Abstract
This study aimed to investigate the protective effects of hydrogen rich water on the intestinal ischemia/reperfusion (I/R) injury in a rat intestinal intussusception (II) model. Ninety Sprague-Dawley rats were randomly assigned into three groups (n = 30 per group). In sham group, rats received laparotomy, and the intestine was exposed for 15 minutes without II. In I/R + saline group and I/R + hydrogen group, rats received II after laparotomy and then intestine was relocated 8 hours later, followed by immediately intraperitoneal injection of normal saline and hydrogen rich water (HRW) (5 mL/kg), respectively. One hour later, the intestine was collected for hematoxylin-eosin staining and immunohistochemistry for apoptotic cells and 8-oxo-deoxyguanosine, and blood was harvested for detection of tumor necrosis factor-α, malondialdehyde and superoxide dismutase. Hematoxylin-eosin staining showed the intestinal mucosa was significantly damaged in I/R + saline group, which was markedly attenuated after HRW treatment. The serum tumor necrosis factor-α content increased significantly in I/R + saline group, but HRW treatment reduced serum tumor necrosis factor-α content as compared to I/R + saline group (P < 0.05). Serum malondialdehyde content and 8-oxo-deoxyguanosine positive cells in the intestine increased dramatically after II, but HRW significantly reduced them in I/R+hydrogen group (P < 0.05). In addition, superoxide dismutase activity reduced markedly and apoptotic cells increased in I/R + saline group as compared to sham group, but they HRW increased superoxide dismutase activity and reduced apoptotic cells significantly in I/R + hydrogen group (P < 0.05). Our results indicate hydrogen rich water is able to attenuate II induced intestinal I/R injury via inhibiting intestinal inflammation, attenuating intestinal/serum oxidative stress and reducing apoptotic intestinal cells.
Keywords: hydrogen rich water, intestinal intussusception, ischemia/reperfusion injury, oxidative stress, inflammation, rat, apoptosis, anti-oxidation
INTRODUCTION
Intestinal ischemia/reperfusion (I/R) injury is a phenomenon that the intestinal injury deteriorates after restoring the blood flow to the intestine following intestinal ischemia.1 The reperfusion of a specific organ following blood flow obstruction or reduction may cause severe damage to this organ, and the small intestine is one of organs susceptible to I/R injury.2 The ischemia and/or inflammation may induce the excess production of reactive oxygen species (ROS). Some ROS such as hydrogen peroxide (H2O2) and nitric oxide (NO) are not only important signal molecules in cells, but also toxic to cells.2 Of these ROS, hydroxyl radical and peroxynitrite are the most toxic and mediate the oxidative damage to cells.3,4 Ohsawa et al.5 found that hydrogen gas at a low dose (2%) could protect the rat brain against I/R injury, which was ascribed to the selective anti-oxidative activity of hydrogen. Increasing evidence demonstrates that hydrogen rich water (HRW) has similar neuroprotective capability in hypoxia/ischemia injury as 2% hydrogen in cerebral I/R injury.6 There is evidence showing that HRW was protective on intestinal I/R injury.7 In the available studies, ischemia I/R is induced by clamping the superior mesenteric artery.7,8 In the present study, intestinal intussusception (II) model was established to mimic intestinal I/R injury in rats which were then intraperitoneally treated with HRW, aiming to investigate the protective effect of HRW on intestinal I/R injury caused by II.
MATERIALS AND METHODS
Animals
Health male Sprague-Dawley (SD) rats (n = 90) aged 7–9 weeks and weighing 200–250 g (specific pathogen free) were purchased from the Experimental Animal Center of Taishan Medical University (license No. Lu[2015012]) and housed for 1 week and given ad libitum access to water and food under the 12-hour/12-hour light-dark cycle. Animals were handled in accordance with the Animal Care and Welfare Guideline, and procedures were taken to reduce the pain of animals. These 90 SD rats were randomly assigned into three groups: sham group, I/R + saline group and I/R + HRW group (n = 30 per group).
Animal modeling
Rat II was prepared as previously reported.9 Rats in I/R + saline group and I/R + HRW goup received laparotomy and the intestine was separated and exposed. A Kirschner wire (Ortho Solutions, UK) was used to intussuscept terminal ileum into the colon. Then, the intussusception was fixed with a 3-0 absorbable suture and the wound was closed. Rats in sham group received laparotomy and the intestine was separated and exposed without II. The wound was closed 15 minutes later.
Criteria for successful establishment of II animal model: At 8 hours after II, rats became listless, crowded together, and showed reduced food intake, and loose and/or bloody stools were observed.9 At 1 hour after reperfusion, pathological examination showed infiltration of inflammatory cells, apoptosis of intestinal villus epithelial cells and loss of intestinal villi.
HRW intervention
In I/R + HRW group, eight hours later, the intestine was relocated and HRW (Institute of Atherosclerosis of Taishan Medical University, Taian, Shandong Province, China) was intraperitoneally injected (5 mL/kg). One hour later, the injured intestine was harvested for examination. And in I/R + saline group, the normal saline was intraperitoneally injected (5 mL/kg).
Sample collection and detections
Serum collection: At 8 hours after ischemia, the intestine was relocated. Then, 5 mL of blood was collected from the heart 1 hour later and serum was harvested by centrifugation at 3,500 r/min for 15 minutes. The serum was aliquoted for the detection of superoxide dismutase (SOD) activity and contents of malondialdehyde (MDA) and tumor necrosis factor-α (TNF-α).
Histological examination: The ileum (about 4–5 cm) at 5 cm proximal to the ileocecal valve was collected and washed in warm saline thrice. The ileum tissues were then fixed in 10% neutral formalin for hematoxylin-eosin staining.
Detection of serum MDA and TNF-α contents and SOD activity
The serum TNF-α content was measured with a commercially available kit according to the manufacturer’s instruction (Dakewei Biotech Co., Ltd., Shanghai, China).
The concentration of MDA, a reliable marker of lipid peroxidation, was estimated in blood serum following the manual of MDA assay kit (Nanjing Jiancheng Institute of Biotechnology, Nanjing, Jiangsu Province, China). Optical density was measured using an ultraviolet spectrophotometer at 532 nm against blanks prepared by using distilled water.
SOD activity in blood serum was measured by using nitro blue tetrazolium as a substrate after suitable dilution with SOD assay kit (Nanjing Jiancheng Institute of Biotechnology), the increase in absorbance value was scanned with an ultraviolet spectrophotometer (BioRad) at 550 nm. One unit of SOD activity was defined as the amount of enzyme that inhibited auto-oxidation by 50% under the given experimental condition and the values were expressed as U/mg protein.
TUNEL staining
At the pre-designed time point, 10 rats from each group were sacrificed and the ileum was collected for TUNEL staining. At least five sections from each rat were obtained for TUNEL staining which was performed with a commercially available kit (Roche) according to the manufacturer’s instructions. In brief, the sections were deparaffined and rehydrated by heating the slides at 60°C. Then these sections were incubated in a 20 μg/mL proteinase K working solution for 15 minutes at room temperature. The slides were rinsed three times with phosphate buffered saline (PBS) followed by incubation with TUNEL reaction mixture for one hour at 37°C. After rinsing with PBS (3 × 5 minutes), color development was performed in the dark with nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indo-lylphosphate (BCIP). TUNEL positive cells had brown granules. Ten fields were randomly selected at a magnification of 400×, and TUNEL positive cells were counted among 100 cells in each field. The apoptotic index (AI) was calculated as follow: AI = (TUNEL positive cells/total cells) × 100%. Here, we used TUNEL positive cells to describe apoptotic cells or necrotic cells.
Immunohistochemical detection
Paraffin sections (5 μm) were deparaffined, washed with 0.01 mM PBS for 5 minutes and boiled in citric acid (pH 6.0) for 2 minutes. Subsequent to cooling, the sections were rinsed three times for 2 minutes in 0.01 M PBS.
Endogenous peroxidase was neutralized with 3% H2O2 for 30 minutes, and the sections were rinsed three times for 2 minutes in 0.01 mM PBS. Bovine serum albumin (3%) was added and incubated for 20 minutes at room temperature. Sections were incubated with anti-8-oxo-deoxyguanosine (8-OHdG) monoclonal antibody (1:100; Beijing Bioss Biotech Co., Ltd., Beijing, China) at 4°C overnight. The sections were rinsed three times for 2 minutes in 0.01 mM PBS prior to the addition of horseradish peroxidase (HRP)-conjugated goat anti-rabbit econdary antibody and incubated at room temperature for 20 minutes. Subsequent to rinsing three times for 2 minutes in 0.01 mM PBS, 50 μL 3,3’-diaminobenzidine solution was added. The sections were then counterstained with hematoxylin and observed using a Leica DM3000 microscope and the Leica image acquisition system (Leica Microsystems, Wetzlar, Germany). The 8-OHdG positive cells had brown granules and were counted in 10 randomly selected fields.
Statistical analysis
Statistical analysis was conducted with SPSS version 18.0 (SPSS, Chicago, IL, USA). Data are expressed as the mean ± SD. Comparisons were conducted with one way analysis of variance (ANOVA) among groups. A value of P < 0.05 was considered statistically significant.
RESULTS
Effect of HRW on the intestinal pathology in an II rat model
Macroscopic findings
Macroscopically, there was no mesenteric vascular pulsation after ischemia for 8 hours and the injured intestine became grey. After relocating the intestine, the mesenteric vascular pulsation was present, suggesting the restoration of blood supply. At 1 hour after relocating the intestine, intestinal flatulence, congestion and edema became obvious. The macroscopic changes in I/R + HRW group were not obvious.
Microscopic findings
Hematoxylin-eosin staining results showed, the intestinal mucosa was clear and complete, and the intestinal villi showed normal structure in sham group. In I/R + saline group, the intestinal mucosa and wall became thinner, loss and shedding of intestinal villi were present, and a lot of inflammatory cells infiltrated the intestine. In I/R + HRW group, the pathology was improved as compared to I/R + saline group: the villus arrangement was relatively regular, there was no evidence loss of intestinal villi, only small gaps formed at the top of intestinal villi and a few inflammatory cells were found in the intestine. These suggest that HRW is protective on the intestinal mucosal injury secondary to II (Figure 1).
Figure 1.
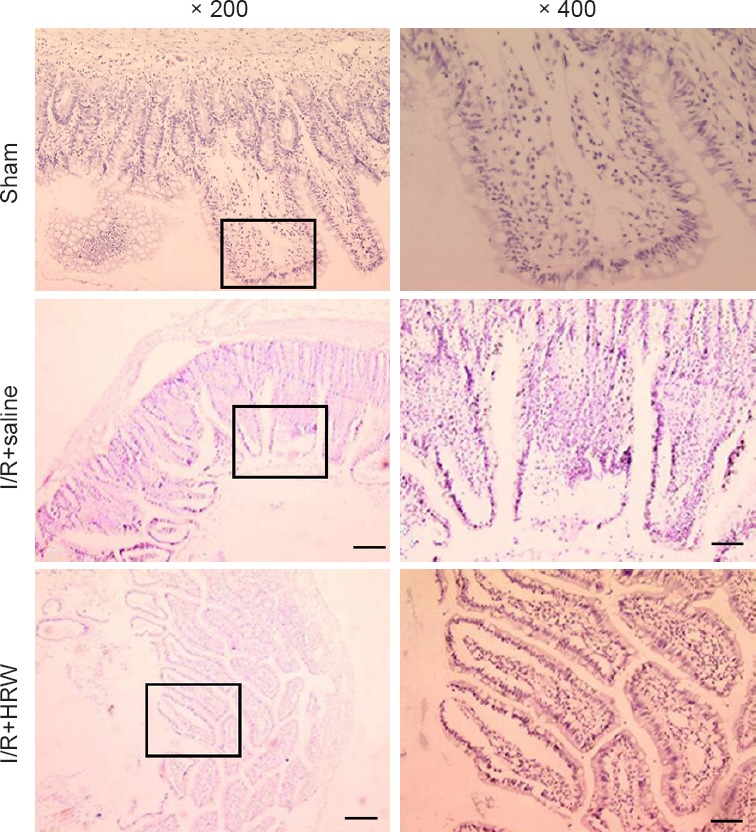
Effect of HRW on the intestinal pathology in an II rat model (hematoxylin-eosin staining).
Note: After I/R (II), the intestinal mucosa and wall became thinner, loss and shedding of intestinal villi were present, and a lot of inflammatory cells infiltrated the intestine. However, HRW treatment in II rats improved the intestinal pathology as compared to I/R (II) + saline group: the villus arrangement was relatively regular, there was no evidence loss of intestinal villi, only small gaps formed at the top of intestinal villi and a few inflammatory cells were found in the intestine. II: Intestinal intussusception; I/R: ischemia/reperfusion; HRW: hydrogen rich water. Scale bars: 200 μm.
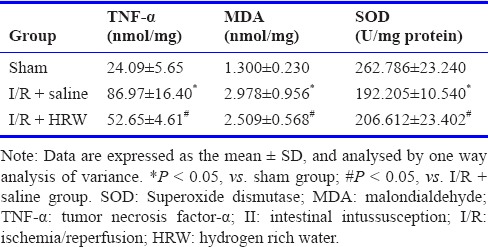
Effect of HRW on the serum TNF-α and MDA contents and SOD activity in an II rat model
As shown in Table 1, the serum TNF-α and MDA contents were the lowest in sham group. The serum TNF-α and MDA contents increased dramatically in I/R + saline group as compared to sham group (P < 0.05). Moreover, the SOD activity was the highest in sham group, but reduced dramatically in the presence of II (P < 0.05). However, after intraperitoneal injection of HRW, the TNF-α and MDA contents reduced significantly and the SOD activity increased markedly compared with I/R + saline group (P < 0.05), indicating that HRW is able to inhibit the release of inflammatory cytokines and suppress the oxidative stress.
Table 1.
Effect of HRW on the serum TNF-α and MDA contents and SOD activity in an II rat model
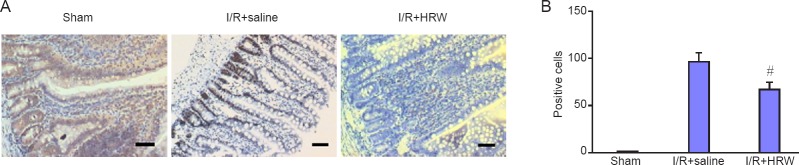
Effect of HRW on the cell apoptosis in an II rat model
In sham group, few TUNEL positive intestinal cells were present. However, the TUNEL positive cells increased significantly after I/R (82.63 ± 16.63, P < 0.05). However, intraperitoneal injection of HRW markedly reduced the AI in I/R + HRW group (49.17 ± 17.78, P < 0.05; Figure 2). This indicates that HRW is able to inhibit the apoptosis after intestinal relocation.
Figure 2.
Effect of HRW on the cell apoptosis in an II rat model.
Note: (A) TUNEL staining of the intestine in different groups (scale bars: 200 μm). Few TUNEL positive cells were observed in Sham group. After II induced I/R, the TUNEL positive cells increased significantly, but intraperitoneal injection of HRW markedly reduced the TUNEL positive cells. (B) Quantitative results of TUNIEL positive cells. Data are expressed as the mean ± SD, and analysed by one way analysis of variance. #P < 0.05, vs. I/R + saline group. II: Intestinal intussusception; I/R: ischemia/reperfusion; HRW: hydrogen rich water.
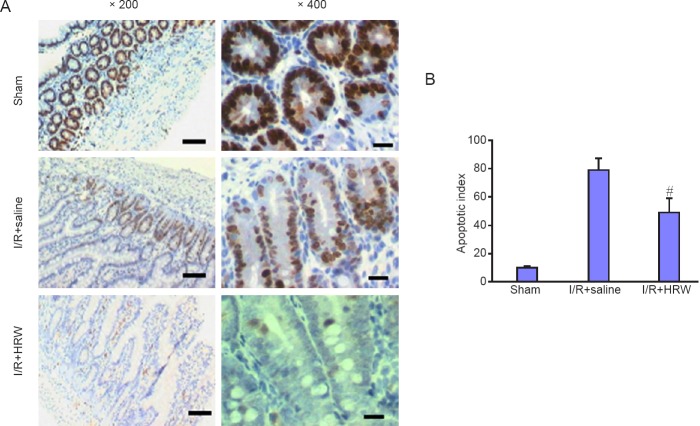
Effect of HRW on the 8-OHdG positive cells in an II rat model
In this study, immunohistochemistry was performed to detect the 8-OHdG positive cells. Results showed 8-OHdG positive cells increased markedly after intestinal II (109.73 ± 4.72; P < 0.05), but intraperitoneal injection of HRW significantly reduced the 8-OHdG positive cells (78.32 ± 7.02; P < 0.05; Figure 3).
Figure 3.
Effect of HRW on the 8-OHdG positive cells in an II rat model.
Note: (A) Immunohistochemistry for 8-OHdG in the intestine of different groups (scale bar: 200 μm; × 200). Few 8-OHdG positive cells were observed in the intestine of Sham group, but II induced I/R dramatically increased these cells. However, intraperitoneal injection of HRW markedly reduced the 8-OHdG positive cells. (B) Quantitative results of 8-OHdG positive cells. Data are expressed as the mean ± SD, and analysed by one way analysis of variance. #P < 0.05, vs. I/R + saline group. II: Intestinal intussusception; I/R: ischemia/reperfusion; HRW: hydrogen rich water; 8-OHdG: 8-oxo-deoxyguanosine.
DISCUSSION
Intussusception is defined as the telescoping of a segment of the gastrointestinal tract into an adjacent one.10 It is the leading cause of intestinal obstruction in children and ranks second only to appendicitis as the most common cause of acute abdominal emergency in children.10 In early phase of intussusception, the veins in the mesentery and intestine are pressed, leading to the blood flow restriction, resulting in intestinal wall congestion, edema and varices. With the deterioration of intestinal wall edema and restriction of blood and lymph flow, the injured intestine becomes ischemic or even necrotic. After reseting the injured intestine, the reperfusion injury of the intestine may be present. Ischemia is easy to cause damage to cells in the intestine, and subsequent reperfusion further aggravates the damage to the intestinal mucosal cells.1 During the ischemia/hypoxia and subsequent reperfusion, a large amount of harmful substances (such as ROS) are produced in the intestine to damage microvessels, release pro-inflammatory cytokines, activate complements and activate neutrophils.1 The ROS and their metabolites are crucial for the pathogenesis of intestinal I/R. In the present study, intussusception was introduced to SD rats and the HRW was intraperitoneally injected to evaluate the protective effect of HRW on the intestinal I/R injury secondary to interstinal intussusception. Hydrogen can be characterized as the lightest and most abundant chemical element. It is colorless, odorless, tasteless and non-toxic. Since Ohsawa et al.5 reported that hydrogen could be used in antioxidant therapy, numerous studies have confirmed that molecular hydrogen is a medical gas with beneficial effects on oxidative stress, inflammation, apoptosis, lipid metabolism, and signaling pathways.11 Basic studies and clinical studies have demonstrated that hydrogen ameliorates the pathological conditions in numerous human diseases or disease models in animals.11,12,13 In available studies, hydrogen is administered through the oral intake of hydrogen water,14 intravenous infusion of hydrogen-rich saline15 and inhalation of air with 2-4% hydrogen gas.16 Study has shown that HRW was protective on I/R induced injury to the intestine in a rat model.7 In this study, intestinal II model was established in rats to mimic the intestinal I/R injury. In our study, we investigated whether HRW was protective on the II induced intestinal injury.
TNF-α is one of major mediators playing important roles in the intestinal I/R injury.17 TNF-α is mainly secreted by activated monocytes and macrophages and has multiple biological activities. In the I/R injury, TNF-α increases early and may not only increase the permeability of intestinal epithelium but mediate the adhesion of neutrophils and the release of other cytokines, results in the deterioration of injury.18 Our results showed II significantly increased the serum TNF-α content which, however, was reduced significantly after intraperitonal injection of hydrogen water. This also confirms the anti-inflammatory effect of molecular hydrogen.
Excessive production of ROS is a major mechanism underlying the pathogenesis of intestinal I/R injury. Excess ROS may cause oxidation of protein, lipid and DNA. MDA results from lipid peroxidation of polyunsaturated fatty acids and the degree of lipid peroxidation can be estimated by the amount of malondialdehyde in tissues.19 8-OHdG is an oxidized derivative of deoxyguanosine and one of the major products of DNA oxidation.20 SOD is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O2-) radical into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2). SOD is an important antioxidant defense in nearly all living cells exposed to oxygen.21 In this study, serum MDA content, serum SOD activity and intestinal 8-OHdG positive cells were detected, aiming to evaluate the anti-oxidative effect of hydrogen in II. Our results showed intraperitoneal injection of HRW significant reduced serum MDA content, increase serum SOD activity and reduced intestinal 8-OHdG positive cells, indicating the anti-oxidative activity of hydrogen in II animal model.
TUNEL is a common method for detecting DNA fragmentation that results from apoptotic signaling cascades.22 TUNEL staining has been widely used to detect the apoptotic cells. In this study, our findings also indicated that intraperitoneal injection was able to reduce the apoptotic intestinal cells. There is a growing consensus that oxidative stress and the redox state of a cell plays a pivotal role in regulating apoptosis.23 The inhibition of cell apoptosis following intraperitoneal injection of HRW may be ascribed to the anti-oxidative effect of molecular hydrogen.
Taken together, our findings indicate that intraperitoneal injection of HRW may inhibit inflammation and suppress oxidative stress following II, leading to the reduction in apoptotic cells and improvement of intestinal pathology. Our findings provide a new strategy for the therapy of II. Of note, there were still limitations in this study, and we did not investigate the optimal dose of hydrogen water and the optimal time point at which hydrogen water was administered in the therapy of II. This will be resolved in our future studies.
Footnotes
Conflicts of interest
The authors declare that there is no conflict of interest in this paper.
Research ethics
The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE). All efforts were made to minimize the number and suffering of the animals used in the experiments. The paper was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines”(ARRIVE Guidelines).
Contributor agreement
A statement of “Publishing Agreement“ has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check
This paper has been checked twice with duplicationchecking software iThenticate.
Peer review
A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewer
Lei Huang, Loma Linda University, USA.
REFERENCES
- 1.Schoenberg MH, Beger HG. Reperfusion injury after intestinal ischemia. Crit Care Med. 1993;21:1376–1386. doi: 10.1097/00003246-199309000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Tassopoulos A, Chalkias A, Papalois A, Iacovidou N, Xanthos T. The effect of antioxidant supplementation on bacterial translocation after intestinal ischemia and reperfusion. Redox Rep. 2017;22:1–9. doi: 10.1080/13510002.2016.1229893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Liu M, Peterson S, Miyake M, Vallyathan V, Liu KJ. Hydroxyl radical formation is greater in striatal core than in penumbra in a rat model of ischemic stroke. J Neurosci Res. 2003;71:882–888. doi: 10.1002/jnr.10534. [DOI] [PubMed] [Google Scholar]
- 4.Nanetti L, Taffi R, Vignini A, et al. Reactive oxygen species plasmatic levels in ischemic stroke. Mol Cell Biochem. 2007;303:19–25. doi: 10.1007/s11010-007-9451-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Kang Z, Liu WW, et al. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167–172. doi: 10.1016/j.neulet.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 7.Zheng X, Mao Y, Cai J, et al. Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res. 2009;43:478–484. doi: 10.1080/10715760902870603. [DOI] [PubMed] [Google Scholar]
- 8.El-Sayyad SM, Soubh AA, Awad AS, El-Abhar HS. Mangiferin protects against intestinal ischemia/reperfusion-induced liver injury: Involvement of PPAR-γ, GSK-3β and Wnt/β-catenin pathway. Eur J Pharmacol. 2017;809:80–86. doi: 10.1016/j.ejphar.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Lei Y, Tong LJ, Wang XH, Qi YF, Tang CS. Taurine decreases the incidence of intussusception in the rat. Beijing Daxue Xuebao: Yixue Ban. 2001;33:35–37. [Google Scholar]
- 10.Marsicovetere P, Ivatury SJ, White B, Holubar SD. Intestinal intussusception: etiology, diagnosis, and treatment. Clin Colon Rectal Surg. 2017;30:30–39. doi: 10.1055/s-0036-1593429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohno K, Ito M, Ichihara M, Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid Med Cell Longev. 2012;2012:353152. doi: 10.1155/2012/353152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Zhang J. Saturated hydrogen saline ameliorates lipopolysaccharide-induced acute lung injury by reducing excessive autophagy. Exp Ther Med. 2017;13:2609–2615. doi: 10.3892/etm.2017.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto Y, Kato T, Ito M, et al. Hydrogen ameliorates pulmonary hypertension in rats by anti-inflammatory and antioxidant effects. J Thorac Cardiovasc Surg. 2015;150:645–654.e3. doi: 10.1016/j.jtcvs.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 14.Fan M, Xu X, He X, et al. Protective effects of hydrogen-rich saline against erectile dysfunction in a streptozotocin induced diabetic rat model. J Urol. 2013;190:350–356. doi: 10.1016/j.juro.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang DQ, Zhu JH. Experimental studies of effects of hydrogen-rich saline in rats with severe acute pancreatitis. Zhonghua Yi Xue Za Zhi. 2012;92:2436–2440. [PubMed] [Google Scholar]
- 16.Ishibashi T, Ichikawa M, Sato B, et al. Improvement of psoriasis-associated arthritis and skin lesions by treatment with molecular hydrogen: A report of three cases. Mol Med Rep. 2015;12:2757–2764. doi: 10.3892/mmr.2015.3707. [DOI] [PubMed] [Google Scholar]
- 17.Nezu Y, Tagawa M, Sakaue Y, Hara Y, Tsuchida S, Ogawa R. Kinetics of endotoxin concentration and tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6 activities in the systemic and portal circulation during small intestinal ischemia and reperfusion in dogs. Am J Vet Res. 2002;63:1680–1686. doi: 10.2460/ajvr.2002.63.1680. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S, Tanabe M, Wakabayashi G, Shimazu M, Matsumoto K, Kitajima M. The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res. 2001;99:134–141. doi: 10.1006/jsre.2001.6106. [DOI] [PubMed] [Google Scholar]
- 19.Domijan AM, Ralić J, Radić Brkanac S, Rumora L, Žanić-Grubišić T. Quantification of malondialdehyde by HPLC-FL-application to various biological samples. Biomed Chromatogr. 2015;29:41–46. doi: 10.1002/bmc.3361. [DOI] [PubMed] [Google Scholar]
- 20.Helbock HJ, Beckman KB, Ames BN. 8-Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods Enzymol. 1999;300:156–166. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 21.McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968-1988) Free Radic Biol Med. 1988;5:363–369. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 22.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002;265:49–72. doi: 10.1016/s0022-1759(02)00070-4. [DOI] [PubMed] [Google Scholar]