50% reduction in fluorescence (indicative of glycan shedding) occurred at the lowest dose of LMWH whereas a 50% increase occurred (indicative of ESL compaction) at the highest dose. Shedding was reduced by LMWH in a dose dependent manner with an EC50 of 0.6 mg/kg. Concomitant WBC-EC adhesion increased over 3-fold for all doses of LMWH. However, at a dose of 1.6 mg/kg, WBC-EC adhesion did not rise significantly during the initial 10 min exposure to fMLP. Correlation of WBC adhesion with intensity of the lectin stain for all measurements revealed a significant 40% reduction in adhesion as intensity increased 50%. This relationship was attributed to LMWH inhibition of heparanase and/or binding to components of the glycocalyx that resulted in mitigation of glycan shedding, compaction of the lectin stain and stabilization of the glycocalyx.
Keywords: Heparin, Endothelium, Glycocalyx, Venules, Mesentery, Inflammation
Graphical Abstract
Introduction
The vascular endothelium is coated with a layer of polysaccharides and transmembrane proteins that serves as a barrier to extravasation of solutes and leukocyte (WBC) adhesion. In light of visualizations by electron microscopy by Bennett and others (Bennett et al., 1959; Luft, 1966) and its predominant polysaccharide constituents, Bennett (Bennett et al., 1959) termed it the “glycocalyx,” as derived from the Latin for “sweet husk.” The fine structure of the glycocalyx has been described as a network of glycoproteins on the order of 50 to 100 nm thick, with a characteristic spacing between proteoglycans (PGs) of 20 nm that accounts for the resistance to filtration of small molecules (Squire et al., 2001). With the addition of an adsorbed layer of proteins the resultant endothelial surface layer (ESL) has been shown to extend into the lumen of microvessels 400–500 nm (Vink and Duling, 1996), which significantly exceeds dimensions obtained in either fixed specimens or cultured cells (Chappell et al., 2009b). The molecular composition and structure of the ESL has been summarized in numerous reviews (Chappell et al., 2009b; Gotte, 2003; Pries et al., 2000; Reitsma et al., 2007; Weinbaum et al., 2007). The most prominent components of the glycocalyx are the glycosaminoglycans (GAGs) heparan sulfate (HS), chondroitin sulfate (CS), and hyaluronan (HA). The GAGs, HS, and CS are covalently linked to membrane-bound proteoglycans (PGs). The density of GAGs on PGs varies, (Reitsma et al., 2007) and each PG may carry multiple chains of HS and CS, typically with a ratio of HS:CS of about 4:1 (Rapraeger, 1989). In addition to GAG-carrying PGs, adsorbed blood-borne soluble proteins comprise substantial components of the glycocalyx and may be decreased by removing plasma proteins.(Adamson and Clough, 1992; Huxley and Curry, 1991). Under normal physiological conditions, the structure of the glycocalyx layer is stable, and its molecular composition results from a dynamic balance between continued biosynthesis of glycans and shear-dependent alterations (Arisaka et al., 1995; Grimm et al., 1988).
Shedding of the ESL has been observed in numerous pathological conditions such as hyperglycemia (Zuurbier et al., 2005), endotoxemia and septic shock (Hofmann-Kiefer et al., 2009), the presence of oxidized LDL (Constantinescu et al., 2001), exposure to TNF-α (Chappell et al., 2009a), exposure to atrial natriuretic peptide (Bruegger et al., 2005), abnormal blood shear stress (Gouverneur et al., 2006; Haldenby et al., 1994), ischemia–reperfusion injury (Mulivor and Lipowsky, 2004), light-induced production of free radicals (Vink and Duling, 1996), and during by-pass surgery (Rehm et al., 2007; Svennevig et al., 2008). Inflammation induces dramatic structural changes in the glycocalyx, manifest by shedding of components and alterations in permeability. Topical stimulation of the endothelium for prolonged periods (20–120 min) with the cytokine TNF-α results in an increased porosity of the glycocalyx in the absence of WBC–EC adhesion (Henry and Duling, 2000). Acute activation of the endothelium with the chemoattractant fMLP results in a rapid (<5 min) shedding of glycans from the EC surface of arterioles, capillaries and venules (Lipowsky et al., 2011).
Shedding of PGs and GAGs from cultured ECs, or their analogs, occurs in response to a broad spectrum of agonists (Colburn et al., 1994; Fitzgerald et al., 2000; Fux et al., 2009; Ihrcke et al., 1993; Park et al., 2000; Platt et al., 1991; Platt et al., 1990). Shedding of HS PGs (namely, the ectodomain of syndecans 1–4) occurs in response to endotoxin (Colburn et al., 1994), serine and/or cystein proteinases (Ihrcke and Platt, 1996), complement activation (Platt et al., 1991), thrombin and growth factors (Subramanian et al., 1997), and activation of protein tyrosine kinase (Fitzgerald et al., 2000). Using hydroxamic acid inhibitors of matrix metalloproteinases, it has been shown that proteolytic cleavage of the syndecan ectodomain results from multiple intracellular pathways that activate a cell surface metalloproteinase (Fitzgerald et al., 2000).
Correlations of ESL shedding with numerous clinical pathologies have led to the suggestion that stabilization of the ESL to mitigate shedding may be advantageous (Becker et al., 2015; Nordling et al., 2015). The specific effectors of shedding are numerous and their relative activities remain to be delineated. Direct in vivo observations have demonstrated inhibition of matrix metalloproteinase (MMP) shedding by application of the broad spectrum inhibitor doxycycline and more specific hydroxamic acid inhibitors (Mulivor and Lipowsky, 2009). Whereas MMP activity presumably cleaves the protein core of GAG bearing proteoglycans, cleavage of GAG chains by EC secretion of heparanase may also contribute to shedding (Becker et al., 2015; Chappell et al., 2008; Fux et al., 2009).
In view of the overwhelming presence of heparan sulfate (HS) within the ESL, the present studies were undertaken to determine if inhibition of HS cleavage by endothelial derived endoglycosidases may also significantly contribute to the glycan shedding previously observed in vivo in response to fMLP (Mulivor and Lipowsky, 2009). To that end, low molecular weight heparin (LMWH) was infused into the circulation to act as a competitive substrate for EC derived heparanase (Fux et al., 2009). Heparin has long been recognized as an inhibitor of heparanase activity (Bar-Ner et al., 1987) and considerable experimental and clinical evidence supports its anti-inflammatory activity (Page, 2013). Shedding of glycans was quantitated by the release of fluorescently labeled lectin bound to the EC of mesenteric post-capillary venules (rat) prior to topical application of fMLP, and concurrent changes in WBC-EC adhesion were examined. The results suggest that as the dosage of LMWH is increased, glycan shedding is significantly reduced and at the maximal dose of LMWH employed ligation of ESL constituents and its compaction correlates significantly with diminished WBC-EC adhesion.
Materials and methods
Animal Preparation
All animal studies conformed to the Guiding Principles in the Care and Use of Animals established by the American Physiological Society, and all protocols have been approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University.
Male Wistar rats, weighing 250–400 g, were anesthetized with Inactin (120 mg/kg, i.p.), tracheostomized, and allowed to breathe under spontaneous respiration. The right jugular vein and its paired carotid artery were cannulated with polyethylene tubing (PE-50, Becton Dickinson, Franklin Lakes, NJ, USA). Supplemental anesthetic was administered via the jugular catheter, as needed, to maintain a surgical plane of anesthesia. The carotid catheter was connected to a strain-gage pressure transducer to monitor central arterial pressure, which averaged a nominal 125 mmHg. Core temperature was monitored by a rectal probe and was maintained between 36 and 37°C with the aid of a heating pad.
Intravital Microscopy
The intestinal mesentery was exteriorized through a midline abdominal incision, placed on a glass pedestal to permit viewing under either epi- or trans-illumination and superfused with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered Ringer’s solution (pH = 7.4) at a temperature of 37.0°C. Visual recordings of the mesentery were made with a Yokogawa CSU-10, spinning disk confocal microscope, (Solamere Technology, Salt Lake City, UT) using an XR/MEGA-10 intensified CCD camera (Stanford Photonics, Palo Alto, CA). Output of the camera was digitized and saved to computer disk in tagged image format (TIF) with each image being 1024 × 1024 pixels in area with a depth in intensity of 10 bits (1024). To measure glycan concentration on the microvessel wall, confocal epi-fluorescence microscopy images were acquired using a Zeiss 20x/0.50NA water immersion objective, and spanned 170 × 170 μm in the focal plane, with an effective pixel size of 0.166 μm (170/1024). Measurements of WBC-EC adhesion were obtained under trans-illumination. The number of stationary WBCs adhered were counted and normalized per 100 μm length of venule. Venule diameters were measured from the digitized images by scaling with reference to a stage micrometer.
Measurement of Endothelial Surface Glycan Concentration
To obtain an index of the glycan concentration on the endothelial cell (EC) surface, the lectin Bandeira Simplicifolia (BS-1, Sigma, St. Louis, MO) was labeled with Alexa Fluor 488 (Invitrogen, Inc., Carlsbad, CA) and infused i.v. via the jugular indwelling catheter. As noted previously (Lipowsky et al., 2011), the concentration of labeled lectin averaged 1.56 ± 0.17 SD mg/ml in PBS with a molar ratio of fluorophore to protein equal to 17.5 ± 3.9 SD. Previous studies have shown that 60% of BS-1 bound to the venular surface could be removed by direct perfusion with 50 U/ml heparinase III (Gao and Lipowsky, 2010). A single bolus of labeled BS-1 was administered i.v. at a dose of 1 ml/kg, and allowed to equilibrate for 10 min prior to intensity measurements on the EC surface. All acquired fluorescence images were corrected for the nonuniformity of the laser illumination by field-flattening and scaling to a reference standard, as described previously (Lipowsky et al., 2011).
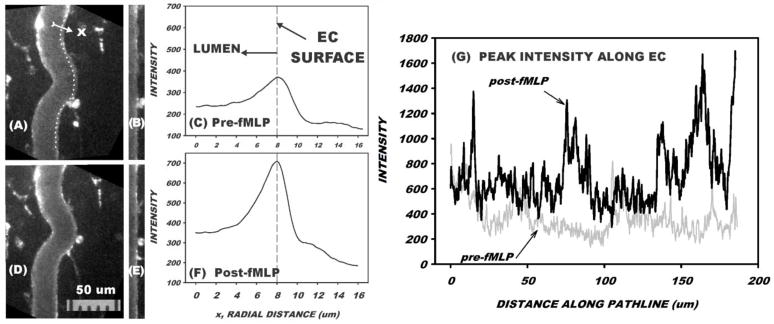
The accumulation of fluorescently labeled BS-1 on the EC wall was quantified by a three-step procedure as illustrated in Fig. 1. First, a measurement path was digitally traced by eye along the curvature of the microvessel wall (panel A) and centered on the lectin stain, which typically ranged from 30–200 μm in length. In this example, the measurement path was 1110 pixels in length (the equivalent of 185 μm). A radial line, normal to the measurement path, was then delineated by prescribing a 100 pixel (16.7 μm) measurement line that spanned the microvessel wall. A custom macro in ImageJ was executed to sample image intensity of each pixel along the radial line. The location of the radial line was then moved to the next pixel along the measurement path and a second set of radial intensities was digitized. This process was repeated along the entire measurement path (in this case, 1110 times) and an average radial intensity distribution was computed. The image formed by the matrix of radial measurement lines is shown in panel B, and the average intensity profile is given in panel C. As shown, the wall delimited by the measurement path became straightened out and the peak intensity at the wall corresponded to the peak in the radial profile of panel C. The peak intensity along the length of the vessel varied considerably, as illustrated in panel (G) for one wall (before and after fMLP). In cases where both walls were in focus in the same image, this process was repeated by drawing a new measurement path line along the opposite wall and its peak intensity determined. The peak intensity at the wall was taken as the average of the values for the two walls. If the opposite wall was not in focus, the intensity was either taken as that of the one wall, or another digitized scene was used in which the wall was brought into sharp focus. In this illustrative case a bolus infusion of LMWH of 1.6 mg/kg was given prior to the intensity measurements. The average peak intensity at the wall increased almost 2-fold following superfusion with 10−7 M fMLP. This dose of fMLP was selected on the basis of in vitro experiments that revealed maximal chemotaxis (Maher et al., 1984), WBC-EC adhesion (Charo et al., 1985), and WBC activation as evidenced by stiffening due to actin polymerization (Kawaoka et al., 1981).
Figure 1.
Illustrative example of measurement of fluorescence of BS-1 lectin on the venular wall prior to superfusion of mesentery with fMLP (A–C) and 10 min following start of fMLP superfusion (D–F). A bolus infusion of LMWH of 1.6 mg/kg was given prior to the measurements. First, a path-line (dotted line in A) was drawn along the luminal edge of the venule and centered on the lectin stain. Second, a radial line normal to and centered on the measurement path was drawn that spanned the wall (16 um in this case). Third, intensity along the radial line was digitized, following which a new radial line was drawn that was centered on the next pixel on the path-line. Panel B shows the matrix of intensities expressed as radial distribution (horizontal) vs location along path-line (vertical). On the order of 1000 radial profiles along the 186 μm path-line were then averaged to obtain the radial profile (C). The distribution of peak intensity along the EC is shown in panel (G), where the average peak intensity increased from 368 ± 136 SD units (C) to 703 ± 240 SD units (F) following 10 min superfusion with fMLP.
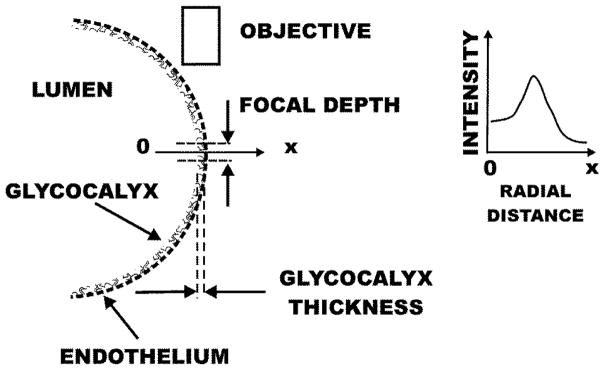
Changes in fluorescence intensity at the wall are proportional to the concentration of lectin within the glycocalyx. To illustrate this point, shown in Fig. 2 is a schematic of the lumen where the endothelial surface is represented by a dashed line. Fluorescence within the glycocalyx is confined to a volume delimited by the thickness of the glycocalyx (typically 0.5 μm) and the focal depth of the microscope objective (approximately 3 μm). The radial intensity scan (along 0-x) exhibits a plateau across the lumen of the vessel, a peak representative of the glycocalyx layer and falls to zero in the extravascular space. The value of intensity within the lumen results from low levels of lectin binding to circulating blood cells (red cells, leukocytes and platelets) and out of focus fluorescence from the vessel wall above the diametral plane. Inasmuch as all of the lectin has been taken up by the blood cells and glycocalyx during the equilibration period following its infusion, a decrease in glycocalyx fluorescence represents a shedding of glycans from the glycocalyx and increases in fluorescence represent increased concentration of fluorophore at the wall. Given the fixed depth of focus at the site of peak intensity, increases in intensity may be interpreted as an increase in concentration arising from compaction of the glycocalyx.
Figure 2.
Schematic representation of fluorescence measurement on the endothelial cell (EC) surface. The EC is represented by a dashed line. The line (0-x) represents the radial coordinate for which the intensity was measured normal to the EC surface. Fluorescence from the glycocalyx emanates from the volume bounded by its thickness (~ 0.5 μm) and the focal depth of the objective (~3 μm). The radial intensity rises from the lumen (due to low level binding to circulating blood cells and out of focus fluorescence from ECs above the diametral plane), peaks at the wall, and falls to zero in the extravascular space. Following complete uptake of the lectin stain, diminishing peak intensity reflects shedding of glycans and increases in peak intensity reflect increased concentration of fluorophore arising from compaction of the glycocalyx.
Experiment Protocol
Following exteriorization of the mesentery, the tissue was allowed to stabilize for 20 min during superfusion with Ringer’s solution (control conditions). Fluorescently labelled BS-1 was then administered via an indwelling catheter in the femoral vein and after 10 min baseline measurements of the fluorescent stain on the endothelial surface were made. A bolus of LMWH (enoxaparin, Lovenox®) was then administered at doses of 0.12, 0.22, 0.60 or 1.6 mg/kg. Enoxaparin (Lovenox®) is a mixture of low molecular weight heparins predominantly ranging from 2000–8000 Da, with an average of 4500 Da. It is used clinically as an antithrombotic in the treatment of deep vein thrombosis, prophylactically in surgical procedures, and myocardial syndromes. To compensate for the circulating half-life of LMWH, following the bolus a steady infusion of 6% of the bolus, per kg/min was maintained using a syringe pump. Following a 10 min equilibration period, measurements of the lectin stain intensity were repeated. The tissue was then superfused with Ringer’s solution containing 10−7M fMLP and measurements of the stain intensity were made every 10–15 min.
Doses of LMWH were selected to cover a range of low, intermediate and high values with one rat at each of 0.12 and 0.22 mg/kg, two rats at 0.60 and two rats at 1.6 mg/kg. Data from the latter two doses, for 6–10 venules per animal, were pooled for analysis. The resultant number of venules at each dose are indicated in the results section. To examine the effect of prolonged exposure to circulating LMWH, a single sham experiment was conducted for 12 venules with a LMWH dose of 1.6 mg/kg over the same period with superfusion by Ringer’s solution without fMLP.
Statistics
Statistical analyses of trends in the data were performed using SigmaStat (Systat Inc., San Jose, CA, USA) with either Student’s t-test for paired measurements or the Holm-Sidak method for ANOVA with multiple comparisons.
Results
Measurements of peak fluorescence intensity and WBC adhesion were obtained for a total of 60 post-capillary venules with a mean diameter of 30.6 ± 11.2 SD μm, ranging in diameter from 14.6 to 63.5 μm. There was no significant difference in diameter size between animals.
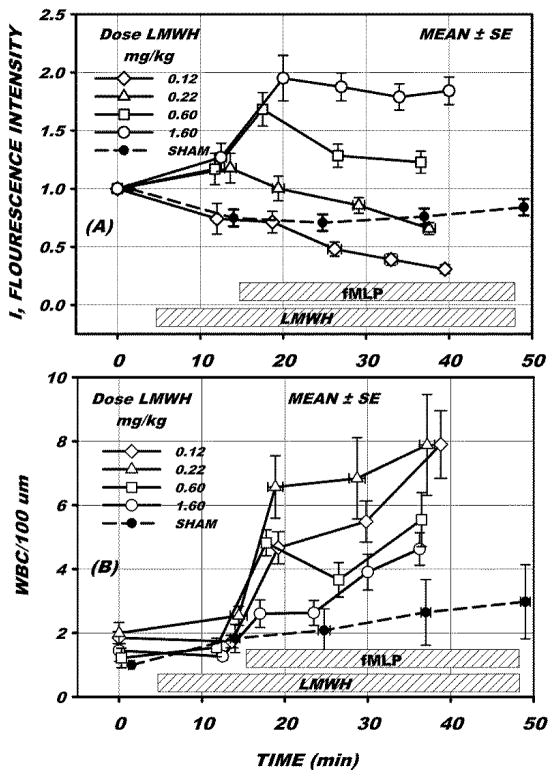
Fluorescence intensity and WBC adhesion during the experiment protocol are illustrated in Fig. 3 for LMWH doses of 0.12, 0.22, 0.60 and 1.60 mg/kg, for 15, 18, 11 and 16 venules, respectively. Following stabilization of the tissue, LMWH infusion was initiated and measurements taken after 10 min. The fluorescence intensity (Fig. 3A) is shown in arbitrary units and normalized to its initial value at time 0. Following 10 min infusion of LMWH no significant changes in either fluorescence intensity (Fig. 3A) or WBC adhesion (Fig. 3B) occurred, as assessed by ANOVA with multiple comparison with time = 0 for all doses (p = 0.287 for intensity and p = 0.929 for adhesion). Following this sampling of intensity and WBC adhesion, superfusion with 10−7 M fMLP was begun. Measurements were then repeated in approximately 10–15 min intervals. The sham intensity response to 1.6 mg/kg LMWH (Fig. 3A) initially fell significantly (p<0.05, t-test) during the period (10 < t <15 min), but due to the variance in the data was not significantly different from the fMLP experiments for all doses in this same time period, p=0.415, as assessed by ANOVA of all dosage values at 10 min following onset of LMWH infusion. WBC adhesion (Fig. 3B) during the sham experiment with 1.6 mg/kg did not rise significantly throughout the entire duration of the fMLP experiments (0 to 50 min, Fig. 3B).
Figure 3.
(A) Average peak fluorescence intensity ± SE, for 8–15 venules following a bolus infusion of LMWH of the indicated dosage. Intensity was adjusted to an arbitrary scale starting with 1 unit at the start of infusion. Following 10–15 min LMWH infusion, superfusion of the mesentery with 10−7M fMLP was begun and maintained for 30–40 min. The dashed line represents sham measurements for 12 venules superfused with Ringer’s solution without fMLP. (B) Measurement of number of WBCs adhered to the endothelium per 100 um length of venule. Both intensity and WBC adhesion did not change significantly prior to onset of fMLP. During the 30 min superfusion with fMLP intensity fell significantly for the lowest dose of LMWH (0.12 mg/kg) whereas it rose significantly at the highest dose (1.60 mg/kg), p <0.05, ANOVA. WBC adhesion rose significantly for all doses over the 30 min period, however at the highest dose (1.6 mg/kg) WBC adhesion did not increase significantly until after 10 min superfusion with fMLP.
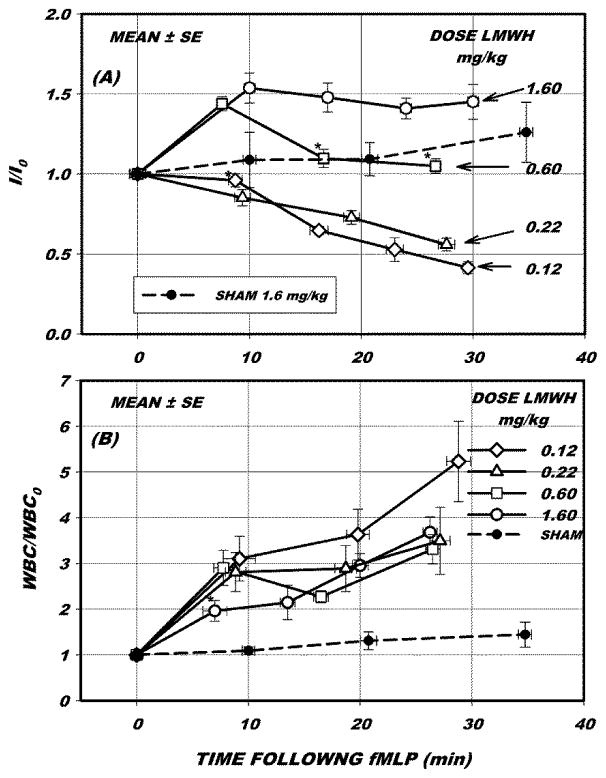
To minimize the effects of tissue heterogeneity and delineate the response to fMLP, for each venule fluorescence intensity and adhesion data were normalized to their values immediately prior to onset of fMLP, as shown in Fig. 4. During the first 10 min (relative to time 0 in Fig. 4A), intensity either rose or fell significantly from its initial value in all cases (p<0.05, ANOVA) except as indicated at 0.12 mg/kg. Over the entire 30 min period of fMLP superfusion, intensity (I/I0) significantly varied from its initial (control) value for all doses of LMWH, p < 0.05, ANOVA. Within the 30 min period, intensities were not significantly different between doses of 0.12 and 0.22 mg/kg at equivalent times from onset of fMLP (ANOVA with multiple comparisons). The time course of intensity at 0.6 mg/kg rose significantly within the first 10 min but fell to its pre-fMLP value after 15 min. In contrast, the response with 1.6 mg/kg remained elevated for the entire duration of the experiments.
Figure 4.
(A) Fluorescence intensity (I) and (B) WBC adhesion per 100 um venule length normalized to initial values prior to start of superfusion of mesentery with 10−7M fMLP, I0 and WBC0, respectively. Intensity either rose or fell significantly from initial values (p < 0.05, ANOVA) in all cases except as indicated *. The transient increase at 0.60 mg/kg LMWH and the sustained increase in intensity for 1.60 mg/kg results from a compaction of the fluorescently labeled glycans. The decrease in intensity represents shedding of glycans. The sham measurements (dashed line) revealed no significant change in intensity at a dose of 1.60 mg/kg LMWH in the absence of fMLP. With fMLP WBC adhesion rose significantly for all doses, except during the first 10 min for 1.60 mg/kg. Following 25–30 min exposure to fMLP adhesion did not vary significantly with dose of LMWH.
Leukocyte adhesion (Fig 4B) rose significantly over the entire 30 min period for all doses of LMWH, p <0.05, ANOVA with multiple comparisons. However, at the highest dose (1.60 mg/kg), adhesion did not rise significantly until after 10 min superfusion with fMLP, whereas it rose immediately with onset of fMLP for the lesser doses. In the absence of fMLP (sham response with 1.6 mg/kg LMWH) intensity did not rise significantly over a time period comparable to that of the fMLP studies, p=0.165, ANOVA.
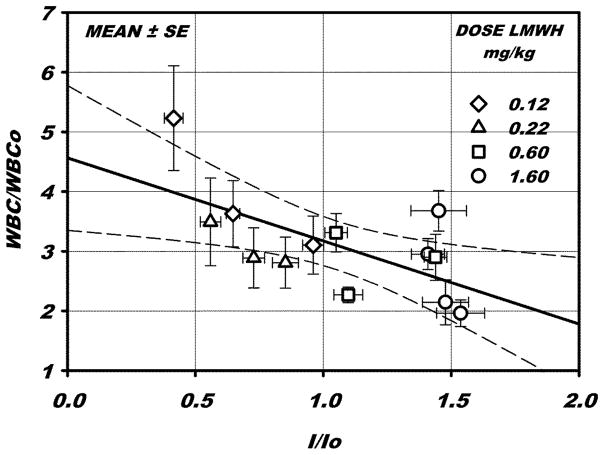
To elucidate the relationship between the change in fluorescence intensity and WBC adhesion following superfusion with fMLP, paired measurements of these parameters were compared for all times and dosages, as shown in Fig. 5. A linear regression indicates an inverse relationship between adhesion and fluorescence intensity, with a significant correlation coefficient r2 = 0.418, p < 0.015 (t-test). These results suggest that increasing intensity of the lectin stain, which is indicative of ligation or compaction of the glycan bearing constituents, significantly mitigates the adhesion response. Conversely, WBC adhesion increases with increased shedding of glycans (decreased intensity).
Figure 5.
WBC adhesion vs fluorescence intensity, for all paired measurements following exposure to fMLP, normalized with respect to their initial values prior to superfusion of fMLP. A linear regression, ±95% confidence interval of the slope (dashed lines) is shown, with a significant correlation coefficient r2= 0.418, p <0.015. An increase in I/I0 may be interpreted as a compaction of the ESL resulting from ligation of proteoglycans by LMWH. The attendant decrease in WBC-EC adhesion is hypothesized to result from diminished availability of adhesion receptors on the EC.
DISCUSSION
The present results reveal that glycan shedding and WBC-EC adhesion in response to topical fMLP were suppressed by LMWH in a dose dependent manner (Fig. 4). As the dose of LMWH was increased, the previously observed reductions in lectin fluorescence in response to fMLP (Lipowsky et al., 2011) were attenuated with a sustained elevation of fluorescence at the highest dose. The rise and fall in intensity at an intermediate dose (0.6 mg/kg) may reflect the continued liberation of heparanase from the EC that could not be inhibited by the limited amount of circulating LMWH. Alternatively, these transients may have resulted from changes in the relative proportions of heparanase and MMP activity with duration of the fMLP stimulus. Previous studies revealed that staining of the dominant HSPG (syndecan-1) on the EC surface with antibody coated microspheres increased with duration of fMLP (Mulivor and Lipowsky, 2004). Assuming that heparanase cleaves HS from its supporting protein core (HSPG), whereas MMPs may cleave the HSPG core itself, it may be hypothesized that for the intermediate dose of LMWH (0.6 mg/kg, Fig. 4) the initial shedding of glycans due to heparanase was suppressed, and was subsequently overwhelmed by shedding due to MMP activity. Further experiments are required to unravel this sequence of events.
The increase in fluorescence intensity at the highest concentration may result from constituents of the ESL becoming more concentrated. Since all of the fluorescent lectin introduced into the circulation was taken up by the EC prior to infusion of LMWH (Lipowsky et al., 2011), increases in peak intensity at the wall can only result from a ligation or compaction of ESL constituents. As shown in Fig. 4A, during the first 10 min of fMLP superfusion fluorescence intensity increased about 50% with increasing doses of LMWH, with a roughly estimated EC50 of 0.6 mg/kg. The EC50 was obtained by fitting a four point logistic curve of the form I =I MIN +(I Max −I MIN) / (1+dose / EC50)n to I/I0 vs dose within the first 10 min of the fMLP response.
Inasmuch as no significant changes in fluorescence intensity occurred during the 10–15 min exposure to LMWH prior to application of fMLP (Fig. 3A), and none occurred in the single sham experiment with 1.6 mg/kg LMWH over entire 30 min superfusion of fMLP (Fig. 4A, dashed line), it is likely that EC activation by fMLP affected the structure of the glycocalyx. It appears that activation of endothelial derived proteases, in concert with inhibition of EC-derived heparanase by LMWH induced a rearrangement of the endothelial glycocalyx.
It is thus hypothesized that cleavage of HS proteoglycans following EC activation was accompanied by binding of LMWH to sites on adjacent proteoglycans. This process may have caused ligation of constituents in a longitudinal direction along the venule length, or a compaction of constituents in the radial direction that is indicative of collapse of the ESL. It should be noted that with the low magnification and resolution of the acquired images, the intensity measurements could not distinguish between these two modes of intensity increase. With the current magnification (20x objective with 0.5 NA) the pixel size of each point in the radial intensity profile was 0.166 μm, which is on the order of one-half the normal thickness of the glycocalyx. Concomitant degradation of the image due to the optical transfer function and point-spread function precluded acquisition of a meaningful radial profile within the thickness of the glycocalyx. Thus, the peak intensity value in the ESL was taken as an index of lectin concentration since it most likely represents an average within the image plane and within the depth of field along the optical axis as depicted in Fig. 2. Acquisition of intensities at higher magnifications precluded the ability to return to the same 3D position at various sample times. Because of their shallow depth of field and greater sensitivity to adjustments in focus, intensity varied considerably with focus adjustments. Further visual evidence in support this hypothesis may be gleaned from the pre- and post-fMLP images, as for example illustrated in Fig. 1. The apparent increased peakedness of the average radial profile following fMLP (Fig. 1F) is consistent with a compaction of the ESL. However, a systematic analysis of the data for 15 venules at the highest concentration of LMWH revealed no significant difference in the half-width of the peak in the radial profile, where the half-width was defined as the radial distance from the location of the peak intensity to the inflection point in the abluminal portion of the radial profile.
As a consequence of these rearrangements, significantly less initial WBC adhesion occurred during the first 10 min exposure to fMLP for 1.6 mg/kg LMWH compared to the lesser doses, p <0.05, ANOVA (Fig. 4B). Due to the variance in the WBC adhesion during the first 10 min exposure to fMLP it was not possible to obtain a meaningful estimate of EC50. It should be noted that the WBC adhesion response to fMLP may be due in part to receptor mediated adhesion in regions of a venule where the glycocalyx is initially very thin. The heterogeneity of lectin stain along the length of a venule (Fig. 1G), and the inexorable rise in WBC adhesion after 10–20 min superfusion with fMLP (Fig. 4B), suggests that factors other than loss of the glycocalyx may also promote adhesion.
The i.v. doses of LMWH used herein were comparable to recommended clinical doses for Lovenox® (enoxaparin). Assuming an 8% blood volume by animal weight an estimated EC50 of 0.006 IU per ml blood volume was calculated, based upon an anti-Factor Xa activity of 1 IU per mg Lovenox®. The range of doses employed here (0.12 – 1.6 mg/kg) spanned the recommended i.v. doses for clinical treatment of acute myocardial syndromes (about 0.4 mg/kg, equivalent to 0.4 IU/kg).
The anti-inflammatory properties of unfractionated and low molecular weight heparins have been studied extensively, although precise mechanisms have not been established (Oduah et al., 2016). Several studies have aimed to delineate the role of heparin in WBC-EC rolling and adhesion, and transmigration trough the microvessel wall. Treatment with LMWH dramatically diminished sepsis-induced neutrophil sequestration in lung (Ning et al., 2015) and attenuated shedding of the glycocalyx in septic shock (Yini et al., 2015). Heparin has been reported to diminish or protect against reperfusion injury in various animal models (Young, 2008). Intradermal administration of heparin attenuated eosinophil accumulation in response to inflammatory stimuli in a dose dependent manner (Teixeira and Hellewell, 1993). Binding of heparins to selectins (Koenig et al., 1998), WBCs (Diamond et al., 1995; Lever et al., 2000; Page, 2013) and HSPGs and other constituents of the EC glycocalyx (Nordling et al., 2015; VanTeeffelen et al., 2007) have been shown to inhibit the inflammatory process.
Heparin oligosaccharides and low molecular weight heparin (LMW, relative molecular mass, Mr = 3000, comparable to the 4500 Da of Lovenox®) have been shown to bind L- and P-selectins (but not E-selectin) and diminish neutrophil extravasation into the peritoneal cavity (mouse) with an IC50 of LMW equal to 0.03 for L-selectin, and 0.10 for P-selectin, mg/ml (Nelson et al., 1993). These concentrations are comparable to the EC50 estimated here for fluorescence intensity vs circulating LMWH concentration.
The dose dependent trends in WBC adhesion were consistent with inhibition of leukocyte rolling noted in prior in vivo studies (rabbit mesentery) where leukocyte rolling on the venular wall was reduced by heparin with doses exceeding 50 μg/ml, with a maximum reduction in WBC rolling of 90% occurring when 5 mg/ml were infused directly upstream (Ley et al., 1991). Similar results were found for systemic i.v. infusions where a bolus of 97 mg/kg heparin resulted in a 90% inhibition of WBC rolling (Tangelder and Arfors, 1991). Further, adhesion of PMNs to fMLP-stimulated human umbilical vein endothelial cells (HUVECS) was inhibited by LMWH (Fragmin®, dalteparin) at doses of 50–1000 U/ml (Lever et al., 2000), which were significantly greater than the concentrations employed herein. Analysis of WBC rolling and adhesion in mesenteric (rat) venules revealed that topically applied heparin had a biphasic effect on WBC rolling flux. At low doses (2–20 IU/ml) heparin reduced fMLP-induced rolling flux, but at higher concentrations (200 and 1000 IU/ml) increased WBC flux due to a dose dependent inhibition of WBC adhesion (Xie et al., 1997). At a concentration of 1.0 mg/ml (100 anti-Xa units/ml), enoxaparin significantly inhibited U937 cell rolling on P-selectin by 51% (Xie et al., 2000). Thus, the significant initial reduction of WBC-EC adhesion with fMLP at high LMWH dose (1.6 mg/kg, Fig. 4B) may be due to inhibition of selectin mediated rolling of WBCs as well as integrin mediated firm adhesion. It has been shown that heparin may bind weakly to PMNs and that activation with fMLP may enhance Mac-1 (CD11b/CD18) PMN binding to heparin (Diamond et al., 1995). Pretreatment with high doses of LMWH inhibited TNF-α-induced leukocyte accumulation by inhibiting the rolling adhesive interaction which may explain, in part, the anti-inflammatory effects of LMWH (Wan et al., 2001). LMWH significantly attenuated leucocyte rolling, adhesion, and migration but did not affect expression of cell adhesion molecules or vascular permeability elicited by TNF-α administration (Salas et al., 2000). Thus the relatively lower doses of LMWH used herein do not appear to directly affect selectin mediated rolling or integrin mediated adhesion.
Within this framework, the trends of Fig. 5 support the hypothesis that LMWH binds to various components in the glycocalyx, such as heparan sulfate proteoglycans or P-selectin (Kumar et al., 2015) in a dose-dependent manner to inhibit WBC binding to receptors on the EC surface by increasing the stiffness of the ESL. It has been suggested that penetration of the ESL by microvilli on the WBC surface is an important determinant of WBC-EC adhesion (Zhao et al., 2001). Thus, reductions in enzymatic degradation of the ESL by either inhibiting heparanase or its secretion (Fux et al., 2009), may lessen reductions in ESL stiffness that limit penetration of the layer (Oberleithner et al., 2011). Also, attenuation of reductions in thickness of the ESL may limit exposure of adhesion receptors on the EC surface (Mulivor and Lipowsky, 2004). Although it has been suggested that reductions in ESL thickness during inflammation are less than that required to expose adhesion receptors (Marki et al., 2015), i.e. only 200 out of the 500 nm total thickness, the analysis therein does not address the process of leukocyte penetration of the surface layer and ESL structural integrity.
In summary, the present studies provide direct in vivo evidence of structural changes in the ESL due to either binding of LMWH to its constituents or inhibition of heparanase during a model of the inflammatory process. While the precise enzymatic processes responsible for shedding of the glycocalyx are intertwined between metalloproteinases that directly cleave proteoglycans on the EC surface (Fitzgerald et al., 2000), and heparanase which may cleave heparan sulfate (Fux et al., 2009), it is clear that stabilizing the ESL to resist cytokine induced changes may pave the way for new therapeutic strategies to thwart inflammatory disorders. It should also be noted that numerous factors may influence the structure and shedding of the glycocalyx, such as circulating hormone and protein content, shear stresses, and gender, to name a few. Further research in these areas is clearly warranted.
Highlights.
The effect of low molecular weight heparin (LMWH) on shedding of the endothelial glycocalyx during inflammation in post-capillary venules is explored
Infusion of LMWH prior to inflammation (modeled by activation of endothelium with fMLP) reduces the shedding of glycans from the EC surface
In the presence of LMWH, an increased intensity of staining of the glycocalyx with fluorescently labelled lectins occurs following EC activation with fMLP
LMWH appears to inhibit EC derived heparanase and bind to proteoglycans resulting in their ligation and compaction of the EC surface layer
These structural rearrangements of the glycocalyx are significantly correlated with a reduction in leukocyte-endothelium adhesion.
Acknowledgments
Supported in part by NIH R01 HL-39286-20.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson RH, Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol. 1992;445:473–86. doi: 10.1113/jphysiol.1992.sp018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisaka T, et al. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- Bar-Ner M, et al. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987;70:551–7. [PubMed] [Google Scholar]
- Becker BF, et al. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br J Clin Pharmacol. 2015;80:389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HS, et al. Morphological classifications of vertebrate blood capillaries. Am J Physiol. 1959;196:381–390. doi: 10.1152/ajplegacy.1959.196.2.381. [DOI] [PubMed] [Google Scholar]
- Bruegger D, et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1993–H1999. doi: 10.1152/ajpheart.00218.2005. [DOI] [PubMed] [Google Scholar]
- Chappell D, et al. Tnf-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009a;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- Chappell D, et al. The glycocalyx of the human umbilical vein endothelial cell: An impressive structure ex vivo but not in culture. Circ Res. 2009b;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- Chappell D, et al. Heparinase selectively sheds heparan sulphate from the endothelial glycocalyx. Biological chemistry. 2008;389:79–82. doi: 10.1515/BC.2008.005. [DOI] [PubMed] [Google Scholar]
- Charo IF, et al. Adherence of human polymorphonuclear leukocytes to endothelial monolayers: Effects of temperature, divalent cations, and chemotactic factors on the strength of adherence measured with a new centrifugation assay. Blood. 1985;65:473–9. [PubMed] [Google Scholar]
- Colburn P, et al. Depleted level of heparan sulfate proteoglycan in the extracellular matrix of endothelial cell cultures exposed to endotoxin. J Cell Physiol. 1994;159:121–130. doi: 10.1002/jcp.1041590116. [DOI] [PubMed] [Google Scholar]
- Constantinescu AA, et al. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized ldl. Am J Physiol Heart Circ Physiol. 2001;280:H1051–H1057. doi: 10.1152/ajpheart.2001.280.3.H1051. [DOI] [PubMed] [Google Scholar]
- Diamond MS, et al. Heparin is an adhesive ligand for the leukocyte integrin mac-1 (cd11b/cd1) The Journal of cell biology. 1995;130:1473–82. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, et al. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a timp-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux L, et al. Heparanase: Busy at the cell surface. Trends Biochem Sci. 2009;34:511–9. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M. Syndecans in inflammation. Faseb Journal. 2003;17:575–591. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- Gouverneur M, et al. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–2. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- Grimm J, et al. Laminar flow induces cell polarity and leads to rearrangement of proteoglycan metabolism in endothelial cells. Thromb Haemost. 1988;60:437–441. [PubMed] [Google Scholar]
- Haldenby KA, et al. Focal and regional variations in the composition of the glycocalyx of large vessel endothelium. J Vasc Res. 1994;31:2–9. doi: 10.1159/000159025. [DOI] [PubMed] [Google Scholar]
- Henry CB, Duling BR. Tnf-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- Hofmann-Kiefer KF, et al. Serum heparan sulfate levels are elevated in endotoxemia. Eur J Med Res. 2009;14:526–531. doi: 10.1186/2047-783X-14-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley VH, Curry FE. Differential actions of albumin and plasma on capillary solute permeability. Am J Physiol. 1991;260:H1645–54. doi: 10.1152/ajpheart.1991.260.5.H1645. [DOI] [PubMed] [Google Scholar]
- Ihrcke NS, Platt JL. Shedding of heparan sulfate proteoglycan by stimulated endothelial cells: Evidence for proteolysis of cell-surface molecules. J Cell Physiol. 1996;168:625–637. doi: 10.1002/(SICI)1097-4652(199609)168:3<625::AID-JCP15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Ihrcke NS, et al. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today. 1993;14:500–505. doi: 10.1016/0167-5699(93)90265-M. [DOI] [PubMed] [Google Scholar]
- Kawaoka EJ, et al. Chemotactic factor-induced effects upon deformability of human polymorphonuclear leukocytes. J Clin Immunol. 1981;1:41–4. doi: 10.1007/BF00915475. [DOI] [PubMed] [Google Scholar]
- Koenig A, et al. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. The Journal of clinical investigation. 1998;101:877–89. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AV, et al. Heparan sulphate as a regulator of leukocyte recruitment in inflammation. Curr Protein Pept Sci. 2015;16:77–86. doi: 10.2174/1573402111666150213165054. [DOI] [PubMed] [Google Scholar]
- Lever R, et al. The effects of heparin and related molecules upon the adhesion of human polymorphonuclear leucocytes to vascular endothelium in vitro. British Journal of Pharmacology. 2000;129:533–540. doi: 10.1038/sj.bjp.0703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, et al. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. The American journal of physiology. 1991;260:H1667–73. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- Lipowsky HH, et al. Shedding of the endothelial glycocalyx in arterioles, capillaries, and venules and its effect on capillary hemodynamics during inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H2235–45. doi: 10.1152/ajpheart.00803.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966;25:1773–1783. [PubMed] [Google Scholar]
- Maher J, et al. The response of human neutrophils to a chemotactic tripeptide (n-formyl-methionyl-leucyl-phenylalanine) studied by microcinematography. Blood. 1984;64:221–8. [PubMed] [Google Scholar]
- Marki A, et al. Role of the endothelial surface layer in neutrophil recruitment. J Leukoc Biol. 2015;98:503–15. doi: 10.1189/jlb.3MR0115-011R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–80. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation. 2009;16:657–66. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- Nelson RM, et al. Heparin oligosaccharides bind l- and p-selectin and inhibit acute inflammation. Blood. 1993;82:3253–8. [PubMed] [Google Scholar]
- Ning F, et al. Low molecular weight heparin may prevent acute lung injury induced by sepsis in rats. Gene. 2015;557:88–91. doi: 10.1016/j.gene.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Nordling S, et al. Vascular repair utilising immobilised heparin conjugate for protection against early activation of inflammation and coagulation. Thrombosis and haemostasis. 2015;113:1312–22. doi: 10.1160/TH14-09-0724. [DOI] [PubMed] [Google Scholar]
- Oberleithner H, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. 2011;462:519–28. doi: 10.1007/s00424-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduah EI, et al. Heparin: Past, present, and future. Pharmaceuticals (Basel) 2016;9 doi: 10.3390/ph9030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page C. Heparin and related drugs: Beyond anticoagulant activity. ISRN Pharmacol. 2013;2013:910743. doi: 10.1155/2013/910743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PW, et al. Cell surface heparan sulfate proteoglycans: Selective regulators of ligand-receptor encounters. J Biol Chem. 2000;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- Platt JL, et al. The role of c5a and antibody in the release of heparan sulfate from endothelial cells. Eur J Immunol. 1991;21:2887–2890. doi: 10.1002/eji.1830211135. [DOI] [PubMed] [Google Scholar]
- Platt JL, et al. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171:1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, et al. The endothelial surface layer. Pflugers Archiv-European Journal of Physiology. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. Transforming growth factor (type beta) promotes the addition of chondroitin sulfate chains to the cell surface proteoglycan (syndecan) of mouse mammary epithelia. J Cell Biol. 1989;109:2509–2518. doi: 10.1083/jcb.109.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- Reitsma S, et al. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 2007;454:345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas A, et al. Heparin attenuates tnf-alpha induced inflammatory response through a cd11b dependent mechanism. Gut. 2000;47:88–96. doi: 10.1136/gut.47.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire JM, et al. Quasi-periodic substructure in the microvessel endothelial glycocalyx: A possible explanation for molecular filtering? Journal of Structural Biology. 2001;136:239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, et al. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- Svennevig K, et al. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion. 2008;23:165–171. doi: 10.1177/0267659108098215. [DOI] [PubMed] [Google Scholar]
- Tangelder GJ, Arfors KE. Inhibition of leukocyte rolling in venules by protamine and sulfated polysaccharides. Blood. 1991;77:1565–71. [PubMed] [Google Scholar]
- Teixeira MM, Hellewell PG. Suppression by intradermal administration of heparin of eosinophil accumulation but not oedema formation in inflammatory reactions in guinea-pig skin. Br J Pharmacol. 1993;110:1496–500. doi: 10.1111/j.1476-5381.1993.tb13991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, et al. Heparin impairs glycocalyx barrier properties and attenuates shear dependent vasodilation in mice. Hypertension. 2007;50:261–7. doi: 10.1161/HYPERTENSIONAHA.107.089250. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- Wan MX, et al. Low molecular weight heparin inhibits tumor necrosis factor alpha-induced leukocyte rolling. Inflammation Research. 2001;50:581–584. doi: 10.1007/PL00000237. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, et al. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Xie X, et al. Inhibition of selectin-mediated cell adhesion and prevention of acute inflammation by nonanticoagulant sulfated saccharides. Studies with carboxyl-reduced and sulfated heparin and with trestatin a sulfate. J Biol Chem. 2000;275:34818–25. doi: 10.1074/jbc.M001257200. [DOI] [PubMed] [Google Scholar]
- Xie X, et al. Inhibitory effect of locally administered heparin on leukocyte rolling and chemoattractant-induced firm adhesion in rat mesenteric venules in vivo. Br J Pharmacol. 1997;122:906–10. doi: 10.1038/sj.bjp.0701454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yini S, et al. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiologica Scandinavica. 2015;59:160–169. doi: 10.1111/aas.12418. [DOI] [PubMed] [Google Scholar]
- Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–52. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Zhao Y, et al. Dynamic contact forces on leukocyte microvilli and their penetration of the endothelial glycocalyx. Biophys J. 2001;80:1124–40. doi: 10.1016/S0006-3495(01)76090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier CJ, et al. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005;99:1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]