ABSTRACT
Objective
To review recent literature on human papillomavirus‐related (HPV‐positive) oropharyngeal squamous cell carcinoma (OPC) and focus on implications of recurrent and metastatic disease.
Methods
Primary articles from 1990 to 2016 indexed in MEDLINE (1) pertaining to the epidemiology of HPV‐positive OPC and (2) providing clinical insight into recurrent and metastatic OPC.
Results
The incidence of HPV‐positive OPC is increasing globally. HPV‐positive OPC is a subtype with distinct molecular and clinical features including enhanced treatment response and improved overall survival. While disease recurrence is less common in patients with HPV‐positive OPC, up to 36% of patients experience treatment failure within eight years. Recurrent and metastatic OPC has historically signified poor prognosis, however recent data are challenging this dogma. Here, we discuss recurrent and metastatic OPC in the context of HPV tumor status.
Conclusion
HPV‐positive OPC exhibits distinct genetic, cellular, epidemiological, and clinical features from HPV‐negative OPC. HPV tumor status is emerging as a marker indicative of improved prognosis after disease progression in both locoregionally recurrent and distant metastatic OPC.
Level of Evidence
N/A.
Keywords: Head and neck, squamous cell carcinoma, HNSCC, human papillomavirus, HPV, oropharyngeal, OPSCC, recurrent, metastatic, prognosis, survival
INTRODUCTION
Oropharyngeal squamous cell carcinoma (OPC) constitutes a subset of head and neck cancers (HNC) arising from the squamous epithelium of the oropharynx. Anatomic subsites of the oropharynx include the base of tongue, pharyngeal tonsils, tonsillar pillars, glossotonsillar sulci, soft palate, uvula, and the pharyngeal wall.1 In 2012, OPC accounted for nearly one quarter of incident cases of HNC and resulted in an estimated 97,000 deaths worldwide.2
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States3 and oral HPV infection is strongly associated with OPC.4 Indeed, HPV is a well‐established cause of OPC.5 Of the approximately 200 HPV types described,6 HPV16 is detectable in 87‐96% of HPV‐positive OPCs in the US.7, 8, 9 Other high‐risk HPV types–including 18, 31, 33, 45, 52 and 58–account for the remainder of HPV‐positive OPCs.10, 11, 12, 13
Despite marked geographic heterogeneity, the global incidence and prevalence of HPV‐positive OPC have risen significantly over the past three decades.7, 8, 9, 14 While the incidence of overall HNC and HPV‐negative HNC have declined substantially in industrialized countries, the incidence of HPV‐positive OPC has risen significantly and is projected to surpass HPV‐related cervical cancer by the year 2020 in the United States.15
Accumulating epidemiological, clinical, histopathological, and molecular evidence indicate that HPV status defines a distinct subtype of OPC.16, 17, 18, 19 HPV‐positive OPC is associated with increasing number of lifetime sex partners, younger age, male sex, and white race.4, 20 Alcohol or tobacco use, and poor oral hygiene do not appear to be independent risk factors for HPV‐positive OPC.16, 21 Conversely, non‐oropharyngeal HNC, malignancies arising in the oral cavity, hypopharynx, and larynx, are typically HPV‐negative and associated with heavy smoking, alcohol use, and poor oral hygiene but not with sexual behavior.10, 16, 22, 23, 24, 25, 26, 27 Recent evidence does, however, support complex interactions between the effects of HPV, alcohol, and tobacco exposure in modifying the risk of OPC.28, 29
HPV‐positive tumor status at diagnosis is an independent prognostic indicator of favorable outcome, conferring increased sensitivity to treatment and improved survival.30, 31, 32, 33, 34 Although disease recurrence is less common in patients with HPV‐positive OPC, up to 36% of patients experience treatment failure within eight years.35
Recurrent and metastatic OPC has historically signified poor prognosis.36, 37, 38, 39 However, the growing proportion of OPCs that are HPV‐positive and the improved survival of HPV‐positive OPC are challenging this paradigm.40, 41 Here, insights into the epidemiology, clinical features, and molecular biology of recurrent and metastatic oropharyngeal squamous cell carcinoma are reviewed in the context of HPV tumor status.
MATERIALS AND METHODS
Review of primary literature from 1990 to 2016 indexed in MEDLINE. Search terms: 1) “epidemiology” AND “human papillomavirus” AND oropharyn* yielded 501 results; 2) oropharyn* AND (metasta* OR recur*) AND “human papillomavirus” yielded 395 results. Additional articles were selected through the review of references in articles selected by the above search method.
RESULTS AND DISCUSSION
Mechanisms of HPV‐mediated oncogenesis and implications for the accurate detection of HPV‐positive OPC
HPV is a DNA virus with a circular genome that exists in the nucleus separate from the host genome.42 Integration of HPV DNA into the host genome is associated with stabilization of viral oncogenes43 and is observed in the majority of HPV‐positive OPCs.44 The role of HPV in carcinogenesis relies primarily on the expression of two oncogenic proteins, E6 and E7. Oncoprotein E6 binds the gene product of TP53 (p53), the most mutated tumor suppressor gene in cancer and “guardian of the genome”,45 targeting it for degradation.46 Similarly, oncoprotein E7 binds and targets the Retinoblastoma tumor suppressor protein (Rb) for degradation.47 Rb protein degradation triggers a regulatory cascade resulting in the compensatory upregulation of another tumor suppressor, p16INK4A (p16).48
The mechanisms of HPV‐mediated oncogenesis guide the strategies contemporary diagnostic laboratories employ to detect HPV in tumor samples. A critical feature of a diagnostic test for HPV‐related cancer is its ability to determine if a detected virus is an oncogenic driver of that tumor.8, 49 Highly sensitive assays such as polymerase chain reaction (PCR) based methods can detect well below one viral copy per cell. This level of sensitivity increases the possibility of detecting cross‐contaminants in clinical samples, which is an unfortunately common occurrence in the conventional diagnostic laboratory. For this reason, if a sensitive assay such as PCR is employed, it is typically performed in combination with a more specific test such as in‐situ hybridization (ISH) or immunohistochemistry (IHC).
Despite the necessity of a reliable method to detect HPV‐driven OPC, variability in HPV tumor detection remains both in research and clinical practice. Available assays detect HPV DNA, HPV RNA, HPV oncoproteins, host cellular proteins dysregulated by viral infection (such as p16), and HPV‐specific serum antibodies. These strategies were comprehensively detailed in two recent reviews.50, 51. It is worth emphasizing that differences in methodology of HPV detection may contribute to the observed heterogeneity in reported epidemiological data52 and that potential misclassification of HPV‐related tumors limits understanding of the impact of HPV‐positive OPC in clinical studies.53
Epidemiology of Oropharyngeal Carcinoma
Trends in incidence
Over the past three decades the relative proportion that each anatomic subsite contributed to the overall incidence of HNC has changed. Analysis of the Surveillance, Epidemiology, and End Results (SEER) data from 1973‐1999 across 9 state registries found that while the incidence of some non‐oropharyngeal HNC declined in the United States (U.S.), the contribution of OPC to total incidence of HNC increased from 17.6% in 1974 to 22.6% in 1999.37
Indeed, a SEER analysis segregated oral tumors into HPV‐related (base of tongue, lingual tonsil, oropharynx, and Waldeyer's ring) and HPV‐unrelated (tongue, gum, floor of mouth, palate, and unspecified parts of the mouth) based on prior associations between anatomic subsite and tumor HPV‐status.54 Consistent with the possibility that HPV was driving trends in HNC, from 1973 to 2004 an increase in the proportion of tumors arising from HPV‐related subsites and a reduction in the proportion of tumors arising from HPV‐unrelated subsites20 was shown. This was confirmed with contemporary gold standard tumor HPV detection in 271 OPCs, demonstrating that from 1984 to 2004 the incidence of HPV‐positive OPC increased by 225%, while the incidence of HPV‐negative OPC declined by 50%.15
A more recent SEER analysis suggested that increases in the incidence of OPC are accelerating. The overall incidence of OPC has increased by 63% from 1975‐2012. However, the most dramatic rise in OPC incidence occurred from 1998‐2012. Specific subsets of the U.S. population have experienced the greatest increases in incidence. Incidence for men and white individuals were observed to increase at annual percent changes (APC) of 0.94%/year (yr) and 0.66%/yr, respectively, from 1975‐1998. From 1998‐2012, the APC rose more than three‐fold to 3%/yr for men and 3.29%/yr for whites.55
Other studies have made similar observations, demonstrating rises in the incidence of OPC in the U.S. and abroad.56, 57, 58, 59, 60, 61 Analysis of the Cancer Incidence in Five Continents (CI5) database performed in 23 countries across 4 continents from 1983‐2002 found that 8 of 9 “more developed” countries (Denmark, Estonia, France, the Netherlands, Poland, Slovakia, Switzerland, and the United Kingdom) experienced median annual increases in the incidence of OPC of 2.5%/yr in men and 3.4%/yr in women.14
The available data suggests that countries defined by the United Nations55 as “less developed” may not have similarly rising trends in HPV‐positive OPC.23, 24, 62 This was explored in a recent analysis of HNC incidence rates in four independent databases: GLOBOCAN 2012, CI5, World Health Organization Mortality Database, and SEER. In this study, Gupta et al found that Western Europe, South‐Central Asia, Central and Eastern Europe, and North America represented regions with the highest incidence rates of HPV‐related HNC subsites: the tonsils and oropharynx. Regions with the highest incidence rates of HPV‐unrelated HNC subsites, the lip and oral cavity, included the Caribbean, Central and Eastern Europe, Southern Europe, and Western Asia.63 The analysis did not, however, directly account for regional differences in the percentage of HNC resulting from HPV‐infection (termed the HPV‐attributable fraction: HPV‐AF) which are known to vary significantly by region.52 Approximately 40‐70% of OPCs are HPV‐positive in more developed countries,9, 15, 62, 64 while fewer than 26% of OPCs are HPV‐positive in less developed regions.23, 24, 62, 65 Combining the analysis by Gupta et al with recent regional estimates of the HPV‐AF of OPC52 is likely to show more profound regional differences in incidence of HPV‐positive OPC.
Collectively, the observations made over the past two decades support the conclusion that increases in HPV‐positive OPC incidence are disproportionately affecting men and individuals in countries designated as “more developed”. These findings suggest an underlying divergence in risk factor exposure among populations that are experiencing rises in OPC relative to those that are not.
Risk factors
Trends in the incidence of oropharyngeal and non‐oropharyngeal HNC can be at least partially attributed to changing exposures to HNC risk factors. Historically, the primary risk factors for HNC have been smoking22 and alcohol consumption.27
Since the mid‐1970s smoking and alcohol consumption have declined by 42%39 and 20%,66 respectively, in the U.S. Although the global prevalence of smoking has also declined significantly between 1980 and 2012, significant variability existed with regard to sex and region. Smoking prevalence was significantly higher in men, with more men smoking in less developed countries.67 Consistent with the notion that smoking played a role in HPV‐unrelated HNC, partial overlap existed between countries (e.g. Eastern and Southern Europe and East and Central Asia) with higher incidence of HPV‐unrelated HNC63 and high smoking prevalence and consumption of cigarettes.67 Declining rates of smoking were observed in parallel with declining rates of HPV‐unrelated HNC in Western Europe14 and Southeastern Asia, regions with rising rates of HPV‐positive OPC.63
In populations with declining alcohol and tobacco consumption yet increasing incidence of OPC, HPV has emerged as a dominant risk factor.16 In an international study, incidence trends for oral cavity cancer (OCC, an HPV‐unrelated HNC) and OPC (an HPV‐related HNC) were evaluated to determine the relative effects of HPV and smoking on the incidence trends of these tumor types.52, 68 Two patterns were suggestive of HPV as a primary cause of OPC: an increasing incidence of OPC accompanied by an unchanging or declining incidence of OCC and statistically stronger increases in the incidence of OPC relative to OCC. Interestingly, all countries exhibiting either of these patterns also displayed significant declines in squamous cell carcinoma of the lung, a strongly tobacco‐related tumor, further supporting a divergent etiology between oropharyngeal and non‐oropharyngeal HNC.14 Consistent with these findings, other studies have reported decreasing incidence of tumors in patients with a history of heavy smoking (>20 pack‐years) and an increasing incidence of OPC arising in never smokers and those smoking fewer than 20 pack‐years.69
In the United States, the prevalence of oral HPV infection among men and women age 14‐69 in 2009‐2010 was approximately 7%.70 One percent of Americans in this age group had a detectable oral HPV16 infection, the most prevalent high‐risk HPV infection detected in oral samples, and the predominant type responsible for HPV‐positive OPCs in the US.70 As would be expected in sexually transmitted infection, the prevalence of oral HPV infection increases with increasing exposure, whether measured by number of sexual partners, oral sex partners, or younger age of sexual debut. For example, prevalence of oral HPV is 21% among individuals who reported 21 or more sexual partners.70 Interestingly, oral infection with high‐risk HPV types showed strong bimodal age distribution with peak prevalence at ages 25‐30 and 55‐64.70 In addition, male sex and current smoking intensity are independently associated with an increasing prevalence of oral HPV infection.70, 71, 72
Prognosis of human papillomavirus‐related oropharyngeal carcinoma
Initial evidence that HPV‐positivity in HNC was associated with improved prognosis came from an early study which foreshadowed that HPV‐positive OPC was a distinct disease entity. In this retrospective study, Gillison and colleagues found that patients with HPV‐positive HNC had a 59% reduction in risk of death from cancer after adjustment for age, alcohol consumption, and lymph node status.5 Consistent with this finding, a meta‐analysis investigating the role of HPV status on prognosis of HNC found HPV‐positive OPC showed a 28% reduced risk of death relative to HPV‐negative OPC. No HPV‐related survival benefit was observed in non‐oropharyngeal HNC.73
The first prospective trial demonstrating the impact of HPV‐status on prognosis in HNC was nested in an Eastern Cooperative Oncology Group (ECOG) chemoradiation trial which investigated the role of tumor HPV‐status on therapeutic response and survival in patients with stage III/IV disease.31 Compared to HPV‐negative tumors, HPV‐positive OPCs were found to have improved response rates to induction chemotherapy (82% vs. 55%) and chemoradiation (84% vs. 57%). At 2 years, patients with HPV‐positive tumors showed significantly improved progression‐free (86% vs. 53%) and overall survival (95% vs. 62%).
These findings have been corroborated by numerous studies in a variety of settings.5, 15, 30, 34, 35, 74, 75, 76, 77, 78, 79 A meta‐analysis of 42 studies investigating the impact of HPV on HNC survival reported improvements of 53% and 72% in overall and disease‐specific survival, respectively, in HPV‐positive OPC compared to HPV‐negative HNC.76 The preponderance of data thus indicates that HPV tumor status in OPC is a favorable prognostic indicator associated with longer overall and disease‐specific survival.
Similarly, a recent population‐based study of 529 oropharyngeal tumors from 1994‐2005 across 6 cancer registries in the United States found that patients with HPV16‐positive OPC showed improved 5‐year overall survival (OS) relative to HPV‐negative tumors (65% vs. 28%). The authors found that OPC unstratified for HPV‐status exhibited subsite specific differences in prognosis. OPC arising from the palatine tonsils was associated with the most favorable 5‐year OS followed by the base of tongue, then by other sites of the oropharynx (62% vs. 50% vs. 31%, respectively). Interestingly, this study suggested the benefits in survival observed in HPV16 tumors may be attenuated by non‐HPV16 oncogenic types (5‐yr OS – HPV16‐positive: 65%, non‐HPV16‐positive: 46%, HPV: 28%).80
Additionally, recent The Cancer Genome Atlas (TCGA) data support the possibility that the improved survival observed in HPV‐positive OPC might be confined to HPV16‐driven tumors. Bratman et al interrogated TCGA transcriptome data for viral gene expression in 515 HNCs. Seventy‐three (14%) of these tumors expressed viral transcripts, of which 61 were HPV16‐positive and 12 were positive for other oncogenic subtypes: HPV 33, 35, and 56. Overall survival of patients with HPV16‐positive HNC was significantly better than non‐HPV16 oncogenic type tumors (3‐yr: 88% vs. 49%).81 These studies suggest the possibility that the underlying tumor biology of HPV16 may differ from that of non‐HPV16 types. Arguing against their findings, a single institution analysis of survival associated with HPV16‐positive OPC versus non‐HPV16 oncogenic HPV‐positive OPC found no difference in survival.82 Given that non‐HPV16 types comprise a small proportion (<8%) of HPV‐positive OPC,12 the surprising observation that HPV16‐positive OPC may display a distinct prognosis from other high‐risk HPV‐positive OPC warrants further investigation.
Trends in prevalence
Epidemiologic phenomena associated with HPV‐positive OPC—including rising incidence rates, significantly prolonged survival, and significant increases in tumors attributable to HPV—are driving an increase in the prevalence of individuals with HPV‐positive OPC (Figure 1).
Figure 1.
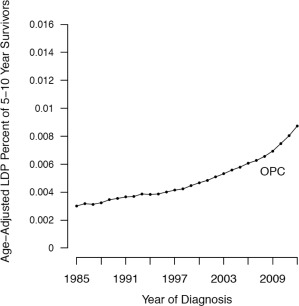
Rising prevalence of long‐term oropharyngeal squamous cell carcinoma survivors. Age‐adjusted limited‐duration prevalence (LDP) in 5‐year to 10‐year survivors of oropharyngeal squamous cell carcinoma (OPC). Adapted figure from Patel et al modified with the removal of curves representing overall oral squamous cell carcinoma and oral cavity cancer55.
The survival advantage associated with HPV‐positive OPC has resulted in significant improvements in OPC survival rates.55, 69 From 1975 to 2007, the average annual percent change in cause‐specific survival for OPC rose by 1%/yr in the United States. Additionally, during 1985‐2012, the average rate of increase in the prevalence of 5‐ to 10‐year survivors reached 18 individuals per 100,000 per year. As a result, the total prevalence of OPC survivors has increased by 115 individuals per 100,000 per year.55 In comparison, the prevalence of survivors of oral cavity cancer—a primarily HPV‐negative HNC68 – decreased by 16 individuals per 100,000 per year during the same period.55
Increases in the proportion of OPC attributable to HPV also help explain the rising prevalence of HPV‐positive OPC. A systematic review of 5,046 HNC from 60 studies reported through the year 2004 calculated the worldwide HPV‐attributable fraction (HPV‐AF) of OPC and non‐OPC to be 35.6% and 24%, respectively.7 Tumor site misclassification in advanced tumors, inclusion of a large portion of small case series, heterogeneity in specimen quality in individual studies, and HPV detection methods limit this analysis and may have resulted in underestimation of HPV‐AF of OPC and overestimation of HPV‐AF of non‐OPC.7 A more recent meta‐analysis found that, while the reported HPV‐AF was 40.5% before 2000, it rose to 64.3% between 2000‐2004, and further increased to 72.2% between 2005‐2009.9 Meanwhile, the prevalence of HPV in non‐oropharyngeal HNC declined from 22.2% to 17.2% to 6.1%,9 respectively, partially addressing the possibility that these findings may be a result of improvements in HPV detection. Consistent with this conclusion, a series of 520 consecutive OPC patients presenting to 10 Australian hospitals from 1985‐2010 also showed an increase in the HPV‐positive rate of OPC from 20.2% in 1987‐1995 to 63.5% in 2006‐2010. Uniform testing for HPV‐status in this study supports the notion that observed increases in HPV‐AF are not a result of improvements in viral detection.64
The collective effects of epidemiologic trends and improved survival rates has led to an accelerating prevalence of HPV‐positive OPC survivors in the United States55 and Europe.83 This increase in OPC survivors represents a growing population of patients susceptible to disease recurrence and highlights the importance of better understanding the influence of HPV‐status in recurrent and metastatic OPC.
The Impact of Human Papillomavirus on Recurrent and Metastatic Oropharyngeal Carcinoma
HPV‐positive tumor status is well established as a significant determinant of favorable outcome.31, 34, 74, 76, 84 Yet 13‐25% of HPV‐positive OPC recur within 2 years31, 34, 74, 79, 84, 85 and up to 36% within 8 years.35 Although less evidence is available to evaluate the role of HPV in the survival of recurrent and metastatic OPC, emerging data suggest that tumor HPV‐status also influences prognosis after disease recurrence.
A secondary analysis of Radiation Therapy Oncology Group (RTOG) trials 0129 and 0522 provided evidence that p16‐status, a reliable surrogate for tumor HPV‐status in OPC,86, 87 showed prognostic relevance after disease progression.40 Patients with OPC who experienced local, regional, or distant progression were eligible for analysis (n=181, p16‐positive=105, p16‐negative=76).88 Consistent with demographic differences observed between HPV‐positive and HPV‐negative OPC patients at primary diagnosis, a greater proportion of p16‐positive patients were younger, white, and had lower tobacco exposure at the time of disease progression. Median time to progression was similar for p16‐positive and p16‐negative OPC (8.2 vs. 7.3 months), with the majority experiencing disease progression in the first year after completion of therapy. Fifty‐five percent of study patients displayed isolated locoregional progression. Despite similar rates of local, regional, and distant progression, patients with p16‐positive OPC survived significantly longer than their p16‐negative counterparts (2.6 vs. 0.8 years). After adjustment for other factors independently associated with overall survival, p16‐positive status was associated with a 52% reduction in risk of death.40
Another analysis of prospective clinical trials evaluating the influence of HPV‐status on HNC (rather than OPC) provided further support for the influence of HPV‐status in recurrent and metastatic (R/M) OPC.30 Analysis of ECOG trials 1395 and 3301 found improved PFS (5.9 vs. 3.2 months) and OS (12.9 vs. 6.7 months) in R/M HPV‐positive relative to HPV‐negative HNC.30
Two reports which evaluated the effect of HPV‐status in R/M HNC suggested little to no effect of HPV‐status on survival.32, 89 However the absence of a difference, may be due to study design.53 Reanalysis of the EXTREME trial was limited by a low proportion (5%) of HPV‐positive tumors and the categorization of OPC with non‐OPC patients.32, 90 The SPECTRUM trial relied on a p16‐positivity threshold (10% of cells) to define HPV‐status, which is substantially lower than the commonly used standards (≥70% of cells).89, 91, 92 These studies underline the importance of applying established principles for HNC subsite stratification and criteria for HPV‐positivity in studies investigating the role of HPV‐status in R/M HNC survival.37, 91, 92, 93
Distant metastatic disease
The influence of HPV‐status on metastatic disease has been a point of recent controversy. A series of retrospective analyses suggested that HPV‐positive OPC displays an atypical pattern of distant metastatic recurrence.94, 95, 96, 97
A single institution retrospective series in which patients were heterogeneously treated generally without systemic therapy, suggested that HPV tumor status influenced the time course, distribution, and propensity of distant recurrence.85 This analysis evaluated any event of disease progression rather than restricting analysis to the initial event of recurrence. Given prolonged PFS associated with HPV‐positive OPC, it is not surprising that Huang et al concluded that HPV‐negative OPC exhibited early distant metastatic (DM) disease. All HPV‐negative OPC showed DM within 2.1 years of therapy, while one third of HPV‐positive OPC DM occurred beyond two years. HPV‐negative OPC was reported to most commonly metastasize to the lung (88% of patients with DM), liver (16%), and bone (12%). Similarly, HPV‐positive OPC also most often metastasized to the lung (78%), with ‘atypical’ metastases found at appreciable frequencies in the skin (22%), brain (14%), and intra‐abdominal lymph nodes (14%). Finally, Huang et al concluded that HPV‐positive OPC more frequently recurred at distant sites, citing that 72% of patients with distant metastasis did not have locoregional recurrence.85 Studies investigating the first event of progression, however, have found no significant difference in HPV‐status on the propensity to recur at locoregional versus distant sites (Figure 2).35, 97, 98, 99
Figure 2.
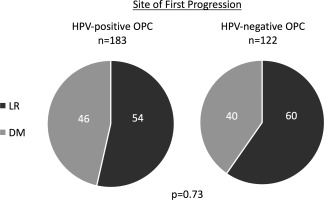
Site of first progression in HPV‐positive and HPV‐negative oropharyngeal squamous cell carcinoma. Published studies35, 98, 99 describing the first event of recurrence were evaluated to determine the proportion of first recurrence events in OPC representing locoregional recurrence or distant metastatic disease. Patients who experienced local or regional recurrence without distant metastases were categorized as locoregional recurrence (LR). Patients who experienced distant metastasis as the first event of recurrence, with or without concurrent local or regional recurrence, were categorized as distant metastasis (DM). Data was pooled across studies and proportion with LR and DM as first event were calculated and compared by HPV tumor status using chi‐square test (GraphPad Prism 7, La Jolla, CA). Pie charts represent the proportional contribution of locoregional recurrence (LR) and distant metastasis (DM) as the site of first progression in HPV‐positive and HPV‐negative oropharyngeal squamous cell carcinoma (OPC). Based on pooled data from Nguyen‐Tan et al, Guo et al, and Sinha et al35, 98, 99. Patients with synchronous DM and LR were included in the DM subgroup.
The retrospective observations by Huang et al85, 97 and others95, 96, 98, 100 seem to imply that the features of HPV‐positive OPC which render it histopathologically, epidemiologically, and clinically distinct from HPV‐negative OPC extend to its metastatic behavior. Suggestive of a potential role for HPV‐related expression profiles in driving a unique metastatic phenotype, distant metastases of HPV‐positive OPC do retain HPV and p16 positivity.96, 97, 101 Nonetheless, tumors arising from the oral cavity and hypopharynx—predominantly HPV‐negative subsites—have also been reported to metastasize to uncommon distant sites including the brain,102 skin,103 muscle,104 and abdominal organs.105 In addition, prospective and retrospective studies alike have consistently demonstrated that the great majority of distant metastases occur within 3 years of primary presentation in both HPV‐positive and HPV‐negative OPC.34, 35, 40, 97, 98
Secondary analysis of RTOG0129 and RTOG052240 found that 41% of HPV‐positive and 38% of HPV‐negative OPC displayed isolated distant metastatic disease at first progression. Median time to distant metastasis did not differ based on p16‐status (11.9 vs. 12.4 months).106 Further contrasting with the study by Huang et al,85 p16‐positive and p16‐negative OPC were found to have similar anatomic distribution of distant metastasis (lung: 73% vs. 70%; bone: 14.6% vs. 15.2%; liver: 8.3% vs. 15.2%; other 16.7% vs. 12.1%). Despite these similarities, patients with HPV‐positive disease showed significantly improved OS relative to those with HPV‐negative disease (2.6 vs. 0.8 years, respectively).40
The discrepancies in findings reported by Huang85 and the RTOG reanalysis40 may be a result of differences in study design. Huang et al derive observations from retrospective review of patients who received curative intent radiotherapy or chemoradiation from 2000‐2010.85 Although also retrospective in nature, the RTOG reanalysis comprised of two randomized, controlled, prospectively collected, and uniformly treated cohorts with a median of 4 years follow‐up after disease progression.40 Whereas the RTOG reanalysis reported survival after the first event of tumor progression, Huang reported survival after distant metastasis.40, 85, 106, 107, 108 Since PFS and OS are significantly diminished in HPV‐negative OPC, it is likely that some HPV‐negative OPC patients with occult distant metastases succumb to other sequelae of disease. As such, the prolonged survival associated with HPV‐positive OPC may allow patients to survive long enough to develop metastases at sites infrequently reported for HPV‐negative OPC (Figure 3). Although median follow‐up of 4 years in the combined secondary analysis of RTOG 0129 and 052240 may have been insufficient to monitor for late metastatic events, reanalysis of RTOG 0129 at 8 years also failed to observe a distinct metastatic pattern or significant differences in distant metastatic disease for HPV‐positive OPC.35
Figure 3.
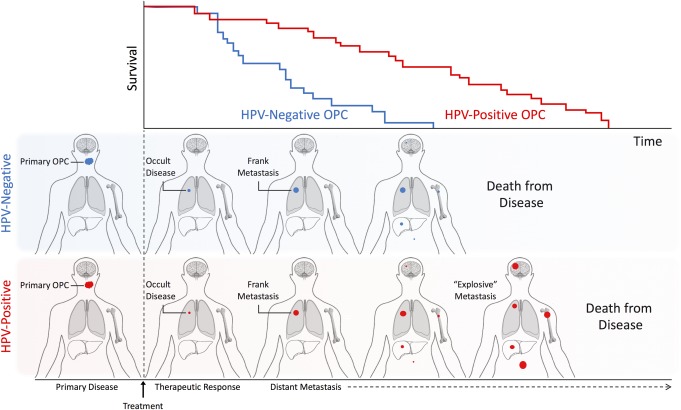
Schematic representation of time‐ and survival‐ dependent differences observed in distant metastatic recurrence of HPV‐positive and HPV‐negative oropharyngeal squamous cell carcinoma. Top panel depicts representative survival curves for HPV‐positive and HPV‐negative squamous cell carcinoma (OPC). The two rows below correspond in time course to the survival curve and represent the typical disease course of HPV‐positive and HPV‐negative OPC. Patients with HPV‐positive and HPV‐negative OPC display approximately cotemporaneous distant recurrence40 at common sites (e.g. lung, bone, liver) with the potential presence of additional occult or subclinical distant disease at rare sites (e.g. brain, abdominal lymph nodes, skin). While patients with HPV‐positive OPC show survival durations adequate to display clinically detectable metastasis at rare distant sites, those with HPV‐negative OPC do not.
Reports suggesting an atypical natural history of metastatic HPV‐positive OPC85, 100 contrast with findings of the RTOG reanalysis40 on two inter‐related points: that HPV‐positive OPC has a distinct anatomic distribution of distant metastasis and a delayed time to distant metastasis. Patients who survive beyond three years, including the minority of HPV‐negative HNC, continue to be susceptible to distant metastatic disease. Observations of the basic biology of metastatic colonization suggest that although specific tumor types display distinct patterns of organotropism,109 single tumor cells disperse widely and may remain dormant for many years.110 In this setting, long‐term survival may serve as the rate‐limiting step to metastasis of atypical or rare organs by providing time for disseminated tumor cells to convert from dormant to actively proliferating111, 112 (Figure 3). As such, retrospective analyses of patients with long follow up periods may inadvertently select for long‐surviving patients who have acquired apparently unique disease features. Consequently, a rise in the prevalence of HPV‐positive OPC‐associated distant metastases is likely to follow the increase in long‐term survival associated with HPV‐positive OPC, and this may be mistaken as a difference in the metastatic behavior of HPV‐positive OPC.
A recent reanalysis of a retrospective OPC cohort demonstrated how such survival bias might occur. Guo et al stratified patients into “early” and “late” survivor groups based on survival less than or greater than 24 months, respectively.33 While late survivors were significantly more likely to be HPV‐positive, 20% of late survivors were HPV‐negative. Additionally, late recurrences were significantly more common among late survivors for both HPV‐positive and HPV‐negative patients. Indeed, recurrence occurred significantly later in late survivors regardless of HPV‐status. With this survival‐based stratification strategy Guo et al explained the notion that late recurrence is dependent on late survivorship.33
CONCLUSION
Epidemiologic trends indicate a global upsurge in the incidence of HPV‐positive oropharyngeal cancer, with the strongest effects observed in white men residing in North America and Western Europe. The rise of HPV‐positive OPC combined with its improved outcomes have resulted in an increased prevalence of OPC survivors. Long‐term follow up of patients with HPV‐positive OPC indicates that greater than one third of survivors will experience locoregional or distant recurrence in the first decade after treatment. Current data suggests HPV‐positive tumor status also confers a survival advantage in recurrent and distant metastatic disease. However, further long‐term prospective studies are required to understand the mechanism of HPV in OPC survival after progression. The success of such studies relies on the standardization of HPV detection strategies and consistent reporting of clinical data, especially patterns of distant metastatic disease and measurements of survival metrics after the first event of tumor progression. Longer‐term observation of HPV‐positive OPC survivor cohorts will clarify the role of HPV‐status in late recurrence and distant metastatic disease and better inform current efforts focused on tailoring therapeutic strategies to HPV‐status.
Acknowledgments
Oral Cancer Foundation
Financial Disclosures: The authors have no financial disclosures.
Conflicts of Interest: The authors have no conflicts of interest to declare.
BIBLIOGRAPHY
- 1. American Joint Committee on Cancer (AJCC) . AJCC Cancer Staging Manual. 7th Ed Chicago, IL: Springer, 2010. [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 2015; 136:E359–386. [DOI] [PubMed] [Google Scholar]
- 3. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40:187–193. [DOI] [PubMed] [Google Scholar]
- 4. D'Souza G, Kreimer AR, Viscidi R, et al. Case‐control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007;356:1944–1956. [DOI] [PubMed] [Google Scholar]
- 5. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92:709–720. [DOI] [PubMed] [Google Scholar]
- 6. de Villiers EM. Cross‐roads in the classification of papillomaviruses. Virol J 2013;445:2–10. [DOI] [PubMed] [Google Scholar]
- 7. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–475. [DOI] [PubMed] [Google Scholar]
- 8. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta‐analysis. Lancet Oncol 2014;15:1319–1331. [DOI] [PubMed] [Google Scholar]
- 9. Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta‐analysis of trends by time and region. Head Neck 2013;35:747–755. [DOI] [PubMed] [Google Scholar]
- 10. Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero‐digestive tract cancers: the ARCAGE study. J Natl Cancer Inst 2013;105:536–545. [DOI] [PubMed] [Google Scholar]
- 11. Michaud DS, Langevin SM, Eliot M, et al. High‐risk HPV types and head and neck cancer. Int J Cancer 2014;135:1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus‐Associated Cancers ‐ United States, 2008‐2012. MMWR Morb Mortal Wkly Rep 2016;65:661–666. [DOI] [PubMed] [Google Scholar]
- 13. Steinau M, Saraiya M, Goodman MT, et al. Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerg Infect Dis 2014;20:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaturvedi AK, Anderson WF, Lortet‐Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013;31:4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16‐positive and human papillomavirus type 16‐negative head and neck cancers. J Natl Cancer Inst 2008;100:407–420. [DOI] [PubMed] [Google Scholar]
- 17. Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol 2008;32:1044–1050. [DOI] [PubMed] [Google Scholar]
- 18. Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV‐positive and HPV‐negative head and neck squamous cell carcinomas. Clin Cancer Res 2015;21:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus‐related and ‐unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26:612–619. [DOI] [PubMed] [Google Scholar]
- 21. Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16‐associated head and neck cancer. J Natl Cancer Inst 2007;99:1801–1810. [DOI] [PubMed] [Google Scholar]
- 22. Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282–3287. [PubMed] [Google Scholar]
- 23. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772–1783. [DOI] [PubMed] [Google Scholar]
- 24. Ribeiro KB, Levi JE, Pawlita M, et al. Low human papillomavirus prevalence in head and neck cancer: results from two large case‐control studies in high‐incidence regions. Int J Epidemiol 2011;40:489–502. [DOI] [PubMed] [Google Scholar]
- 25. Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 2013;31:2708–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Combes JD, Franceschi S. Role of human papillomavirus in non‐oropharyngeal head and neck cancers. Oral Oncol 2014;50:370–379. [DOI] [PubMed] [Google Scholar]
- 27. Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 2007;99:777–789. [DOI] [PubMed] [Google Scholar]
- 28. Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol 2012;2012:571862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anantharaman D, Muller DC, Lagiou P et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int J Epidemiol 2016;45:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Argiris A, Li S, Ghebremichael M, et al. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol 2014;25:1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus‐positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–269. [DOI] [PubMed] [Google Scholar]
- 32. Vermorken JB, Psyrri A, Mesia R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol 2014;25:801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo T, Rettig E, Fakhry C. Understanding the impact of survival and human papillomavirus tumor status on timing of recurrence in oropharyngeal squamous cell carcinoma. Oral Oncol 2016;52:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen‐Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long‐term report of efficacy and toxicity. J Clin Oncol 2014;32:3858–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vermorken JB, Mesia R, Rivera F, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–1127. [DOI] [PubMed] [Google Scholar]
- 37. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site‐specific analysis of the SEER database. Int J Cancer 2005;114:806–816. [DOI] [PubMed] [Google Scholar]
- 38. Viani L, Dammeijer P, Jones AS, Dalby JE, Stell PM. Recurrence of oropharyngeal carcinoma after radiotherapy. J Laryngol Otol 1991;105:24–28. [DOI] [PubMed] [Google Scholar]
- 39. Yueh B, Feinstein AR, Weaver EM, Sasaki CT, Concato J. Prognostic staging system for recurrent, persistent, and second primary cancers of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg 1998;124:975–981. [DOI] [PubMed] [Google Scholar]
- 40. Fakhry C, Zhang Q, Nguyen‐Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol 2014;32:3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jayaram SC, Muzaffar SJ, Ahmed I, Dhanda J, Paleri V, Mehanna H. Efficacy, outcomes, and complication rates of different surgical and nonsurgical treatment modalities for recurrent/residual oropharyngeal carcinoma: A systematic review and meta‐analysis. [published online July 13 2016]. Head Neck 2016. doi: 10.1002/hed.24531. [DOI] [PubMed] [Google Scholar]
- 42. Favre M, Orth G, Croissant O, Yaniv M. Human papillomavirus DNA: physical map. Proc Natl Acad Sci U S A 1975;72:4810–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A 1995;92:1654–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim MY, Dahlstrom KR, Sturgis EM, Li G. Human papillomavirus integration pattern and demographic, clinical, and survival characteristics of patients with oropharyngeal squamous cell carcinoma. Head Neck 2016;38:1139–1144. [DOI] [PubMed] [Google Scholar]
- 45. Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991;253:49–53. [DOI] [PubMed] [Google Scholar]
- 46. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990;63:1129–1136. [DOI] [PubMed] [Google Scholar]
- 47. Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus‐16 induces degradation of retinoblastoma protein through the ubiquitin‐proteasome pathway. Cancer Res 1996;56:4620–4624. [PubMed] [Google Scholar]
- 48. Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell‐cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993;366:704–707. [DOI] [PubMed] [Google Scholar]
- 49. Holzinger D, Schmitt M, Dyckhoff G, Benner A, Pawlita M, Bosch FX. Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res 2012;72:4993–5003. [DOI] [PubMed] [Google Scholar]
- 50. Westra WH. Detection of human papillomavirus (HPV) in clinical samples: evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol 2014;50:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marur S, D'Souza G, Westra WH, Forastiere AA. HPV‐associated head and neck cancer: a virus‐related cancer epidemic. Lancet Oncol 2010;11:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Castellsague X, Alemany L, Quer M et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst 2016;108:djv403. [DOI] [PubMed] [Google Scholar]
- 53. Misiukiewicz K, Camille N, Gupta V et al. The role of HPV status in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Adv Hematol Oncol 2014;12:812–819. [PubMed] [Google Scholar]
- 54. Gillison ML. Human papillomavirus‐associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol 2004;31:744–754. [DOI] [PubMed] [Google Scholar]
- 55. Patel MA, Blackford AL, Rettig EM, Richmon JD, Eisele DW, Fakhry C. Rising population of survivors of oral squamous cell cancer in the United States. Cancer 2016;122:1380–1387. [DOI] [PubMed] [Google Scholar]
- 56. Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978‐2007: focus on human papillomavirus associated sites. Int J Cancer 2011;129:733–741. [DOI] [PubMed] [Google Scholar]
- 57. Braakhuis BJ, Visser O, Leemans CR. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol 2009;45:e85–89. [DOI] [PubMed] [Google Scholar]
- 58. Forte T, Niu J, Lockwood GA, Bryant HE. Incidence trends in head and neck cancers and human papillomavirus (HPV)‐associated oropharyngeal cancer in Canada, 1992‐2009. Cancer Causes Control 2012;23:1343–1348. [DOI] [PubMed] [Google Scholar]
- 59. Hammarstedt L, Lindquist D, Dahlstrand H et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer 2006;119:2620–2623. [DOI] [PubMed] [Google Scholar]
- 60. Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975‐2009, featuring the burden and trends in human papillomavirus(HPV)‐associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mork J, Moller B, Dahl T, Bray F. Time trends in pharyngeal cancer incidence in Norway 1981‐2005: a subsite analysis based on a reabstraction and recoding of registered cases. Cancer Causes Control 2010;21:1397–1405. [DOI] [PubMed] [Google Scholar]
- 62. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607–615. [DOI] [PubMed] [Google Scholar]
- 63. Gupta B, Johnson NW, Kumar N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016;91:13–23. [DOI] [PubMed] [Google Scholar]
- 64. Hong A, Lee CS, Jones D, et al. Rising prevalence of human papillomavirus‐related oropharyngeal cancer in Australia over the last 2 decades. Head Neck 2016;38:743–750. [DOI] [PubMed] [Google Scholar]
- 65. Shaikh MH, McMillan NA, Johnson NW. HPV‐associated head and neck cancers in the Asia Pacific: A critical literature review & meta‐analysis. Cancer Epidemiol 2015;39:923–938. [DOI] [PubMed] [Google Scholar]
- 66. Greenfield TK, Midanik LT, Rogers JD. A 10‐year national trend study of alcohol consumption, 1984‐1995: is the period of declining drinking over? Am J Public Health 2000;90:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980‐2012. JAMA 2014;311:183–192. [DOI] [PubMed] [Google Scholar]
- 68. Zafereo ME, Xu L, Dahlstrom KR, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol 2016;56:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Das LC, Karrison TG, Witt ME, et al. Comparison of outcomes of locoregionally advanced oropharyngeal and non‐oropharyngeal squamous cell carcinoma over two decades. Ann Oncol 2015;26:198–205. [DOI] [PubMed] [Google Scholar]
- 70. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009‐2010. JAMA 2012;307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chaturvedi AK, Graubard BI, Broutian T et al. NHANES 2009‐2012 Findings: Association of Sexual Behaviors with Higher Prevalence of Oral Oncogenic Human Papillomavirus Infections in U.S. Men. Cancer Res 2015;75:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fakhry C, Gillison ML, D'Souza G. Tobacco use and oral HPV‐16 infection. JAMA 2014;312:1465–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta‐analysis. Int J Cancer 2007;121:1813–1820. [DOI] [PubMed] [Google Scholar]
- 74. Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010;28:4142–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol 2007;25:3971–3977. [DOI] [PubMed] [Google Scholar]
- 76. O'Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta‐analysis. Oral Oncol 2012;48:1191–1201. [DOI] [PubMed] [Google Scholar]
- 77. Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV‐DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer 2007;120:1731–1738. [DOI] [PubMed] [Google Scholar]
- 78. Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol 2014;50:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Posner MR, Lorch JH, Goloubeva O et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol 2011;22:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Goodman MT, Saraiya M, Thompson TD, et al. Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer 2015;51:2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bratman SV, Bruce JP, O'Sullivan B et al. Human Papillomavirus Genotype Association With Survival in Head and Neck Squamous Cell Carcinoma. JAMA Oncol 2016;2:823–826. [DOI] [PubMed] [Google Scholar]
- 82. Varier I, Keeley BR, Krupar R, et al. Clinical characteristics and outcomes of oropharyngeal carcinoma related to high‐risk non‐human papillomavirus16 viral subtypes. Head Neck 2016;38:1330–1337. [DOI] [PubMed] [Google Scholar]
- 83. Fakhry C, Andersen KK, Eisele DW, Gillison ML. Oropharyngeal cancer survivorship in Denmark, 1977‐2012. Oral Oncol 2015;51:982–984. [DOI] [PubMed] [Google Scholar]
- 84. Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006;24:736–747. [DOI] [PubMed] [Google Scholar]
- 85. Huang SH, Perez‐Ordonez B, Weinreb I et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV‐related oropharyngeal cancer. Oral Oncol 2013;49:79–85. [DOI] [PubMed] [Google Scholar]
- 86. El‐Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV‐related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck 2012;34:459–461. [DOI] [PubMed] [Google Scholar]
- 87. Mooren JJ, Gultekin SE, Straetmans JM, et al. P16(INK4A) immunostaining is a strong indicator for high‐risk‐HPV‐associated oropharyngeal carcinomas and dysplasias, but is unreliable to predict low‐risk‐HPV‐infection in head and neck papillomas and laryngeal dysplasias. Int J Cancer 2014; 134:2108–2117. [DOI] [PubMed] [Google Scholar]
- 88. Shi W, Kato H, Perez‐Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol 2009;27:6213–6221. [DOI] [PubMed] [Google Scholar]
- 89. Vermorken JB, Stohlmacher‐Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck (SPECTRUM): an open‐label phase 3 randomised trial. Lancet Oncol 2013;14:697–710. [DOI] [PubMed] [Google Scholar]
- 90. Misiukiewicz K, Bonomi M, Demicco E, Posner M. Controversies and role of HPV16 in recurrent/metastatic squamous cell cancers of the head and neck. Ann Oncol 2014;25:1667–1668. [DOI] [PubMed] [Google Scholar]
- 91. Jordan RC, Lingen MW, Perez‐Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol 2012;36:945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schache AG, Liloglou T, Risk JM, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res 2011;17:6262–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol 2014;32:3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Daly ME, Le QT, Maxim PG, et al. Intensity‐modulated radiotherapy in the treatment of oropharyngeal cancer: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys 2010;76:1339–1346. [DOI] [PubMed] [Google Scholar]
- 95. Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with HPV‐related head and neck cancer. J Neurooncol 2013;112:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Muller S, Khuri FR, Kono SA, Beitler JJ, Shin DM, Saba NF. HPV positive squamous cell carcinoma of the oropharynx. Are we observing an unusual pattern of metastases? Head Neck Pathol 2012;6:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang SH, Perez‐Ordonez B, Liu FF, et al. Atypical clinical behavior of p16‐confirmed HPV‐related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:276–283. [DOI] [PubMed] [Google Scholar]
- 98. Sinha P, Thorstad WT, Nussenbaum B, et al. Distant metastasis in p16‐positive oropharyngeal squamous cell carcinoma: a critical analysis of patterns and outcomes. Oral Oncol 2014;50:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Guo T, Qualliotine JR, Ha PK, et al. Surgical salvage improves overall survival for patients with HPV‐positive and HPV‐negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 2015;121:1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Trosman SJ, Koyfman SA, Ward MC, et al. Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA Otolaryngol Head Neck Surg 2015;141:457–462. [DOI] [PubMed] [Google Scholar]
- 101. Vainshtein J, McHugh JB, Spector ME, et al. Human papillomavirus‐related oropharyngeal cancer: HPV and p16 status in the recurrent versus parent tumor. Head Neck 2015;37:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bulut OC, Lindel K, Hauswald H, et al. Clinical and molecular characteristics of HNSCC patients with brain metastases: a retrospective study. Eur Arch Otorhinolaryngol 2014;271:1715–1722. [DOI] [PubMed] [Google Scholar]
- 103. Pitman KT, Johnson JT. Skin metastases from head and neck squamous cell carcinoma: incidence and impact. Head Neck 1999;21:560–565. [DOI] [PubMed] [Google Scholar]
- 104. Smeets R, Grosjean MB, Heiland M, Riediger D, Maciejewski O. Distant metastases of a squamous cell carcinoma of the tongue in peripheral skeletal muscles and adjacent soft tissues. Head Face Med 2008;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cruz I, Mamel JJ, Brady PG, Cass‐Garcia M. Incidence of abdominal wall metastasis complicating PEG tube placement in untreated head and neck cancer. Gastrointest Endosc 2005;62:708–711; quiz 752, 753. [DOI] [PubMed] [Google Scholar]
- 106. Fakhry C, Zhang Q, Nguyen‐Tan PF, et al. Reply to B. O'Sullivan et Al. J Clin Oncol 2015;33:1708–1709. [DOI] [PubMed] [Google Scholar]
- 107. Pfister DG, Baxi SS, Dunn LA, Fury MG. Reply to B. O'Sullivan et al. J Clin Oncol 2015;33:1710. [DOI] [PubMed] [Google Scholar]
- 108. O'Sullivan B, Adelstein DL, Huang SH, et al. First Site of Failure Analysis Incompletely Addresses Issues of Late and Unexpected Metastases in p16‐Positive Oropharyngeal Cancer. J Clin Oncol 2015;33:1707–1708. [DOI] [PubMed] [Google Scholar]
- 109. Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med 2013;19:1450–1464. [DOI] [PubMed] [Google Scholar]
- 110. Aguirre‐Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 2007;7:834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Paez D, Labonte MJ, Bohanes P et al. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res 2012;18:645–653. [DOI] [PubMed] [Google Scholar]
- 112. Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer 2010;10:871–877. [DOI] [PubMed] [Google Scholar]


