Abstract
Objectives
As indications for cochlear implantation have expanded to include patients with more residual hearing, increasing emphasis has been placed on minimally traumatic electrode insertion. Histopathologic evaluation remains the gold standard for evaluation of cochlear trauma, but advances in imaging techniques have allowed clinicians to determine scalar electrode location in vivo. This review will examine the relationship between scalar location of electrode arrays and audiologic outcomes. In addition, the impact that surgical approach, electrode design, and insertion depth have on scalar location will be evaluated.
Data Sources: PubMed literature review
Review Methods: A review of the current literature was conducted to analyze the relationship between scalar location of cochlear implant electrode arrays and speech perception outcomes. Further, data were reviewed to determine the impact that surgical variables have on scalar electrode location.
Results
Electrode insertions into the scala tympani are associated with superior speech perception and higher rates of hearing preservation. Lateral wall electrodes, and round window/extended round window approaches appear to maximize the likelihood of a scala tympani insertion. It does not appear that deeper insertions are associated with higher rates of scalar translocation.
Conclusion
Superior audiologic outcomes are observed for electrode arrays inserted entirely within the scala tympani. The majority of clinical data demonstrate that lateral wall design and a round window approach increase the likelihood of a scala tympani insertion.
Level of Evidence
N/A.
Keywords: cochlear implant, scala tympani, electrode location, electrode design, surgical approach, speech perception
INTRODUCTION
In recent years, cochlear implantation indications have expanded to include patients with greater degrees of residual hearing. As evidence has demonstrated that combined electric and acoustic stimulation results in improved audiologic outcomes, particularly with respect with speech understanding in complex listening environments, music perception, and sound localization, hearing preservation has become a fundamental concept of cochlear implant (CI) surgery.1, 2, 3, 4, 5 Further, there is evidence to suggest that minimally traumatic surgery is associated with superior audiologic outcomes in those patients destined for electric‐only stimulation.6 This has led to increased emphasis on the preservation of cochlear structures and minimization of trauma during electrode insertion.
Insertion trauma can occur secondary to various mechanisms, both mechanical and physiologic. Table 1 provides a general overview of possible mechanisms of injury related electrode insertion. The gold standard for assessing cochlear trauma remains histologic evaluation. A widely referenced classification system utilizes a five‐point scale to grade insertion trauma as follows: 0– no trauma, 1–basilar membrane elevation, 2–basilar membrane rupture, 3–electrode contacts within the scala vestibuli, and 4–osseous spiral lamina fracture, modiolus fracture or stria vascularis tear.7 Applying histologic assessment of trauma to routine clinical practice, however, is challenging since the implanted cochlea must either be studied post‐mortem or in cadaveric specimens.
Table 1.
Potential mechanisms of trauma related to electrode insertion in cochlear implantation. ST = scala tympani; SV = scala vestibule.
| Acute mechanical trauma |
| Fracture of the osseous spiral lamina, with injury to dendrite processes |
| Damage to the modiolus, with injury to spiral ganglion cells along medial wall of ST |
| Damage to the lateral wall, with injury to spiral ligament, organ of Corti, or stria vascularis |
| Rupture of cochlear partitions with electrode translocation from the ST to the SV |
| Compression or tearing of cochlear vasculature |
| Acute non‐mechanical trauma |
| Acoustic trauma related to drilling |
| Disruption of cochlear fluid hemostasis (mixing of endolymph and perilymph with injury to cochlear partitions, excessive suctioning, introduction of blood into the ST) |
| Sub‐acute or delayed events |
| Labyrinthitis secondary to spread of middle ear flora into the cochlea |
| Foreign body reaction to the electrode |
| Fibrosis or ossification |
| Molecular activation of apoptotic pathways with resultant delayed neural injury |
With recent advances in imaging techniques, clinicians can now determine scalar location of an electrode array in vivo.8, 9 It is widely accepted that to limit trauma during electrode insertion, the electrode array should be positioned entirely within the scala tympani (Figure 1). An electrode that translocates from the scala tympani into the scala vestibuli damages both the basilar and Reissner's membranes, and potentially injures the Organ of Corti and osseous spiral lamina (Figure 2). As such, damage to cochlear partitions is inherent when imaging demonstrates that an electrode array has contacts located within the scala vestibuli. It follows that classification schemes of histologic trauma, as described above, generally grade scalar translocation as severe trauma.
Figure 1.
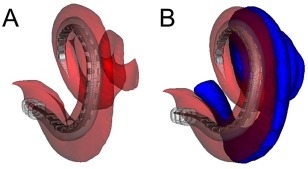
Reconstructed CT images showing a cochlear implant completely within the scala tympani (ST). (A) The electrode is positioned entirely within the ST, shown in red. (B) The scala vestibuli (SV), shown in blue, is added to the reconstruction. Used with permission from Wiley Publishers.
Figure 2.

Reconstructed CT images showing translocation of an electrode array from the scala tympani (ST) into the scala vestibuli (SV). (A) The electrode crosses the basilar membrane and exits the ST, shown in red, in the basal turn. (B) Electrode translocation from the ST (red) to the SV (blue) is shown. Used with permission from Wiley Publishers.
In addition to translocation, electrodes can also be directly inserted into the scala vestibuli. Despite the theoretical possibility that less trauma would occur with direct scala vestibuli insertions, studies have shown that significant trauma is also present in such cases. In a cadaveric temporal bone study, Adunka et al intentionally inserted electrodes directly into the scala vestibuli; in all cases, rupture of Reissner's membrane was noted.10 The osseous spiral lamina was also fractured in the majority of specimens, but this was attributed to drilling the cochleostomy, which was placed in an abnormal location (inferior aspect of the oval window) so as to achieve an immediate scala vestibuli insertion. Taken together, electrodes should be positioned entirely within the scala tympani to minimize trauma associated with electrode insertion.
The purpose of this review is therefore to examine factors that are associated with achieving a scala tympani electrode insertion. Particular attention will be given to the influence that electrode design, surgical approach, and insertion depth have on scalar location, as these are surgically modifiable variables. Lastly, given that ultimate performance is paramount, the literature assessing the association between scalar location and audiologic outcomes will also be reviewed.
The Impact of Surgical Variables on Scalar Electrode Location
Recent research has focused on identification of surgical factors that impact intracochlear trauma. Electrode design, surgical approach, and insertion depth have all been suggested as potentially important variables.
Electrode design
Electrode design can be classified as three general types: lateral wall, perimodiolar, and mid‐scala. The majority of available data support the notion that lateral wall electrodes enter the scala vestibuli much less frequently than perimodiolar electrode arrays. Reported rates of positioning within the scala tympani range between 89‐97% for lateral wall arrays, and 10‐74% for perimodiolar arrays.9, 11, 12, 13, 14, 15
Studies that directly compare rates of scala tympani insertion between lateral wall and perimodiolar electrodes arrays are detailed in Table 2. Wanna et al studied 116 adults undergoing cochlear implantation with either lateral wall or perimodiolar arrays. Using data from micro‐CT scans of cadaveric cochlea, an active shape statistical model was created which allowed for identification of the scala tympani and vestibuli on an individual patients' pre‐operative CT imaging. Electrode contacts were localized on post‐operative CT using either semi‐automated or fully automated approaches.16, 17 The pre‐operative and post‐operative sequences were then fused, and the scalar position for each electrode contact was determined. The described methodology was validated using cadaveric models.18 They found that higher rates of scala tympani insertion were observed for lateral wall electrodes (89%) when compared to perimodiolar arrays (58%).
Table 2.
Overview of studies that compare rates of scala tympani insertion between different types of electrodes. UV = univariate; MV = multivariate; ST = scala tympani; SV = scala vestibuli; LW = lateral wall; MS = mid‐scala; PM = perimodiolar; CT = computed tomography.
| Electrodes included | ||||||
|---|---|---|---|---|---|---|
| Study (year) | Method for determining electrode location | Statistical analysis | LW (n) | PM (n) | MS (n) | Findings |
| Wanna et al (2014) | Pre‐ and post‐operative CT | UV | 47 | 69 | Higher rates of ST insertion with LW (89%) compared to PM (58%) | |
| Boyer et al (2015) | Post‐operative CT | UV | 30 | 31 | Higher rates of ST insertion with LW (97%) compared to PM (74%) | |
| O'Connell et al (2016) | Pre‐ and post‐operative CT | MV | 91 | 115 | 14 |
Higher rates of ST insertion with LW (96%) compared to PM (49%) and MS (43%) PM and MS electrodes were 22.4 and 55.0 times (respectively) more likely to have SV insertion than LW electrodes in MV analysis |
More recently, O'Connell et al evaluated a larger subset of patients (n=220) using the same technique to determine scalar location. Multivariate analysis was performed to control for surgical variables, including surgical approach and insertion depth, that could otherwise bias results.11 The superiority of lateral wall electrodes was clearly demonstrated, as perimodiolar electrode arrays were 22 times more likely to have at least one electrode contact within the scala vestibuli when compared to lateral wall arrays. These findings have also been corroborated by Boyer et al who analyzed electrode location in 61 patients using post‐operative cone beam CT imaging.12 While their technique was not formally validated, there is evidence to suggest that high‐resolution post‐operative CT imaging can be used to accurately determine electrode position.19 They also showed that rates of scala tympani insertion were significantly higher with lateral wall electrodes (97%) when compared to perimodiolar electrodes (74%).
The most likely explanation for these findings has to do with electrode mechanics. Specifically, the flexible properties of lateral wall electrodes decrease the likelihood of disrupting and crossing the basilar membrane. While perimodiolar arrays may theoretically be advantageous due to increased modiolar proximity and more localized neural excitation, it is possible that the pre‐curved shape causes the tip to contact the outer scalar wall beneath the basilar membrane roughly 10 mm from the insertion site, potentially predisposing to translocation.20 The variability associated with performance of the advance off‐stylet technique required for perimodiolar arrays should also be considered. Given that cochleae vary considerably in size, the fact that the advance off‐stylet technique relies on an external electrode marker to begin stylet removal may impact translocation rates. While the most experienced advance off‐stylet surgeons may obtain better results than what has been reported in the literature, the aforementioned data likely represent a reasonable cross‐section of high‐volume CI surgeons.
Less data are available regarding the scalar location of mid‐scala electrode arrays. In cadaveric temporal bone studies, electrode contacts were located entirely within the scala tympani in the vast majority of specimens.21, 22, 23 Interestingly, Frisch et al noted that 50% of electrode contacts were actually located in a mid‐scala position, while the remaining electrodes tended to occupy the perimodiolar region.21 In vivo studies examining position of mid‐scala electrodes radiographically have suggested higher rates of electrode translocation, with only 43% of insertions achieving a scala tympani location.11 It should be noted that the cohorts of mid‐scala patients in all the previously cited studies were small, which may explain the discrepancies in results. Further investigations of mid‐scala electrode positioning in vivo are needed before definitive conclusions regarding scalar location outcomes can be made.
Surgical approach
Surgical approach has also been identified as a potentially important factor in predicting scalar electrode location. Three types of surgical approach are typically described: 1) round window, 2) extended round window, defined as enlarging and then opening the round window by drilling the anterior‐inferior margin, and 3) cochleostomy. Most large clinical studies have shown that the rate of scala tympani insertion is considerably higher when either a round window, or extended round window approach, is employed as compared to cochleostomy approaches.9, 11
Since electrode design has been shown to be independently predictive of a scala tympani insertion, it is very important to account for this covariate when assessing the impact that surgical approach has on scalar location. Wanna et al demonstrated that round window (91%) and extended round window (84%) approaches were more likely to result in scala tympani insertion than cochleostomy approaches (37%).9 Importantly, this finding held true regardless of whether the electrode was a lateral wall or perimodiolar design. Similarly, O'Connell et al studied the impact of surgical approach controlling for electrode design and insertion depth, and found that round window and extended round window approaches were associated with a 70% reduction in the rate of scala vestibuli insertion when compared to cochleostomy approaches.11 Connor et al prospectively studied patients undergoing implantation (n=65) with lateral wall electrodes, and studied translocation rates in the basal turn of the cochlea using post‐operative CT imaging.15 The rate of scalar translocation was higher for cochleostomies (9%) than round window insertions (0%), but this difference did not achieve significance. It is possible that confining analysis to the basal turn of the cochlea limited their ability to identify all cases of crossover, regardless of approach. This notion is supported by evidence that electrode dislocations tend to occur around 370 degrees for lateral wall arrays.12
One potential explanation for these in vivo findings is that surgical misplacement of the cochleostomy can result in either direct insertion into the scala vestibuli or scalar translocation soon after the distal portion of the electrode has been inserted into the cochleostomy. Proponents of the cochleostomy technique cite that discrepancies in the literature regarding scalar location after cochleostomy stem from the wide range of techniques used in creating a cochleostomy.24 In a survey of cochlear implant surgeons, Adunka et al found that considerable variability amongst surgeons exists with respect to where the cochleostomy is placed.25
Briggs et al studied the relation between cochleostomy location and intracochlear electrode position in temporal bone specimens.26 An increased risk of scala vestibuli insertions was observed for cochleostomies positioned anterior to the round window when compared to those located inferior to the round window. This finding can be explained by the anatomy of the hook region of the cochlea, in which the osseous spiral lamina and basilar membrane rotate from a vertical position at the level of the round window, to a more horizontal position further along the basal turn. A cochleostomy performed inferior to the round window through the crista fenestra should enter into the scala tympani and minimize potential for injury to other intracochlear structures. It should be noted that only perimodiolar arrays were used in this study, which may confound results.
Adunka et al also studied cochlear trauma in relation to cochleostomy position in cadaveric specimens. While they did not find differences in rates of scalar translocation between inferior and anteroinferior cochleostomies, the former were associated with less trauma overall.27 Drilling an inferiorly placed cochleostomy does necessitate more complete facial recess dissection than would be required for either a round window or anterior cochleostomy approach. Anatomic differences between patients may also account for some degree of variability in cochleostomy location among even the most experienced surgeons. Compared with the cochleostomy, exposure of the round window is straight forward in most cases, and it seems that unintentional insertions into the scala vestibuli can largely be avoided by using the round window, which is in direct continuity with the scala tympani.
Several other studies have reported rates of scalar dislocation in relation to surgical approach. These reports are generally limited by small sample sizes and lack of robust comparison groups precluding direct head to head comparisons of approaches. Souter et al studied temporal bone specimens implanted with perimodiolar arrays through a round window approach and noted that 20% (2/10) electrode arrays translocated into the scala vestibuli.28 Coordes et al prospectively evaluated 21 patients that underwent round membrane insertion of a perimodiolar array, and reported a similar electrode translocation rate of 19%.29 Zhou studied 15 cadaveric specimens implanted with lateral wall electrodes through either round window, extended round window, or cochleostomy approaches.24 One round window insertion was characterized by translocation, while all other insertions showed no evidence of trauma; statistical analysis was not performed given the small cohort.
Insertion depth
In general, the cross‐sectional area of the scala tympani decreases from the base to the apex.30 Biedron et al studied the internal dimensions of cochlear scala and demonstrated that the diameter of the scala tympani decreases by approximately 300 microns during the ascending portion of the basal turn.31 Verbist et al also showed that an increase in steepness of the spiraling cochlea is present at the junction between the second and apical turn, around 405 to 450 degrees of insertion.32 In light of these findings, a deep electrode insertion theoretically may increase the risk of electrode translocation from the scala tympani into the scala vestibuli.
However, most available data do not demonstrate a significant relationship between electrode insertion depth and scalar translocation. In their study of 220 implants, O'Connell et al found no significant association between greater angular insertion depth and scalar location when controlling for electrode design and surgical approach.11 Other studies examining insertion depth with lateral wall electrodes have similarly failed to demonstrate a significant relationship between deeper insertions and higher rates of electrode translocation.14 In contrast to the aforementioned clinical studies, however, a cadaveric temporal bone study suggested that electrode translocation from the scala tympani into the scala vestibuli is associated with greater angular insertion depths. It should be noted that this finding only pertained to perimodiolar electrodes inserted through cochleostomy approaches, both of which independently predispose to translocation and may bias results.33 In the same study, electrode translocation of lateral wall arrays was not found to be associated with angular insertion depth.
Electrode Location and Audiologic Outcomes
Postoperative audiometric performance is ultimately of greatest interest to clinicians involved in the care of patients undergoing cochlear implantation. Studies have suggested audiologic outcomes are influenced by scalar electrode location, thus available evidence will be reviewed.
Speech perception
Whether scalar position of an electrode impacts speech performance has important clinical implications. Multiple studies have demonstrated that scala tympani insertions are associated with better speech perception performance when compared to scala vestibuli insertions.8, 9, 11, 13, 34, 35 Relevant studies have been detailed in Table 3.
Table 3.
Overview of studies that examine speech perception in relation to scalar location of electrode arrays. UV = univariate; MV = multivariate; ST = scala tympani; SV = scala vestibuli; LW = lateral wall; MS = mid‐scala; PM = perimodiolar; CT = computed tomography.
| Study (year) | Method for determining electrode location | Statistical analysis | Number Implants (n) | Findings |
|---|---|---|---|---|
| Skinner et al (2007) | Pre‐ and post‐operative CT | UV | 15 | Negative correlation between number of electrode contacts in the scala vestibuli and CNC score |
| Aschendorff et al (2007) | Rotational tomography | UV | 43 | In patients with short duration deafness, Freiburg numbers and Oldenburg sentence scores were higher for ST insertions than SV insertions |
| Finley et al (2008) | Pre‐ and post‐operative CT | MV | 14 | Overall scalar position of the electrode array and number of electrode contacts in the scala vestibuli accounted for significant variance in CNC score |
| Holden et al (2013) | Pre‐ and post‐operative CT | MV | 114 | The percentage of electrode contacts in the SV inversely correlated with CNC score |
| Wanna et al (2014) | Pre‐ and post‐operative CT | UV | 116 | CNC score was higher for ST insertion (49%) than SV insertion (36%) |
| O'Connell et al (2016) | Pre‐ and post‐operative CT | MV | 220 | CNC score was higher for ST insertion (51%) than SV insertion (39%) |
| AzBio score was higher for ST insertion (61%) than SV insertion (50%) | ||||
| SV insertion associated with 12% decrease in CNC score in MV analysis |
There are several possible mechanisms by which scala vestibuli insertions negatively impact speech perception outcomes. Perhaps most obviously, trauma to terminal sensorineural structures and spiral ganglion cells is more likely with scala vestibuli insertions. Alternatively, if monopolar‐coupled electrodes are located within the scala vestibuli, they are more likely to stimulate ganglion cells in the next more‐apical turn, in addition to ganglion cells in the immediate cochlear turn, which could result in cross‐turn stimulation and pitch confusion.35
Hearing preservation
Avoiding trauma is of paramount importance in patients undergoing hearing preservation surgery. For many years, authors postulated that a scala vestibuli insertion or dislocation from one scala to the other would probably destroy residual hearing.10, 13 Recent research examining hearing preservation as a function of scalar location has confirmed this notion. Wanna et al studied 45 implants in patients with residual hearing at the time of cochlear implantation, defined as an 80 dB HL or less unaided air conduction threshold at 250 Hz.36 Hearing was preserved in 58% of scala tympani insertions at short‐term follow‐up, which also was defined by 80 dB HL or less at 250 Hz at 1 month post‐activation audiometric testing. In contrast, all seven patients with scala vestibuli insertions lost hearing; this difference was statistically significant. Other authors have also noted loss of residual hearing in patients with electrode translocation, but small cohorts limited the ability to perform robust statistical comparisons.37, 38 Taken together, it appears that scalar excursion is a strong predictor for loss of residual hearing. This can be explained by the fact that direct trauma to cochlear structures likely contributes to the loss of residual hearing observed in patients with scala vestibuli insertions. It is also possible that mixing of endolymph and perilymph subsequent to disruption of cochlear partitions impacts the ability to preserve hearing.
As efforts to understand which variables minimize trauma during electrode insertion are ongoing, it is important to note that a multitude of factors contribute, which are beyond the scope of this article. While we have the ability to determine scalar electrode position in vivo, identification of cochlear micro‐trauma during electrode insertion remains challenging given the need for histologic examination of specimens. In this light, it should be emphasized that controlling for scalar excursion addresses only one mechanism of insertion trauma, and does not imply that an insertion was entirely atraumatic. Current research investigating the use of electrocochleography during electrode insertion to provide surgeons with information regarding the functional status of the cochlea in real‐time is promising.39, 40 This may prove to be a valuable tool in providing feedback on electrode behavior and location within the cochlea during insertion.
CONCLUSIONS
Avoiding scalar translocation is associated with superior speech perception and higher rates of hearing preservation in patients undergoing cochlear implantation. Lateral wall electrodes, and round window/extended round window approaches appear to maximize the likelihood of a scala tympani insertion. It does not appear that deeper insertions are associated with higher rates of scalar translocation. Further research is needed to establish more comprehensive methods of identifying cochlear trauma during electrode insertion.
Financial Material & Support: No funding or other support was required for this study.
Financial Disclosures: Dr. Wanna is a consultant for MED‐EL, Advanced Bionics, Oticon Medical, and Cochlear Americas.
Editor's Note: This manuscript was received
BIBLIOGRAPHY
- 1. Buchner A, Schussler M, Battmer RD, Stover T, Lesinski‐Schiedat A, Lenarz T. Impact of low‐frequency hearing. Audiol Neurootol 2009;14(Suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 2. Turner C, Gantz BJ, Reiss L. Integration of acoustic and electrical hearing. J Rehabil Res Dev 2008;45:769–778. [DOI] [PubMed] [Google Scholar]
- 3. Skarzynski H, Lorens A, Matusiak M, Porowski M, Skarzynski PH, James CJ. Cochlear implantation with the nucleus slim straight electrode in subjects with residual low‐frequency hearing. Ear Hear 2014;35:e33–e43. [DOI] [PubMed] [Google Scholar]
- 4. Gifford RH, Dorman MF, Brown CA. Psychophysical properties of low‐frequency hearing: implications for perceiving speech and music via electric and acoustic stimulation. Adv Otorhinolaryngol 2010;67:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gifford RH, Dorman MF, Skarzynski H et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear 2013;34:413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlson ML, Driscoll CL, Gifford RH et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol 2011;32:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eshraghi AA, Yang NW, Balkany TJ. Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope 2003;113:415–419. [DOI] [PubMed] [Google Scholar]
- 8. Skinner MW, Holden TA, Whiting BR et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol 2007;116(4):2–24. [PubMed] [Google Scholar]
- 9. Wanna GB, Noble JH, Carlson ML et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014;124(Suppl 6):S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adunka O, Kiefer J, Unkelbach MH, Radeloff A, Gstoettner W. Evaluating cochlear implant trauma to the scala vestibuli. Clin Otolaryngol 2005;30:121–127. [DOI] [PubMed] [Google Scholar]
- 11. O'Connell BP, Cakir A, Hunter JB et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol 2016;37(8):1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyer E, Karkas A, Attye A, Lefournier V, Escude B, Schmerber S. Scalar localization by cone‐beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol 2015;36:422–429. [DOI] [PubMed] [Google Scholar]
- 13. Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear 2007;28:75S–79S. [DOI] [PubMed] [Google Scholar]
- 14. Fischer N, Pinggera L, Weichbold V, Dejaco D, Schmutzhard J, Widmann G. Radiologic and functional evaluation of electrode dislocation from the scala tympani to the scala vestibuli in patients with cochlear implants. AJNR Am J Neuroradiol 2015;36:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Connor SE, Holland NJ, Agger A et al. Round window electrode insertion potentiates retention in the scala tympani. Acta Otolaryngol 2012;132:932–937. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y, Dawant BM, Labadie RF, Noble JH. Automatic localization of cochlear implant electrodes in CT. Med Image Comput Comput Assist Interv 2014;17:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noble JH, Dawant BM. Automatic graph‐based localization of cochlear implant electrodes in CT. Med Image Comput Comput Assist Interv 2015; 9350:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuman TA, Noble JH, Wright CG, Wanna GB, Dawant B, Labadie RF. Anatomic verification of a novel method for precise intrascalar localization of cochlear implant electrodes in adult temporal bones using clinically available computed tomography. Laryngoscope 2010;120:2277–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saeed SR, Selvadurai D, Beale T et al. The use of cone‐beam computed tomography to determine cochlear implant electrode position in human temporal bones. Otol Neurotol 2014;35:1338–1344. [DOI] [PubMed] [Google Scholar]
- 20. Briggs RJ, Tykocinski M, Saunders E et al. Surgical implications of perimodiolar cochlear implant electrode design: avoiding intracochlear damage and scala vestibuli insertion. Cochlear Implants Int 2001;2:135–149. [DOI] [PubMed] [Google Scholar]
- 21. Frisch CD, Carlson ML, Lane JI, Driscoll CL. Evaluation of a new mid‐scala cochlear implant electrode using microcomputed tomography. Laryngoscope 2015;125:2778–2783. [DOI] [PubMed] [Google Scholar]
- 22. Hassepass F, Bulla S, Maier W et al. The new mid‐scala electrode array: a radiologic and histologic study in human temporal bones. Otol Neurotol 2014;35:1415–1420. [DOI] [PubMed] [Google Scholar]
- 23. Dietz A, Gazibegovic D, Tervaniemi J, Vartiainen VM, Lopponen H. Insertion characteristics and placement of the Mid‐Scala electrode array in human temporal bones using detailed cone beam computed tomography. Eur Arch Otorhinolaryngol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24. Zhou L, Friedmann DR, Treaba C, Peng R, Roland JT, Jr. Does cochleostomy location influence electrode trajectory and intracochlear trauma? Laryngoscope 2015;125:966–971. [DOI] [PubMed] [Google Scholar]
- 25. Adunka OF, Buchman CA. Scala tympani cochleostomy I: results of a survey. Laryngoscope 2007;117:2187–2194. [DOI] [PubMed] [Google Scholar]
- 26. Briggs RJ, Tykocinski M, Stidham K, Roberson JB. Cochleostomy site: implications for electrode placement and hearing preservation. Acta Otolaryngol 2005;125:870–876. [DOI] [PubMed] [Google Scholar]
- 27. Adunka OF, Radeloff A, Gstoettner WK, Pillsbury HC, Buchman CA. Scala tympani cochleostomy II: topography and histology. Laryngoscope 2007;117:2195–2200. [DOI] [PubMed] [Google Scholar]
- 28. Souter MA, Briggs RJ, Wright CG, Roland PS. Round window insertion of precurved perimodiolar electrode arrays: how successful is it? Otol Neurotol 2011;32:58–63. [DOI] [PubMed] [Google Scholar]
- 29. Coordes A, Ernst A, Brademann G, Todt I. Round window membrane insertion with perimodiolar cochlear implant electrodes. Otol Neurotol 2013;34:1027–1032. [DOI] [PubMed] [Google Scholar]
- 30. Walby AP. Scala tympani measurement. Ann Otol Rhinol Laryngol 1985;94:393–397. [PubMed] [Google Scholar]
- 31. Biedron S, Prescher A, Ilgner J, Westhofen M. The internal dimensions of the cochlear scalae with special reference to cochlear electrode insertion trauma. Otol Neurotol 2010;31:731–737. [DOI] [PubMed] [Google Scholar]
- 32. Verbist BM, Ferrarini L, Briaire JJ et al. Anatomic considerations of cochlear morphology and its implications for insertion trauma in cochlear implant surgery. Otol Neurotol 2009;30:471–477. [DOI] [PubMed] [Google Scholar]
- 33. Radeloff A, Mack M, Baghi M, Gstoettner WK, Adunka OF. Variance of angular insertion depths in free‐fitting and perimodiolar cochlear implant electrodes. Otol Neurotol 2008;29:131–136. [DOI] [PubMed] [Google Scholar]
- 34. Finley CC, Holden TA, Holden LK et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol 2008;29:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holden LK, Finley CC, Firszt JB et al. Factors affecting open‐set word recognition in adults with cochlear implants. Ear Hear 2013;34:342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wanna GB, Noble JH, Gifford RH et al. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: preliminary results. Otol Neurotol 2015;36:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nordfalk KF, Rasmussen K, Hopp E, Greisiger R, Jablonski GE. Scalar position in cochlear implant surgery and outcome in residual hearing and the vestibular system. Int J Audiol 2014;53:121–127. [DOI] [PubMed] [Google Scholar]
- 38. Dalbert A, Huber A, Veraguth D, Roosli C, Pfiffner F. Assessment of cochlear trauma during cochlear implantation using electrocochleography and Cone Beam Computed Tomography. Otol Neurotol 2016;37:446–453. [DOI] [PubMed] [Google Scholar]
- 39. Adunka OF, Giardina CK, Formeister EJ, Choudhury B, Buchman CA, Fitzpatrick DC. Round window electrocochleography before and after cochlear implant electrode insertion. Laryngoscope 2016;126:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell L, Kaicer A, Sly D et al. Intraoperative real‐time cochlear response telemetry predicts hearing preservation in cochlear implantation. Otol Neurotol 2016;37:332–338. [DOI] [PubMed] [Google Scholar]


