Abstract
Objective
Hollow nerve conduits made of natural or synthetic biomaterials are used clinically to aid regeneration of peripheral nerves damaged by trauma or disease. To support healing, conduit lumen patency must be maintained until recovery occurs. New methods to study conduit structural integrity would provide an important means to optimize conduits in preclinical studies. We explored a novel combined technique to examine structural integrity of two types of nerve conduits after in vivo healing.
Study Design
Micro‐CT imaging with iodine contrast was combined with histological analysis to examine two different nerve conduits after in vivo nerve reconstruction in rats.
Materials and Methods
Sciatic nerve gaps in adult Lewis rats were reconstructed with poly(caprolactone) (PCL, 1.6 cm gap, 14‐week survival) or silicone (1 cm gap, 6‐week survival) conduits (N = 12 total). Conduits with regenerating tissues were imaged by micro‐CT with iodine contrast and compared to the histology (hematoxylin and eosin, immunostaining for axons) of regenerated tissues after iodine removal.
Results
PCL nerve conduits showed extensive breakage throughout their length, but all showed successful nerve growth through the conduits. The silicone conduits remained intact, although significant constriction was uniquely detected by micro‐CT, with 1 of 6 animals showing incomplete tissue regeneration.
Conclusions
Micro‐CT with iodine contrast offers a unique and valuable means to determine 3D structural integrity of nerve conduits and nerve healing following reconstruction. Furthermore, this paper shows that even if conduit compression and degradation occur, nerve regeneration can still take place.
Keywords: peripheral nerve regeneration, nerve repair, nerve conduits, micro‐CT, contrast agent, biomaterial implants, rat
INTRODUCTION
Traumatic peripheral nerve gaps (< 2.5cm) can be reconstructed using nerve autografts and/or hollow conduits made of natural or synthetic biomaterials to reconstitute an injured nerve. Autografts are usually the first choice to reconstruct peripheral nerve gaps that cannot be repaired with direct end‐to‐end coaptation. However, autografts require a separate surgical site, a functional deficit of the donor nerve, and prolonged surgery time. Use of hollow conduits made of synthetic biomaterials (also called nerve guides) provides advantages over these negative factors. Biocompatible biomaterial conduits have been shown to serve as a substitute for autografts for relatively short injury gaps, of <2.5 cm in humans (in some cases <1.5 cm in animals).1 The design of these hollow tubes, which are placed to encase both the proximal and distal stumps, has been studied extensively and many important features defined.2, 3, 4 The optimal conduit allows the ingrowth of fibroblasts and Schwann cells from both stumps, to nourish and form a supportive tissue bridge between the nerve stumps. Schwann cells then specifically support axonal regrowth by rearranging into fascicles called the Bands of Bungner. The conduits also prevent regenerating axons from innervating inappropriate structures or from sprouting into the surrounding soft tissues, thereby causing neuromas. They also guide regenerating axons to make appropriate contact with extracellular matrix materials and support cells in the distal stump that are crucial for further axonal regrowth.
Several types of conduits are currently FDA approved and used clinically.5, 6 Poly(caprolactone) (PCL) and its copolymers, especially a copolymer combining lactic acid and caprolactone, poly(DL‐lactide‐co‐caprolactone) (PLCL), have been studied extensively and a PLCL conduit is in clinical use, sold under the brand name of Neurolac©.5, 6, 7 However, further improvement is needed, because of some reports of difficulties with current versions8 and because none of the currently used FDA‐approved conduits support successful nerve regrowth across gaps longer than around 2.5 cm.5
A successful nerve conduit has many properties.1, 9, 10 It must have appropriate porosity to allow diffusion of growth factors, nutrients, and waste products, but restrict infiltration of fibrous cells, which could block formation of a tissue bridge across the gap. The material must be biocompatible and biodegradable, and not hinder peripheral nerve growth. Finally, conduits must be rigid enough to remain open to resist surrounding transmural soft tissue constrictive forces that would collapse the conduit before peripheral nerve regeneration.
To date, for optimizing conduits, it has been difficult to study all aspects of the patency and therefore all measures of success in preclinical animal studies. Challenges for histological studies include the large size and cylindrical shape of the conduits, which preclude examining the entire structure via histological sections, making reconstruction of a complete 3D structure technically challenging. For this reason, we explored a novel means to measure nerve conduit patency and structure in rodent models, using micro‐computed tomography (micro‐CT) after infiltrating tissues with iodine contrast agent, to visualize soft tissues.11 We previously showed that the iodine could be removed and the tissues successfully processed via standard methods to also examine their histology.11 This makes it now possible to view the entire regenerating nerve and conduit, yet also view the cellular status of the same tissues. Herein, these techniques were used to better understand how nerve conduits made of two different materials, PCL and silicone, can be involved in nerve reconstruction in vivo.
MATERIALS AND METHODS
Animals and Surgical Procedures
Twelve young adult Lewis rats were used in 2 in vivo experiments, one using PCL (females) and in the second, silicone (males), conduits. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee (IACUC) and were in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health.
Procedures were described previously,12 but in brief, for surgery, rats were anesthetized with continual isoflurane and oxygen exposure and given an analgesic. On one leg, the sciatic nerve was exposed, the nerve removed and a conduit was placed over both the proximal and distal nerve stumps, sutured in place and the conduit space was filled with sterile saline by injection. Muscle and skin were closed with sutures and awake animals were returned to their cages. Skin sutures were removed at 14 days.
Nerve Conduits
The PCL conduits were prepared by dipping a glass mandrel into a solution of PCL and then air‐drying, as described previously.13 The PCL conduits were 1.8 cm long, around 0.23 mm in average wall thickness, the injury gap was 1.6 cm long and the animals survived to 14 weeks. The silicone conduits used were 1.46 mm ID and 1.96 mm OD (Thermo Fisher Scientific, Florence, KY [Fisher]), 1.2 cm in length, the injury gap was 1 cm long and the animals survived to 6 weeks. No signs of autophagy, discomfort or weight loss were detected in any animal.
Tissue Preparation
At sacrifice, animals were euthanized via approved protocols and the conduits with a few mm of both nerve stumps were removed and fixed in 4% paraformaldehyde in phosphate buffered saline (PBS). Tissues with silicone conduits were imaged by micro‐CT at 1‐3 hrs after sacrifice and then placed back into fixative. After 48 hrs, tissues were rinsed in PBS and placed in a 2% Lugol's iodine solution (6% total iodine, “diluted Lugol's” from Fisher) for 48 h at room temperature, rinsed and then all were imaged via micro‐CT. Following imaging, the iodine was removed by incubation in 2.5% sodium thiosulfate (STS) in PBS (Fisher) for 48 h. After rinsing, tissues were paraffin embedded and axial sections were cut (10 micron thickness), retaining multiple sections at 500 micron distances along the length of the conduits. Some sections were stained with hematoxylin and eosin (H&E), dehydrated and coverslipped with Permount (Fisher). Adjacent sections were immunostained as previously described,12 labeling axons with a rabbit antibody to the 200 MW neurofilament protein (anti‐NF, 1:500 dilution, Sigma, St. Louis, MO) and a fluorescent secondary antibody (anti‐rabbit antibody labeled with Alexa 594, 1:1000 dilution, Invitrogen, Grand Island, NY). Nuclei were labeled with 4',6‐diamidino‐2‐phenylindole (DAPI, 1:1000 dilution, Sigma) and sections were coverslipped with Fluoromount (Fisher).
Micro‐CT
All tissues were maintained in PBS while being imaged via micro‐CT, as previously described.12 Briefly, imaging was done using a Siemens Inveon Multimodality System (San Diego, CA) in the University of Cincinnati Vontz Imaging Laboratory facility (VCIL), with high magnification, resulting in an effective voxel size of 17.3 microns. Image analysis and figure preparation were done with an Inveon software package or ImageJ (NIH), from DICOM image file stacks, using either 3D reconstructions or single frames. A movie of one stack was made using ImageJ (Supplementary information).
Microscopy
Stained sections were photographed using a wide field upright Zeiss Axioplan Imaging 2e fluorescence microscope (Zeiss, Germany). Grayscale images (for H&E or fluorescent staining) were taken with a QICam cooled CCS camera (Q Imaging, Canada). For fluorescently labeled slides, images taken in three channels were combined into a composite and pseudo colored in Photoshop (Adobe Systems, Inc.) Color images (H&E stained sections) were taken with a Zeiss Axiocam digital camera (Zeiss).
Image Processing
Images were arranged in Photoshop and enhanced to ensure that features discussed were visible to the reader, but the nature of the features was not altered.
RESULTS
Tissues Reconstructed With PCL Conduits Were Imaged by Iodine‐Enhanced Micro‐CT
Similar to views shown previously,11 many tissue features were visible inside nerve conduits using micro‐CT imaging with iodine as a contrast agent. The PCL conduit material (Figure 1) could be differentiated from internal (regenerated) tissues and external tissues that included a connective tissue sheath, fat, and muscle. In either an axial view (1A) or a longitudinal view (1E), the conduit appears as a dark region surrounded on both sides by a lighter layer, giving it a tri‐laminar appearance. These lighter layers are accumulations of inflammatory and infiltrating cells at the edges, as seen in the corresponding H&E stained section (1B). Further comparisons allowed identification of fragments of conduit (top structure in 1A, B, and C). These fragments were surrounded by inflammatory cells including foreign body giant cells (FBGCs, 1C), indicating that degradation was occurring. The lower high‐density (white) structure seen inside the conduit in 1A corresponded to a blood vessel in H&E, with fat deposits surrounding some sides of the vessel (1B and 1D). These fat deposits (fat has a very high affinity for iodine) allowed visualization of a long length of this blood vessel running down the center of the conduit (1E). Similar white densities, some paired with dark circles, were detected throughout the conduit (see Supplementary Information), showing extensive vascularization within regenerating tissues.
Figure 1.
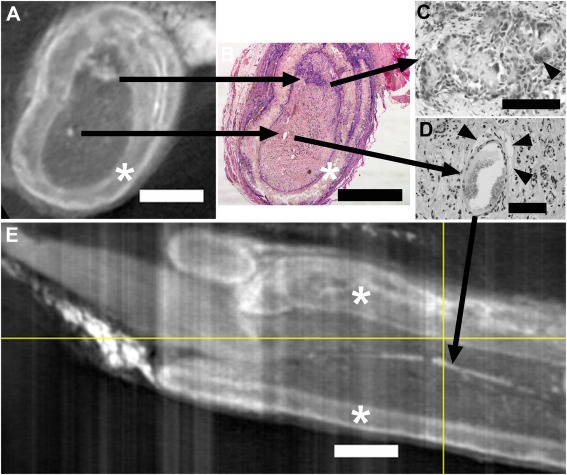
A and E are axial and longitudinal (respectively) micro‐CT images of iodine infused PCL nerve conduits with regenerating tissues. The location of the slice in A is indicated in E by the vertical yellow line (proximal is to the left in E). B, C, and D are images from an H&E stained paraffin section that was matched to the axial view in A. The black arrows starting in A connect micro‐CT structures to their respective structures in B and to higher magnification views in C and D. The uppermost black arrow series illustrates PCL fragments surrounded by and infiltrated with inflammatory cells, including foreign body giant cells (FBGCs, arrowhead in C). The lower black arrow series Illustrates that a high‐density (white) spot in A corresponds to a large blood vessel (B) surrounded by fat deposits (arrowheads in D). This white density marking a blood vessel shows as a white streak running longitudinally within the conduit in E (black arrow). PCL conduit walls are marked by asterisks. Bars equal 1 mm in A, B, and E and 0.1 mm in C and D.
Multiple Breaks Occurred in PCL Conduits
In animals with PCL conduits, significant breaks were observed at multiple points along the conduits. The conduit shown in Figure 2 demonstrates several types of these breaks. A longitudinal view is shown in (A), and axial micro‐CT sections of this conduit are shown in the second row (B, C, E, G, and H). In the third row are paraffin sections stained for axons and matched to the axial slices (D, F, and I). At the proximal (left) end of the conduit, the axial view (2B) shows a semi‐intact circle of conduit, although there are multiple breaks in the conduit walls. In the slightly more distal axial view (2C), the conduit is missing sections and immunostained axons (2D) were both inside the original central conduit area and outside the conduit, covering the left and lower exterior sides. This indicates nerves escaping the conduit and following the exterior. In the next region (2E), the conduit was again essentially intact, just compressed and with breaks. Axons were again detectable both inside and outside the conduit, forming a band around the periphery. Further distally (2G), multiple walls of the conduit can be seen that, by examination of both axial and longitudinal micro‐CT views, represent an area where one section had been pushed into the interior of the next in a telescoping fashion. This compressed the area available for internal regenerating tissues. Distally, the conduit was complete, but had a common breakage pattern (2H, see also 2E), consisting of one fairly intact semicircle of conduit, while the other half has two major pieces broken off and pushed inward. Despite all the breaks and constrictions, examination of the entire stack showed that an intact channel of internal regenerating tissues was present throughout the conduit (see Supplementary Information). This tissue continuity is consistent with the immunostaining (2I), which shows axons present in this distal section. In this distal section, axons were no longer present outside the conduit.
Figure 2.
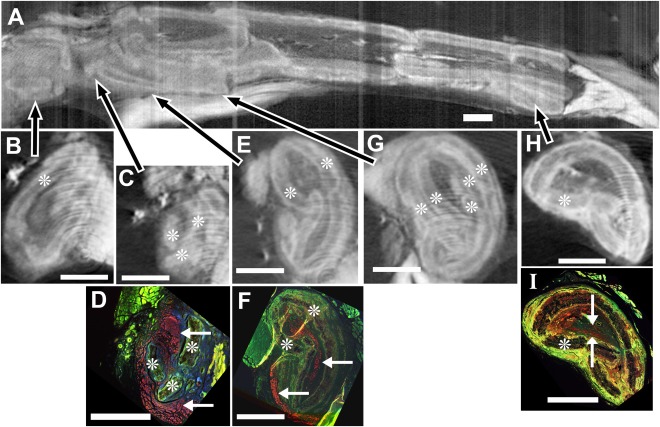
Breaks in PCL conduits. In a second PCL conduit, removed after 14 weeks in vivo, micro‐CT images with iodine infiltration (A, B, C, E, G, and H) were matched (after iodine removal and paraffin embedding) to sections immunostained for axons (red, anti‐neurofilament 200 protein) and nuclei (blue, DAPI) (non‐specific green staining enhanced to show tissue features) (D, F, and I). A) In the longitudinal image of the entire conduit (proximal to the left), arrows point to locations of the respective axial images (B, C, E, G, H). B) At a proximal axial level, the conduit (asterisks throughout indicate conduit material) is fairly complete, although with breaks. C) Slightly more distal, areas of conduit are missing, allowing contact between central tissues and the surrounding capsular tissues. D) In the immunostained section corresponding to C, nerve fibers are present essentially within the conduit (top arrow), but also outside the conduit material (red along the entire left side and lower arrow). E) In the next most distal region, the conduit again surrounds the tissues, although it is broken in several places. F) In the immunostained section corresponding to E, axons are both inside and outside the conduit (upper and lower arrows, respectively). G) A further distal region shows multiple conduit walls indicating where two sections of conduit have telescoped together and overlapped. H) In a very distal section, conduit breakage is still apparent. I) In the immunostained section matching the view in H, axons are present within (between the white arrows), but not outside the conduit. Bars equal 1 cm in all images.
Imaging of Silicone Nerve Conduits
In another series of animals, reconstructions were done with silicone conduits. This material is considered suboptimal because of both its lack of degradation and lack of porosity, which prevents nutrient inflow and waste removal, but it does avoid tissue compression. Silicone is denser than soft tissues so micro‐CT imaging was done shortly after sacrifice, without iodine enhancement, and the silicone material was visible (Figure 3A and B). Both the longitudinal (3A) and axial (3B) views show a central indentation of the conduit that suggests that the conduit was subjected to significant compressive forces in vivo. Several days later and after immersion in iodine, the tissue was re‐imaged (3C and D). The conduit was no longer compressed; it had regained a circular shape. It was now obvious that the nerve stumps (T in 3C) had not reconnected in the middle and the conduit appeared to be fluid‐filled. By viewing the entire micro‐CT stack (and subsequent histology), we confirmed the lack of intact tissue between the stumps. There also was no evidence that outgrowth would continue if time in vivo was extended. This occurred in one out of the six animals in this group, while 6 out of 6 animals with PCL conduits had tissue throughout, with no fluid‐filled areas.
Figure 3.
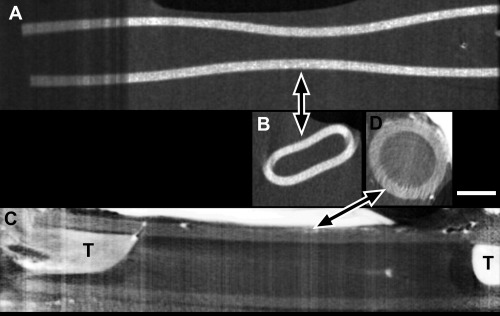
Nerve reconstruction through a silicone nerve conduit. A and B) Silicone appeared dense (white) in micro‐CT imaging done shortly after sacrifice, without iodine contrast. Compression of the conduit was evident in both the longitudinal view (A) and one axial view (B) taken at the longitudinal level indicated by the arrow. C and D) After iodine infusion, micro‐CT images of the same conduit show the tissue (T) of both nerve stumps but no tissue between them (one suture is evident in the proximal (left) stump). The conduit was no longer compressed, as seen in either longitudinal (C) or axial (D) views. Bar equals 1 cm and applies to all images.
DISCUSSION
This paper shows that micro‐CT imaging with iodine contrast provided detailed images of the 3D structural integrity of biomaterial nerve conduits in preclinical nerve reconstruction studies. The search for clinically optimal conduits requires understanding how each new biomaterial conduit can resist surrounding transmural pressures within the body to stay patent long enough to allow nerve growth. It is also important to examine whether the conduit degradation processes are rapid or severe enough to interfere with subsequent nerve regeneration. Conduits made with PLCL, a well‐studied and faster‐degrading co‐polymer of PCL, began to lose tensile strength after three weeks in vivo and had minimal tensile strength by 8 weeks.14 In rats, supporting cells cross a one cm gap by 2‐3 weeks, followed by axons by approximately 4 weeks,15 so this timetable of conduit degradation appears sufficient. In our study, with a 1.6 cm nerve gap, PCL conduits remained patent long enough for regenerating tissues to cross the gaps, because axons reached the distal nerve stump throughout all conduits, with a few exceptions where external nerve growth occurred. With respect to human nerve reconstruction, a longer time lag occurs before nerve growth begins16 and the nerve gaps are often wider. Thus, future conduits designed for human use will undoubtedly need to remain patent longer and degrade later.
The porosity of a conduit has an impact on nerve regrowth. PCL conduits can be structurally tailored to the most appropriate porosity, which holds a distinct advantage in nerve reconstruction strategies.17, 18 Towards this goal, the porosity of our PCL conduits was favorable for nerve growth.13
Evidence of conduit cracking, early conduit breakdown, swelling, and complete collapse have been reported previously with PCL or PLCL.17, 19, 20, 21 These can compromise nerve growth to various degrees, and the extent can vary with the conduit material formulation.17, 19, 20, 21 Even the FDA approved PLCL conduit (the Neurolac© conduit),5, 6, 7, 18 has been shown to exhibit significant swelling and breakage leading to compromised nerve growth.14, 19, 22 Significant inflammatory responses to PCL or copolymers of PCL have also been reported, including infiltration with fibroblasts, inflammatory cells, and FBGCs.16, 17, 19, 20, 23 In our study, micro‐CT imaging with contrast allowed us to identify fragments of conduit detached from the walls and then use subsequent histology to confirm the inflammatory response to the fragments.
The thickness of the conduit wall also affects collapse, degradation rates and the diffusion of nutrients. PLCL conduits with a wall thickness of approximately 0.17 mm collapsed completely, diverting all axons to the outside of the conduit.24 Our conduit thickness averaged 0.23 mm prior to surgery, which is somewhat thicker and, thus, closer to conduits with a thickness of 0.34 mm, which did not collapse.23 This is consistent with our observations that conduit breakage did not block tissue regrowth, as outlined above.
In previous studies, researchers were only able to examine the conduit at a few places along its longitudinal path via histological examination,21, 24 which could lead to incomplete information and conclusions about how the regenerating tissues are affected by cracks, collapse or degradation. The micro‐CT imaging not only documented breaks, it allowed us to study the various types of breaks along its axis and confirm that a continuous tissue path existed throughout, adding significantly to our understanding of how a regenerating peripheral nerve interacts with its conduit in vivo.
In terms of silicone conduits, advantages of using micro‐CT imaging showed that these dense conduits can be examined relatively quickly (1‐3 hours) after removal from the animal. While silicone is non‐porous, its rigid nature is valuable for testing nerve healing variables, by reducing surrounding transmural tissue pressures. Our first imaging sequences showed significant compression, presumably due to the in vivo transmural soft tissue pressure. However, the second imaging sequence, done several days later, did not show compression because this elastic material rebounds back to its original shape. Thus, without micro‐CT imaging, this phenomenon of early conduit compression during early in vivo healing would not have been recognized by histology alone.
Micro‐CT with contrast gave detailed images of nerve tissue growth through the conduit, in a relatively rapid fashion, without requiring tissue fixation and prolonged processing. This distinct advantage of micro‐CT imaging minimizes artefactual changes from tissue processing that could skew in vivo analysis. We believe that the image detail we obtained from micro‐ CT will enable us in the near future to quantify important variables of nerve healing (e.g. nerve extension into the conduit, nerve strand size within the conduit, and other variables) that would not be possible with histological analysis.
CONCLUSION
Micro‐CT with iodine contrast is a valuable novel tool to visualize and analyze the 3D structural integrity of nerve graft biomaterials after in vivo implantation, and to analyze how this effects peripheral nerve reconstruction. Recently characterized techniques11 were used to document the extent of cracks and defects in PCL nerve conduits, the extent of nerve healing after reconstruction and how compression affected silicone conduits. Despite latent significant breakage of PCL conduits, nerve growth still occurred in 6 out of 6 animals, across a 1.6 cm gap. In contrast, in silicone conduits where only compression occurred, tissue growth was not successful in 1 out of 6 animals across a 1 cm gap. Micro CT imaging will allow us to further optimize nerve conduits, with the goal of improving peripheral nerve reconstruction.
Supporting information
Legend for supporting movie: Pixley‐Hom‐MicroCTmovie.avi
This movie demonstrates the novel nature of how imaging via micro‐CT with iodine contrast can assist in analysis of the 3D structural integrity of nerve conduits in nerve healing experiments. Using NIH ImageJ, a movie (avi format) was made of the entire stack of 1,381axial images taken by micro‐CT, after iodine infusion, of the tissues inside one PCL nerve conduit. Selected axial images and a reconstructed longitudinal image of this conduit and its regenerating tissues are shown in Figure 2. Each axial slice is one pixel thick, which is 56.8 microns thick. The movie begins at the proximal end (to the left of the longitudinal view in Figure 2A). It is suggested that the movie be compared to the longitudinal view (Fig. 2A), to appreciate both perspectives. Details of Figure 2 are discussed in the text.
Acknowledgments
Thanks to Dr. Danielle Minteer and Dr. Kacey Marra for generous production of PCL conduits. Thanks to Kathleen LaSance, CNMT, ARRT(N), Director of the Vontz Core Imaging Laboratory, for providing micro‐CT imaging and guidance on CT image manipulation and James Liggett for technical assistance. Grants supporting this research included the NSF ERC for Revolutionizing Metallic Biomaterials (EEC‐0812348 and NCAT 260116C supplement) and funding from the University of Cincinnati Department of Otolaryngology‐Head and Neck Surgery. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any of the funding agencies.
Financial Disclosure: The authors declare that they have no financial competing interests.
Conflict of interest: The authors declare that they have no conflicts of interest.
Portions of this work have appeared previously in abstract and poster format
BIBLIOGRAPHY
- 1. Schmidt CE, Leach JB. Neural tissue engineering: Strategies for repair and regeneration. Ann Rev Biomed Eng 2003;5:293–347. [DOI] [PubMed] [Google Scholar]
- 2. de Ruiter GCW, Malessy MJA, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus 2009;26:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khaing ZZ, Schmidt CE. Advances in natural biomaterials for nerve tissue repair. Neurosci Lett 2012;519:103–114. [DOI] [PubMed] [Google Scholar]
- 4. Bell JHA, Haycock JW. Next generation nerve guides: Materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev 2012;18:116–128. [DOI] [PubMed] [Google Scholar]
- 5. Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014;35:6143–6156. [DOI] [PubMed] [Google Scholar]
- 6. Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012;43:553–572. [DOI] [PubMed] [Google Scholar]
- 7. Bliley JM, Marra KG. Polymeric Biomaterials as Tissue Scaffolds In: Stem Cell Biology and Tissue Engineering in Dental Sciences. Waltham, MA: Elsevier; 2014:149–161. [Google Scholar]
- 8. Meek MF, Jansen K, Steendam R, et al. In vitro degradation and biocompatibility of poly(DL‐lactide‐ε ‐caprolactone) nerve guides. J Biomed Mater Res Part A 2004;68:43–51. [DOI] [PubMed] [Google Scholar]
- 9. Chiono V, Tonda‐Turo C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog Neurobiol 2015;131:87–104. [DOI] [PubMed] [Google Scholar]
- 10. Chiono V, Tonda‐Turo C, Ciardelli G. Chapter 9 artificial scaffolds for peripheral nerve reconstruction. Int Rev Neurobiol 2009;87:173–198. [DOI] [PubMed] [Google Scholar]
- 11. Hopkins TM, Heilman AM, Liggett JA, et al. Combining micro‐computed tomography with histology to analyze biomedical implants for peripheral nerve repair. J Neurosci Methods 2015;255:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vennemeyer JJ, Hopkins T, Hershcovitch M, et al. Initial observations on using magnesium metal in peripheral nerve repair. J Biomater Appl 2015;29:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kokai LE, Lin Y, Oyster NM, Marra KG. Diffusion of soluble factors through degradable polymer nerve guides: Controlling manufacturing parameters. Acta Biomaterialia 2009;5:2540–2550. [DOI] [PubMed] [Google Scholar]
- 14. Den Dunnen WFA, Meek MF, Grijpma DW, Robinson PH, Schakenraad JM. In vivo and in vitro degradation of poly[50/50 (85/15 L/D)LA/e‐CL], and the implications for the use in nerve reconstruction. J Biomed Mater Res 2000;51:575–585. [DOI] [PubMed] [Google Scholar]
- 15. Williams LR, Longo FM, Powell HC. Spatial‐temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J Comp Neurol 1983;218:460–470. [DOI] [PubMed] [Google Scholar]
- 16. Den Dunnen WFA, Meek MF, Grijpma DW, Robinson PH, Schakenraad JM. In vivo and in vitro degradation of poly[50/50 (85/15 L/D)LA/e‐CL], and the implications for the use in nerve reconstruction. J Biomed Mater Res 2000;51:575–585. [DOI] [PubMed] [Google Scholar]
- 17. Den Dunnen WFA, Robinson PH, Van Wessel R, et al. Long‐term evaluation of degradation and foreign‐body reaction of subcutaneously implanted poly(DL‐lactide‐e‐caprolactone). J Biomed Mater Res 1997;36:337–346. [DOI] [PubMed] [Google Scholar]
- 18. Dash TK, Konkimalla VB. Poly‐e‐caprolactone based formulations for drug delivery and tissue engineering: A review. J Control Release 2012;158:15–33. [DOI] [PubMed] [Google Scholar]
- 19. Meek MF, Den Dunnen WFA. Porosity of the wall of a neurolac® nerve conduit hampers nerve regeneration. Microsurgery 2009;29:473–478. [DOI] [PubMed] [Google Scholar]
- 20. Duda S, Dreyer L, Behrens P, et al. Outer electrospun polycaprolactone shell induces massive foreign body reaction and impairs axonal regeneration through 3D multichannel chitosan nerve guides. BioMed Res Int 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R. Long‐term in vivo biomechanical properties and biocompatibility of poly(2‐hydroxyethyl methacrylate‐co‐methyl methacrylate) nerve conduits. Biomaterials 2005;26:1741–1749. [DOI] [PubMed] [Google Scholar]
- 22. Hernández‐Cortés P, Juan G, Cámara M, Ravassa FO. Failed digital nerve reconstruction by foreign body reaction to neurolac®: Nerve conduit. Microsurgery 2010;30:414–416. [DOI] [PubMed] [Google Scholar]
- 23. Den Dunnen WFA, Van der Lei B, Robinson PH, et al. Biological performance of a degradable poly(lactic acid‐e‐caprolactone) nerve guide: Influence of tube dimensions. J Biomed Mater Res 1995;29:757–766. [DOI] [PubMed] [Google Scholar]
- 24. Meek MF, Den Dunnen WFA, Bartels HL, et al. Peripheral nerve regeneration and functional nerve recovery after reconstruction with a thin‐walled biodegradable poly(dl‐lactide‐e‐ caprolactone) nerve guide. Cells Mater 1997;7:53–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legend for supporting movie: Pixley‐Hom‐MicroCTmovie.avi
This movie demonstrates the novel nature of how imaging via micro‐CT with iodine contrast can assist in analysis of the 3D structural integrity of nerve conduits in nerve healing experiments. Using NIH ImageJ, a movie (avi format) was made of the entire stack of 1,381axial images taken by micro‐CT, after iodine infusion, of the tissues inside one PCL nerve conduit. Selected axial images and a reconstructed longitudinal image of this conduit and its regenerating tissues are shown in Figure 2. Each axial slice is one pixel thick, which is 56.8 microns thick. The movie begins at the proximal end (to the left of the longitudinal view in Figure 2A). It is suggested that the movie be compared to the longitudinal view (Fig. 2A), to appreciate both perspectives. Details of Figure 2 are discussed in the text.


