Abstract
Objectives/Hypothesis
Aural rehabilitation is not standardized for adults after cochlear implantation. Most cochlear implant (CI) centers in the United States do not routinely enroll adult CI users in focused postoperative rehabilitation programs due to poor reimbursement and lack of data supporting (or refuting) the efficacy of any one specific approach. Consequently, patients generally assume a self‐driven approach toward rehabilitation. This exploratory pilot study examined rehabilitation strategies pursued by adults with CIs and associated these strategies with speech recognition and CI‐specific quality of life (QOL).
Study Design
Cross‐sectional study of 23 postlingually deafened adults with CIs.
Methods
Participants responded to an open‐ended questionnaire regarding rehabilitation strategies. A subset underwent in‐depth interviews. Thematic content analysis was applied to the questionnaires and interview transcripts. Participants also underwent word recognition testing and completed a CI‐related QOL measure. Participants were classified as having good or poor performance (upper or lower quartile for speech recognition) and high or low QOL (upper or lower quartile for QOL). Rehabilitation themes were compared and contrasted among groups.
Results
Five rehabilitation themes were identified: 1) Preimplant expectations of postoperative performance, 2) personal motivation, 3) social support, 4) specific rehabilitation strategies, and 5) patient‐perceived role of the audiologist. Patients with good speech recognition and high QOL tended to pursue more active rehabilitation and had greater social support. Patient expectations and motivation played significant roles in postoperative QOL.
Conclusion
Postoperative patient‐driven rehabilitation strategies are highly variable but appear to relate to outcomes. Larger‐scale extensions of this pilot study are needed.
Keywords: Adult, auditory rehabilitation, cochlear implants, sensorineural hearing loss, thematic content analysis
INTRODUCTION
Cochlear implants (CIs) have restored some degree of auditory function and enhanced verbal interactions for thousands of patients.1 However, current postoperative speech‐recognition measures still reveal that average recognition of words in isolation and words in sentences in quiet remain at approximately 60% and 70% correct, respectively, with substantial unexplained variability among individuals.2, 3, 4
Patients with CIs generally do not show speech recognition performance on par with normal‐hearing peers, an effect believed to be mediated by the degraded speech signals presented through their devices.5, 6, 7 This degradation of speech continues to be a major limitation with CIs, even with significant strides in hardware and processing over the past 30 years. Nevertheless, the majority of patients are able to make sense of the degraded speech signal. For many CI users, this learning process requires extended practice (6 months or more) with their devices.8, 9, 10 Although for some patients the process of learning to listen through their implants comes naturally during daily life, for others it may require more intentional, focused rehabilitation.
A variety of aural rehabilitation strategies have been developed, but the field lacks a standardized method for adult CI patients. Although surgeons and audiologists are increasingly recognizing the need for rehabilitation for patients after CI activation, questions and barriers remain regarding the most efficient and cost‐effective approach. A primary reason is that aural rehabilitation provided by audiologists, outside of device programming and patient counseling, is rarely reimbursed by insurance providers.11, 12 Additionally, there is a paucity of speech‐language pathologists who perform rehabilitation for adult CI users. Consequently, for patients with suboptimal speech recognition, audiologists often recommend patient‐driven rehabilitation approaches known anecdotally to have been helpful to other users (e.g., use of audiobooks, computerized auditory training, and CI support groups). Moreover, it is unclear what type of rehabilitation approach is most effective. Many forms of rehabilitation have been studied in adults with hearing impairment, including group therapy and individualized computer‐based auditory training, but benefits have been inconsistent.13, 14, 15
In addition, clinical programs for adult CI patients typically evaluate speech recognition as their only dependent measures before and after rehabilitation. This narrow approach is insufficient to assess the benefits and/or limitations of CIs and aural rehabilitation programs. Quality of life (QOL) evaluations provide information beyond speech recognition and capture a wider range of ways in which a CI may affect a patient's everyday communication.16, 17, 18 Even for patients who do not demonstrate substantial improvement in speech recognition abilities, subjective QOL may improve after implantation.19 Quality‐of‐life questionnaires are straightforward tools with which to evaluate rehabilitation and CI‐specific health‐related QOL (HRQOL).16 Health‐related QOL questionnaires also provide a unique opportunity to examine the benefits of CIs and postoperative aural rehabilitation in the hopes of increasing support from clinicians and insurers. Unfortunately, until there is evidence of overall benefit for CI patients, there will be no reimbursement or support, which will lead to fewer patients participating in aural rehabilitation programs.11, 12
The aim of this study was twofold: to explore the patient‐initiated postoperative aural rehabilitation approaches used by adult CI users in a tertiary CI center, and to attempt to identify commonalities and differences among those patients who had either good versus poor speech recognition outcomes or high versus low QOL with their devices. To accomplish these goals, patients provided responses to an open‐ended questionnaire inquiring about postoperative rehabilitation approaches. A subset of patients were then interviewed for an in‐depth examination of personal rehabilitation strategies following implantation. Thematic content analysis was applied to these responses to identify the primary rehabilitation themes discussed by participants. Following this, themes were re‐examined, comparing only those patients who demonstrated good versus poor speech recognition outcomes (the upper quartile vs. the lower quartile on a measure of word recognition), or patients with high versus low QOL (again, upper and lower quartile on a CI‐related HRQOL questionnaire). The overarching hypothesis tested was that the groups with good speech recognition (or high QOL) would report more active rehabilitation strategies as compared with those with poor speech recognition (or low QOL), suggesting a beneficial role of postoperative rehabilitation in adult CI users.
Thematic content analysis is a methodology applied to interview transcripts that involves identifying patterns and generating a list of themes.20 In the context of CI users, this approach has been previously employed in interviews of samples of pediatric CI users (ages 12–20 years) who did not demonstrate benefit following sequential second CI and in adult CI candidates who did not meet implant criteria and were not implanted.21, 22 To our knowledge, thematic content analysis has not been used to explore the patient‐driven rehabilitation strategies used by adult CI users.
MATERIALS AND METHODS
Participants
Adult CI users from a pool of research participants at The Ohio State University, Columbus, Ohio, were contacted by e‐mail. Twenty‐three patients with CIs enrolled. Participants had varying etiologies of hearing loss and ages of implantation, although most experienced a progressive decline in hearing during adulthood. All used Cochlear (Cochlear Americas, Centennial, CO) devices, except one who wore an Advanced Bionics device (Advanced Bionics, LLC, Valencia, CA). All had CI‐aided thresholds better than 35‐dB hearing level for the frequencies 0.25 to 4 kHz, as measured by audiologists within the 12 months prior to testing. All users had been preoperatively counseled regarding realistic expectations and the learning curve after implantation. All had at least 9 months of experience using their implants. Four participants had bilateral implants, 11 used a right implant, and eight used a left implant. A hearing aid was worn on the ear contralateral to the CI by 10 participants. All participants used an advanced combined encoder (ACE) (Cochlear Americas, Centennial, CO) speech‐processing strategy, except for the Advanced Bionics (Advanced Bionics, LLC) user who employed a HiRes Fidelity 120 processing strategy (Advanced Bionics, LLC). Participants wore devices using their everyday mode during testing.
All participants underwent screening tests of unaided audiometry to evaluate both residual hearing after implantation and cognitive function to ensure that none had evidence of dementia. The Mini‐Mental State Examination (MMSE) is a validated screening assessment to rule out cognitive impairment. Raw scores were converted to T‐scores, based on age and education, with a T‐score less than 29 being suggestive of cognitive impairment.23 None of the participants had a T‐ score less than 29, with mean MMSE T‐score of 47.3 (standard deviation 8.5). All participants were adults whose first language was American English and who had graduated from high school. The data regarding demographics and audiologic testing are shown for the 23 CI participants in Table 1.
Table 1.
Participant Demographics.
| Participant | Gender | Age (years) | Implantaton Age (years) | Side of Implant | Hearing Aid | Etiology of Hearing Loss | MMSE (T score) | Better ear PTA (dB HL) | Word Recognition (% correct) | NCIQ (total score) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | 54 | B | N | Genetic | 50 | 105 | 96 | 465 |
| 2 | F | 64 | 62 | R | Y | Genetic, progressive as adult | 50 | 75 | 76 | 460 |
| 3 | M | 64 | 61 | L | N | Noise, Meniere's | 50 | 80 | 78 | 455 |
| 4 | F | 64 | 58 | R | Y | Genetic, progressive as adult | 44 | 105 | 84 | 483 |
| 6 | M | 67 | 65 | R | N | Genetic, progressive as adult | 30 | 84 | 80 | 440 |
| 7 | M | 56 | 52 | B | N | Rubella, progressive | 43 | 105 | 46 | 485 |
| 8 | F | 54 | 48 | R | Y | Genetic, progressive | 55 | 105 | 72 | 338 |
| 9 | M | 77 | 67 | L | N | Genetic, progressive | 50 | 93 | 58 | 358 |
| 11 | M | 88 | 83 | R | Y | Progressive as adult | 50 | 88 | 38 | 335 |
| 16 | F | 61 | 59 | R | N | Progressive as adult | 35 | 105 | 68 | 380 |
| 18 | F | 75 | 63 | R | N | Genetic, progressive as adult | 43 | 95 | 56 | 445 |
| 19 | F | 73 | 67 | L | N | Genetic, autoimmune | 56 | 105 | 68 | 433 |
| 20 | M | 76 | 74 | L | N | Ear infections | 38 | 105 | 50 | 425 |
| 21 | M | 80 | 58 | L | Y | Meniere's | 50 | 69 | 58 | 330 |
| 23 | F | 79 | 73 | R | N | Progressive as adult | 56 | 86 | 92 | 403 |
| 24 | F | 58 | 53 | B | N | Progressive as adult | 50 | 105 | 88 | 295 |
| 25 | M | 57 | 56 | R | Y | Autoimmune, sudden | 57 | 76 | 84 | 310 |
| 26 | M | 53 | 50 | B | N | Noise, progressive as adult | 50 | 98 | 92 | 489 |
| 29 | F | 59 | 58 | R | N | Sudden hearing loss | 37 | 80 | 44 | 245 |
| 30 | M | 80 | 79 | R | Y | Progressive as adult | 50 | 66 | 58 | 340 |
| 31 | F | 66 | 62 | L | Y | Progressive as child and adult | 50 | 84 | 52 | 303 |
| 32 | M | 68 | 67 | L | Y | Progressive as adult | 29 | 73 | 68 | 308 |
| 34 | M | 59 | 54 | L | Y | Meniere's, noise | 43 | 81 | 80 | 233 |
PTA: Unaided four‐tone pure tone average at .5, 1, 2, and 4 kHz.
B = both; F = female; L = left; M = male; MMSE = Mini‐Mental State examination; N = No; NCIQ = Nijmegen Cochlear Implant Questionnaire; PTA = pure tone average; R = right; Y = yes.
Procedures
Testing took place at the Eye and Ear Institute of the Ohio State University Wexner Medical Center, Columbus, Ohio; data presented here are from a larger study of variability in outcomes for adults with CIs.6 Approval was obtained from the institutional review board of the Ohio State University, and written informed consent was obtained. Participants were tested on the screening and word recognition measures presented here over approximately 45 minutes.
Screening measures were performed first, followed by word recognition with the Central Institute for the Deaf‐22 (CID‐22) words.24 The CID‐22 words were used in testing because they were unfamiliar to the participants, who had undergone word and sentence recognition testing numerous times in the clinic. Responses were video‐ and audio‐ recorded; participants wore FM transmitters, which provided direct input to the camera. Responses were scored at a later time by two research assistants scoring responses to check reliability. All participants were tested wearing their usual auditory prostheses (single CI, two CIs, or CI with hearing aid), which were checked at the start of testing by having the tester confirm sound detection. Individuals were later contacted by e‐mail and asked to respond to questionnaires regarding their QOL and rehabilitative strategies. Twenty‐three patients provided responses and were invited to participate in informal interviews. Five participants agreed to participate in interviews.
Word Recognition
The CID‐22 testing was conducted in an acoustically insulated room. Words were presented at 68‐dB sound pressure level over a loudspeaker positioned 1 meter from the participant at zero‐degrees azimuth. The percent of correct words was used to stratify patients with speech recognition in the upper and lower quartiles.
Nijmegen Cochlear Implant Questionnaire
Participants completed this assessment at home with no time limit. The Nijmegen Cochlear Implant Questionnaire (NCIQ) is a validated HRQOL instrument designed specifically for CI users and to encompasses hearing and speech, psychological, and social domains.16 Six subdomain scores are computed from responses to 60 questions, with responses from 1 (never) to 5 (always). The score for each subdomain was then computed by summing scores for the 10 items corresponding to that subdomain and dividing by the number of items. Total scores across subdomains (for a maximum of 600) were used to stratify patients with scores in the upper and lower quartiles of responses, with larger scores representing better QOL.
Rehabilitation Questionnaire
All participants completed a rehabilitation questionnaire developed by the authors (see online Appendix). This questionnaire was completed at home with no time limit and mailed back to the investigators. This consisted of mostly open‐ended questions inquiring about self‐driven rehabilitation strategies used. For example, participants were asked what rehabilitative activities they participated in postoperatively, such as watching television with or without captions, using the telephone, listening to audiobooks, or using computerized auditory training programs. They were asked about how long it took them to reach a subjective plateau in various abilities postoperatively (e.g., success using the telephone, understanding conversations in quiet), as well as what techniques seemed most beneficial. Questions also asked about support groups and assistance from friends and family in rehabilitation. Finally, participants were asked what types of goals they set.
Interviews
From the larger pool, five participants agreed to participate in individual 30‐minute in‐person interviews with the authors. Interviews were designed to examine participants’ responses regarding rehabilitation in greater depth and without potential bias imposed by the wording of the questionnaire. During interviews, two of the authors (a.c.m. and m.a.p.) and the participant discussed the participants’ rehabilitation strategies, using the questionnaire responses as a starting point. For example, if participants had responded that they used a number of the listed rehabilitation strategies, we would inquire more about which strategies seemed most helpful. Alternatively, if participants did not use any of the listed rehabilitation strategies, we would ask about the individual approaches that they did use. We also inquired as to whether additional factors seemed to impact their postoperative rehabilitation course, with the goal of exploring factors that might not be evident from the questionnaires alone. Responses were audio‐ and video‐recorded and later transcribed.
Data Analyses
Questionnaire responses and interview transcripts were reviewed by two nonblinded investigators and thematic content analysis was applied.20, 25 Two of the authors (a.c.m. and m.s.h.) performed these analyses independently and then came to a consensus. Thematic content analysis is a method for identifying, analyzing, and reporting themes within data.20, 26 Thematic analysis is an analysis method whereby meaning units—patterned responses within the data set, based on prevalence or importance—are distilled by individual reviewers and consolidated into themes. A theme captures something important about the data as it relates to the research question. Thematic analysis provides a flexible and useful tool, which can provide a rich and detailed account of data.20 There are various conventions for representing the prevalence of a theme within a data set that does not provide a quantified measure, for example, the majority of participants, many participants, or a number of participants.20 For the present study, thematic content analysis was performed using the six‐phase approach discussed by Braun and Clarke.20
The first question of interest, which regarded what types of patient‐driven rehabilitative approaches were used by this sample of CI users, was addressed by identifying themes resulting from thematic content analysis of questionnaires and interviews.
The second question of interest was whether differences in patient‐driven rehabilitation techniques were associated with differences in speech recognition or differences in QOL following implantation. Therefore, scores of word recognition were used to assign listeners to extreme groups of the upper quartile (“good” performers) or lower quartile (“poor” performers). Patients were similarly assigned to extreme groups based on QOL scores; upper quartile (“high” QOL) or lower quartile (“low” QOL). Although the use of this type of extreme‐groups analysis has limitations (e.g., loss of individual variability from removal of a portion of the distribution), this approach can be useful for determining the existence of relationships between variables.27, 28 Based on the extreme‐group divisions of patients into good/poor performance and high/low QOL, commonalities and differences were sought in reported themes regarding rehabilitation strategies.
RESULTS
Thematic Content Analysis
Our first aim was to explore the patient‐driven postoperative rehabilitation approaches taken by this group of adult CI users. Thematic content analysis of questionnaires and interviews identified the following core set of themes for the entire group of CI users: 1) preimplant expectations of postoperative performance, 2) personal motivation, 3) social support, 4) specific rehabilitation strategies, and 5) patient‐perceived role of the audiologist. Each of these themes will be explored below:
Preimplant expectations of postoperative performance. Most patients cited the importance of realistic expectations for postactivation progress. These expectations pertained to the degraded and/or robotic quality of the speech sound delivered through a CI, the length of time it would take for the brain to learn to understand speech again (varying from instantaneous at the time of CI activation to still improving after 5 years), and the amount of work and practice it would take to understand speech through an implant. Most patients stated they wished they had been advised more thoroughly concerning what they should expect postoperatively. This theme became most apparent during participant interviews because the questionnaire did not specifically inquire about patient expectations.
Personal motivation. Most patients expressed motivation after implantation to “have better conversation.” However, the majority of patients did not set any personal iterative or long‐term performance goals, nor did they set benchmarks toward a particular endpoint. Commitment to rehabilitation strategies was highly variable. At the time of the questionnaire or interview, the majority of patients stated that they still engaged in rehabilitative activities in some form.
Social support. Most patients stated that family support was important and helpful in pursuing implantation, and to a lesser extent in personal rehabilitation and learning to optimally use the device. Very few patients, however, actively solicited feedback from family and friends regarding their implant use or performance. A slight majority of patients utilized CI community support groups, whether in person or online.
Specific rehabilitation strategies. A wide range of postoperative rehabilitative strategies was used by patients in this sample. Most of these involved passive, real‐life activities rather than specific training programs. For example, over half of CI users specifically used watching the television or movies with or without captions, listening to familiar music, or talking on the telephone as rehabilitation techniques. Approximately one‐third of participants listened to audiobooks or used structured computerized auditory training programs. The majority found that actively controlling their sound environments and making use of different CI programs were helpful strategies.
Patient‐perceived role of the audiologist. Patients almost unanimously had a very favorable view of their relationship with their audiologist, but surprisingly only a minority of patients cited their audiologist as the primary source of information or guidance on rehabilitation strategies. The role of the audiologist, as viewed by this particular sample of CI users, seemed largely to consist of mapping and making CI programming adjustments.
Relating Rehabilitation Themes to Speech Recognition and Quality of Life
Beyond characterizing what self‐driven approaches CI users were using for rehabilitation following activation, the next question of interest was whether patients with good word recognition used rehabilitation strategies that differed from those with poor word recognition, with the hypothesis being that use of more active rehabilitation strategies would be associated with better performance. A similar question was whether patients with high QOL made use of different rehabilitation strategies than those with low QOL, again with the hypothesis that more active rehabilitation would be associated with better QOL.
Mean word recognition for all 23 participants was found to be 69% correct, with patients in the upper quartile scoring between 82% and 96% and those in the lower quartile scoring between 38% and 46%. The median score on the NCIQ questionnaire was a score of 380 points, with patients in the upper quartile scoring between 460 and 488 and those in the lower quartile scoring between 233 and 308. Scores of word recognition and QOL did not correlate significantly (P = .24) (Fig. 1).
Figure 1.
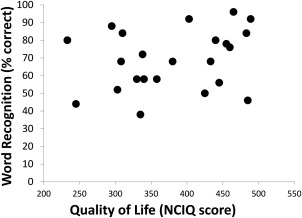
Scatter plot of word recognition scores and quality of life scores.
NCIQ = Nijmegen Cochlear Implant Questionnaire.
Before relating speech recognition and QOL to rehabilitation strategies, it was important to examine other patient factors that might reasonably contribute to outcomes of word recognition and QOL. Therefore, correlation analyses were performed between those outcome measures and age, duration of hearing loss, duration of hearing aid use, duration of CI use, and a measure of socioeconomic status—as well as one‐way analysis of variance analyses to identify differences in outcomes based on side of implant (right, left, or bilateral) or continued use of a contralateral hearing aid (yes or no). The only significant finding was that older age correlated with poorer word recognition (r = −.44, P = .038).
Next, themes identified from thematic content analysis for the entire group were re‐examined for those CI users in the upper and lower quartiles for either speech recognition or QOL. Both speech recognition and QOL were considered here because it has been demonstrated that clinical speech recognition tests in quiet do not correlate broadly with CI‐related QOL measures, suggesting that it is reasonable to consider them independently.29
Several interesting findings emerged related to the previously identified themes: A preoperative understanding and expectation that a degree of work and effort would be necessary in the postoperative period was repeatedly cited by those who did well with their device both from a QOL and a word recognition standpoint. Those who demonstrated poorer word recognition following implantation, and those with poorer QOL, tended to have expectations that the device would automatically restore normal hearing—or that user effort following activation was not required or not a factor that could influence outcomes.
High‐QOL individuals seemed to be personally more oriented (or motivated) toward making use of rehabilitation strategies than their low‐QOL counterparts. Participants who fell into the top quartile either for word recognition or QOL stated that they used a mean of 6.8 different types of rehabilitation strategies (range 4 to 12) compared to a mean of five different strategies (range 2 to 9) used among those in the lower quartile groups. Overall, patients with better speech recognition performance tended to pursue more active rehabilitation methods, whereas poorer performers tended to approach postoperative rehabilitation more passively (such as through everyday living techniques). Good performers were more active in CI support meetings or groups (4 endorsed using these vs. 2 participants in the “poor” group), and their family members appeared to play a greater role in their rehabilitation than for poor performers.
DISCUSSION
This study was performed to explore the postoperative patient‐driven rehabilitation strategies used by adult CI recipients and to examine whether these strategies related to performance on word recognition and CI‐specific QOL. The value of this knowledge is in characterizing the impact of various approaches on real world success with a device.
The first finding of interest was that many patients did not use any particular focused or active rehabilitation strategies at all. Rather, the most common rehabilitation approach consisted of general exposure to sounds and voices through everyday living: watching television or movies, listening to the radio or familiar music, or talking on the telephone. Controlling the acoustic environment and making use of different CI programs were also relatively common in this sample.
A pervading theme that emerged from patient interviews was the importance of preoperative expectations. One patient stated, “I think one reason people do not seek active rehabilitation approaches goes back to the expectation of instantaneous results. People think you put CIs in the ear and you can hear, and that's not true.” A poor performer mentioned, “I really had wrong expectations. I expected when I got this thing it would just take care of business for me. That just isn't the case. You have to invest, too.” Our CI center gives written materials discussing the need to actively practice using the CI postoperatively, and our audiologists consistently discuss postoperative expectations with patients considering implantation. Clearly, however, preoperative expectations need to be addressed more frequently and/or extensively with the patient by all members of the CI team.
Another theme that emerged from this study involved patient motivation. Good performers commented, “I just decided I was going to do everything I could to hear well,” and “I found myself seeking different situations to practice listening.” On the other hand, a poor performer stated, “I just didn't work at it … I figured I would just learn to get by.” High levels of motivation to work toward the goal of optimal CI use appear to be tied to one's choice of an active versus passive rehabilitation strategy. Research into use of cognitive training tasks among young adult CI users has found a significant effect of motivation on training and transfer of trained skills to new contexts.30 Potential sources of motivation influencing choice of rehabilitation strategy may include a desire to maintain or re‐establish social connectedness and intimacy, to achieve career goals, or simply the appeal of the challenge.
Intimately related to the new CI user's motivation is the patient's implicit belief about the malleable versus fixed nature of intelligence. This concept seemed to underlie responses in two ways: First, CI users who were highly motivated to improve their abilities with their devices made statements reflecting the acknowledgment that they could influence their CI performance (“I committed to doing anything it took to hear better”; “I would look for people to talk to so I could practice using my implant”). On the other hand, some CI users, who may not share the belief in the malleable nature of cognitive and listening skills, saw their device more akin to eyeglasses, resulting in immediate benefit.
There is some experimental evidence in CI users of the role of an individual's perspective on the malleable versus fixed nature of intelligence. For example, in CI users undergoing working memory training, a belief that intelligence is fixed and immutable is associated with a lower likelihood of persisting in the face of a challenge (i.e., more likely to give up on the training task). A belief that intelligence can be modified by experience is associated with a higher likelihood of persisting.30, 31 This quality of perseverance and drive toward long‐term goals is referred to as grit, a noncognitive quality that may also underlie pursuit of active rehabilitation strategies and good outcomes following implantation. Studies examining this trait outside the CI literature have found that it can account for 4% of variance in success outcomes, implying that attainment of difficult goals entails not only intelligence and talent but sustained and intentional effort applied over time.32 Future work examining post‐CI rehabilitation strategies as well as preoperative CI counseling or candidacy may benefit from detailed assessment of the nature of the patient's motivation, implicit notion of intelligence, and level of grit.
Although for many patients everyday living serves as sufficient aural rehabilitation to yield reasonably good speech recognition and QOL, findings from this exploration did suggest that patients with both better performance and better QOL were generally more motivated to pursue active as opposed to passive rehabilitation strategies; however, this was not unanimously true because some patients had good speech recognition abilities immediately postactivation of their devices. Good performers and those with high QOL were also more likely to have family support and to participate in CI support meetings or groups.
It is important to keep in mind when interpreting these results that the performance status of patients (i.e., good vs. poor word recognition or high vs. low QOL) was not blinded to the reviewers performing thematic content analysis. This may have influenced interpretations of ambiguous or equivocal statements made by patients. Likewise, a selection bias is also inherent in this study because it was composed entirely of volunteer participants. Furthermore, recall bias may also come into play because CI users were asked in some cases to reflect back several years, an exercise that could easily be influenced by current performance status and overall CI satisfaction.
Other limitations of this study include the fact that the use of open‐ended questionnaires leads to difficulty in analyzing data, which may have complicated interpretations. On the other hand, this design permits a more detailed account of the data, serving to more thoroughly explore a research question.20 In addition, the limited data collected in this pilot study precluded evaluation of the roles of other factors that may contribute to patients’ likelihood of pursuing rehabilitation, such as family interactions and amount of time spent on various rehabilitation approaches. Future work could attempt to quantify responses in a more rigorous fashion, as well as to collect data with regard to frequency or duration of rehabilitation used. Also, although some rehabilitation strategies seemed to be associated with better word recognition or better QOL, these associations cannot be seen as causal in nature. Nonetheless, the findings do suggest that patients should be encouraged to set goals and to actively pursue their own postoperative rehabilitation. Clinicians should support the postoperative rehabilitative process for CI users, such as by using handouts discussing local resources, Web sites, and support groups. Moreover, surgeons and audiologists should reinforce the need for rehabilitation during pre‐ and postoperative counseling to dispel misconceptions on the part of the patient that verbal communication will automatically return without it.
CONCLUSION
Adult postlingually deafened CI users made use of a variety of postoperative self‐driven rehabilitation strategies after implantation; many did not use any particular rehabilitation strategies at all. This pilot study revealed that those who had realistic expectations, were motivated to approach their rehabilitation actively, had family support, and participated in CI support meetings or groups tended to perform better and had higher QOL with their implants. Findings reinforce the role of both surgeons and audiologists in improving counseling, and suggest the need for improved postoperative rehabilitation approaches to optimize outcomes, and also for larger‐scale studies assessing rehabilitative strategies used.
Supporting information
Supporting Information
Acknowledgment
The authors wish to acknowledge Susan Nittrouer, Ph.D., for providing word recognition stimuli and scoring materials for use in this study.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Gaylor JM, Raman G, Chung M, et al. Cochlear implantation in adults: a systematic review and meta‐analysis. JAMA Otolaryngol Head Neck Surg 2013;139:265–272. [DOI] [PubMed] [Google Scholar]
- 2. Firszt JB, Holden LK, Skinner MW, et al. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear 2004;25:375–387. [DOI] [PubMed] [Google Scholar]
- 3. Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol 2008;13:193–205. [DOI] [PubMed] [Google Scholar]
- 4. Holden LK, Finley CC, Firszt JB, et al. Factors affecting open‐set word recognition in adults with cochlear implants. Ear Hear 2013;34:342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friesen LM, Shannon RV, Baskent D, et al. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am 2001;110:1150–1163. [DOI] [PubMed] [Google Scholar]
- 6. Moberly AC, Lowenstein JH, Nittrouer S. Word recognition variability with cochlear implants: “perceptual attention” versus” auditory sensitivity”. Ear Hear 2016;37:14–26. doi: 10.1097/AUD.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson BS, Dorman MF. Cochlear implants: current designs and future possibilities. J Rehabil Res Dev 2008;45:695–730. [DOI] [PubMed] [Google Scholar]
- 8. Dillon MT, Buss E, Adunka MC, et al. Long‐term speech perception in elderly cochlear implant users. JAMA Otolaryngol Head Neck Surg 2013;139:279–283. [DOI] [PubMed] [Google Scholar]
- 9. Manrique MJ, Espinosa JM, Huarte A, et al. Cochlear implants in post‐lingual persons: results during the first five years of the clinical course. Acta Otorrhinolaringol Esp 1998;49:19–24. [PubMed] [Google Scholar]
- 10. Massa ST, Ruckenstein MJ. Comparing the performance plateau in adult cochlear implant patients using HINT and AzBio. Otol Neurotol 2014;35:598–604. [DOI] [PubMed] [Google Scholar]
- 11. Sweetow R, Palmer CV. Efficacy of individual auditory training in adults: a systematic review of the evidence. J Am Acad Audiol 2005;16:494–504. [DOI] [PubMed] [Google Scholar]
- 12. Sweetow RW, Sabes JH. Technologic advances in aural rehabilitation: Applications and innovative methods of service delivery. Trends Amplif 2007;11:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humes LE, Burk MH, Strauser LE, et al. Development and efficacy of a frequent‐word auditory training protocol for older adults with impaired hearing. Ear Hear 2009;30:613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stacey PC, Raine CH, O'Donoghue GM, et al. Effectiveness of computer‐based auditory training for adult users of cochlear implants. Int J Audiol 2010;49:347–356. [DOI] [PubMed] [Google Scholar]
- 15. Stacey PC, Summerfield AQ. Comparison of word‐, sentence‐, and phoneme‐based training strategies in improving the perception of spectrally distorted speech. J Speech Lang Hear Res 2008;51:526–538. [DOI] [PubMed] [Google Scholar]
- 16. Hinderink JB, Krabbe PF, Van den Broek P. Development and application of a health‐related quality‐of‐life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngol Head Neck Surg 2000;123:756–765. [DOI] [PubMed] [Google Scholar]
- 17. Klop WM, Briare JJ, Stiggelbout AM, et al. Cochlear implant outcomes and quality of life in adults with prelingual deafness. Laryngoscope 2007;117:1982–1987. [DOI] [PubMed] [Google Scholar]
- 18. Zaidman‐Zait A. Quality of life among cochlear implant recipients In: Stone JH, Blouin M, eds. International Encyclopedia of Rehabilitation. Available at: http://cirrie.buffalo.edu/encyclopedia/article.php?id=293&language=en. Accessed May 21, 2016. [Google Scholar]
- 19. Ramos A, Guerra‐Jimenez G, Rodriguez C, et al. Cochlear implants in adults over 60: a study of communicative benefits and the impact on quality of life. Cochlear Implants Int 2013;14:241–245. [DOI] [PubMed] [Google Scholar]
- 20. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. [Google Scholar]
- 21. Emond A, Moore M, Tjornby C, Kentish R. Factors associated with poor use of sequential bilateral cochlear implants in young people: a preliminary audit of poor users. Cochlear Implants Int 2013;14(suppl 4):S40–S43. [DOI] [PubMed] [Google Scholar]
- 22. Athalye S, Mulla I, Archbold S. The experiences of adults assessed for cochlear implantation who did not proceed. Cochlear Implants Int 2014;15:301–311. doi: 10.1179/1754762814Y.0000000067. [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24. Hirsh IJ, Davis H, Silverman SR, Reynolds EG, Eldert E, Benson RW. Development of material for speech audiometry. J Speech Hear Disord 1952;17:321–337. [DOI] [PubMed] [Google Scholar]
- 25. Green J, Thorogood N. Qualitative Methods for Health Research. London, UK: Sage; 2004. [Google Scholar]
- 26. Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 27. Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: a methodological review and user's guide. Psychon Bull Rev 2005;12:769–786. [DOI] [PubMed] [Google Scholar]
- 28. Tamati TN, Gilbert JL, Pisoni DB. Some factors underlying individual differences in speech recognition on PRESTO: a first report. J Am Acad Audiol 2013;24:616–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capretta NR, Moberly AC. Does quality of life depend on speech recognition performance for adult cochlear implant users? Laryngoscope 2016;126:699–706. doi: 10.1002/lary.25525. [DOI] [PubMed] [Google Scholar]
- 30. Jaeggi SM, Buschkuehl, Shah P, Jonides J. The role of individual differences in cognitive training and transfer. Mem Cognit 2014;42:464–480. [DOI] [PubMed] [Google Scholar]
- 31. Blackwell LS, Trzesniewski KH, Dweck CS. Implicit theories of intelligence predict achievement across an adolescent transition: a longitudinal study and intervention. Child Dev 2007;78:246–263. [DOI] [PubMed] [Google Scholar]
- 32. Duckworth AL, Peterson C, Mathews MD, Kelly DR. Grit: perseverance and passion for long‐term goals. J Pers Soc Psychol 2007;92:1087–1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


