Abstract
Stroke is a cerebrovascular disease with high mortality and morbidity. Despite extensive research, there are only a very limited number of therapeutic approaches suitable for treatment of stroke patients as yet. Mounting evidence has demonstrated that such gases as oxygen, hydrogen and hydrogen sulfide are able to provide neuroprotection after stroke. In this paper, we will focus on the recent two years’ progress in the development of gas therapies of stroke and in understanding the molecular mechanisms underlying protection induced by medical gases. We will also discuss the advantages and challenges of these approaches and provide information for future study.
Keywords: medical gases, stroke, neuroprotection
INTRODUCTION
Stroke is a leading cause of death and acquired adult disability worldwide. Approximately 80% of all strokes are ischemic, which result from obstruction of the cerebral arteries. Currently, tissue plasminogen activator (tPA) is the only approved drug by the US Food and Drug Administration (FDA) for treatment of acute ischemic stroke.1 However, encouraging data from recent clinical studies have demonstrated that endovascular management strategies such as thrombectomy can restore the blood flow and, hence, be beneficial for the acute stroke treatment.2,3 Although blood supply recovery can rescue distant penumbra area, it is inevitable that penumbra cells in the rim of infarct core will undergo death triggered by complex ischemic cascades.4 These underscore the needs of development of additive treatments that are able to increase the therapeutic window of advanced reperfusion strategies and, on this way, to preserve brain functions after stroke.2
In past decades, neuroprotective abilities of certain gases have been observed. Several publications reported about neuroprotective effects of oxygen, hydrogen, carbon monoxide (CO) and nitric oxide (NO) as well as some volatile anesthetics (isoflurane, sevoflurane, xenon and nitrous oxide).5,6,7,8 Protective properties of inert gases (helium and argon) and even gases classically considered as toxic (hydrogen sulfide, H2S; CO) have been also investigated (Table 1).9
Table 1.
Original articles of medical gases in stroke from 2015 to 2016
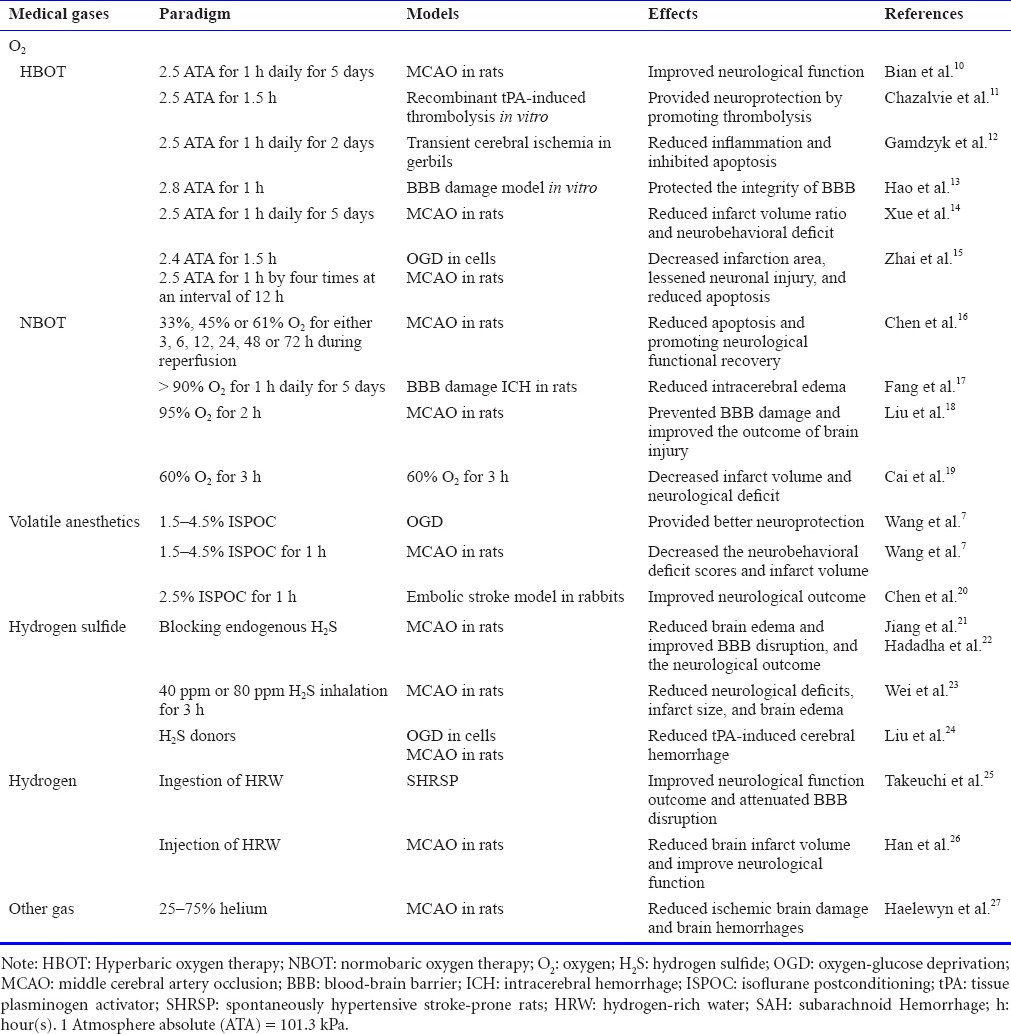
Among protective medical gases, oxygen, isoflurane, H2S and hydrogen are the most studied ones. In this paper, we briefly summarize the newest reports regarding application of these medical gases in the stroke treatment and discuss their challenges and advantages needed for future study.
OXYGEN
Oxygen can be administrated normobaric (normobaric oxygen therapy, NBOT) or under pressure (hyperbaric oxygen therapy, HBOT). Neuroprotective effects of oxygen therapy have been observed in various experimental models of brain injury and neurological diseases.11,28,29,30,31 A systematic review and meta-analysis of the literature published prior to September 2015 showed hyperbaric oxygen (HBO) had a neuroprotective effect and improved survival in animal models of middle cerebral artery occlusion (MCAO), especially in animals given more than 6 hours of HBO and given HBO at 2.0 ATA (101.3 kPa) immediately after MCAO.32 In tPA-induced thrombolysis in vitro, HBO increased tPA-induced thrombolysis; and in rats subjected to thromboembolic MCAO, 5-minute HBO reduced infarct volume and brain edema.11 However, HBOT in human stroke is still not sufficiently evidence-based, due to the insufficient randomized double-blind controlled clinical studies.33
HBO has also been investigated as a pre-conditioning agent.34 HBO pre-conditioning is neuroprotective and able to attenuate hemorrhagic transformation after MCAO by upregulation of peroxisome proliferator activated receptor gamma (PPAR-γ), downregulation of aquaporin-4 (AQP-4) and oxidative stress reduction.10,12,13,14,15,17 Yan et al.35 reviewed the status of clinical and experimental HBOT research in China and concluded that HBOT could increase the oxygen supply to ischemic tissue, improve blood oxygen partial pressure and reduce irreversible tissue damage.
Similar to HBOT, NBOT provides therapeutic benefits and is likewise effective in the treatment of stroke.36 NBOT can inhibit the apoptotic pathway by reducing the expression of caspase-3 and -9, thereby promoting neurological functional recovery in MCAO rats.16 In transient ischemic attack rat model, normobaric oxygen therapy (NBO) administered during ischemia nearly completely prevented the neuronal death, microglial inflammation and sensorimotor impairment.18 Cai et al.19 reported that combining NBO (60% for 3 hours) with ethanol (1.0 g/kg) or hypothermia (33°C for 3 hours) reduced post-stroke hyperglycolysis in thromboembolic stroke rats. NBOT is a promising therapy for short-lasting ischemia. Since it can be initiated at home in at-risk patients or in the ambulance in subjects suspected of transient ischemic attack/early stroke, it is clinically very attractive. It may also be a straightforward support or combination to reperfusion therapies, and help prevent brain damage, attenuating the long-term cognitive and sensorimotor impairment in at-risk populations.37 The major concern with oxygen therapy in acute ischemic stroke is the potential increase of reactive oxygen species (ROS), however, the review paper on NBOT in animal models of stroke by Weaver and Liu38 showed that NBO does not increase ROS or oxidative stress if applied for a short duration.
It has been reported recently that chronic hypoxia activated the hypoxia inducible factor-1α (HIF-1α) response in zebrafish embryos and alleviated death caused by mitochon-drial dysfunction.39 Hypoxia may represent the promising therapeutic strategy with vital clinic significance by triggering innate adaptive programs.5 Repetitive intermittent hypoxic exposures have been showed protective effects against ischemic stroke in pre-clinical models.40,41 The major hurdle in translating of hypoxia into the clinical settings is the negative attitude of clinicians and patients towards the breathing of low levels of oxygen.42
Volatile Anesthetics
Volatile anesthetics routinely used in clinic. Hence, the volatile anesthetics such as isoflurane and sevoflurane, are considered as low risk-bearing gaseous agents. Application of commonly used volatile anesthetics after brain ischemia onset provides neuroprotection in experimental stroke research.43 Wang et al.44 reported that post-conditioning with 3.0% isoflurane provided better neuroprotection than 1.5% and 4.5% isoflurane. Effects of isoflurane post-conditioning were mediated by activation of activin A/Smad 2/3 and activin A/extracellular signal-regulated kinase (ERK) 1/2 signaling pathway. However, in another study, 1.5% isoflurane post-conditioning was showed to be more effective than 3.0%, and 4% isoflurane in reducing infarct volume and improving neurological deficits, which were associated with up-regulated expression of transforming growth factor beta 1 (TGF-β1) and down-regulated phosphorylated c-Jun N-terminal kinase (p-JNK) expression.45 To determine the translational potential, Chen et al.20 used 2.5% isoflurane post-conditioning in rabbit model of embolic stroke and confirmed its neuroprotection. Rabbit post-conditioned with isoflurane tolerated more clots than control animals in the dose-response study, and isoflurane post-conditioning reduced infarct volume and improved neurological deficit scores in animals received intra-carotid injection of 5 mg clots. Although preclinical studies provide strong evidences that isoflurane induces neuroprotection, clinical results, especially long-term neurological outcome, are disappointing. Translation of positive animal findings to patients has been hampered by the associated clinical comorbidity and concurrent medication of patients.46
H2S
H2S is a signaling molecule and exert antioxidant, anti-inflammatory and vasodilatory actions.47,48,49 These results lend credence to the notion that H2S provide neuroprotection in ischemic brain tissue. Wei et al.23 revealed that 40 ppm and 80 ppm H2S inhalation reduced brain edema and infarct volume through inhibiting the expression of AQP-4 via activating protein kinase C (PKC) in MCAO rats. Co-administration of two H2S donors, 5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentene-3-thione (ADT-OH) and sodium hydrosulfide (NaHS) with tPA attenuated hemorrhagic transformation following MCAO in mice.24 Controversially, Hadadha et al.22 indicated that the administration of oxyacetic acid, an inhibitor of H2S synthesis, at a low dose significantly reduced the infarct volume brain edema and improved the neurological outcome in transient MCAO rats. Similarly, Jiang et al.21 found that endogenous production of H2S results in post-ischemic cerebral vasodilation and early blood-brain barrier (BBB) disruption in MCAO mice. These results suggest that the role of H2S in cerebral ischemic might be double-sided depends on the concentration. Hadadha et al.22 believed that while low-concentration of H2S is beneficial, the high-concentration is harmful and aggravates brain injury after ischemic stroke. At present, H2S inhalation has not found its use in clinic practice. Further investigations leading to better understanding of whether modulating of H2S activity can be an option for the treatment of cerebral ischemia, are needed.
HYDROGEN
After the discovery of antioxidant properties of hydrogen by Ohsawa et al.50 in 2007, hydrogen therapy has been a new hotspot of medical gas application in stroke.51 Most studies in regards of the hydrogen application after experimental stroke have been conducted in Japan, China, and the USA.52 The mechanisms underlying the hydrogen-induced neuroprotection include inhibition of various inflammatory molecules, such as ERK, inducible nitric oxide synthase and nuclear factor-кB.52,53 Takeuchi et al.25 demonstrated that hydrogen-rich water improved neurological function and survival rate in spontaneously hypertensive stroke-prone rats, which was associated with reduction of ROS production and suppressing the activity of matrix metalloprotein-ase-9, leading to the stabilization of BBB. Furthermore, Han et al.26 demonstrated that hydrogen-rich water reduced infarct volume and improved neurological function of rats after MCAO. They also demonstrated that hydrogen-rich water maintained the levels of parvalbumin and hippocal-cin (two calcium buffering proteins) and attenuated the glutamate toxicity-induced elevation of intracellular Ca2+ levels.26 The usage of therapeutic hydrogen for treatment of ischemic stroke is promising, but its positive effects need to be replicated in the clinic studies and underlying mechanisms need to be further investigated.
OTHER GASES
Besides oxygen, isoflurane, H2S and hydrogen, neuroprotective effects of other gases also have been studied recently. Abraini’s group reported that helium is an efficient neuroprotective agent able to attenuate tPA-induced thrombolysis consequently decreasing brain injury in thromboembolic model of stroke in rats.27 In the clinic practice, helium would be a safe treatment for patients receiving thrombolytic agent.
ADVANTAGES AND CHALLENGES
Medical gases are one of the promising therapy strategies for stroke. Among the medical gases, oxygen and volatile anesthetics (isoflurane and sevoflurane) are of the greatest interest, for all because they are routinely used in clinical settings. H2S, hydrogen and helium are more popular than other medical gases due to numerous pre-clinical studies demonstrating benefits of these gases. Even though NBO and HBO has been studied in clinical trials, the usage of therapeutic gases in clinic has received little attention.35,54,55,56,57 Given the complex pathophysiology of stroke, it is unlikely that a single intervention strategy will result in clinical relevant protection. Medical gases have distinct advantages over pharmaceutical drugs such as the ease of diffusibility across the BBB and the mechanism of action may be via multiple pathways. Medical gases might be a valuable support therapy to the tPA therapy or thrombectomy after stroke. However, timing and dosing of gas administration investigated in animal studies not necessarily extrapolate with clinical settings.58 These facts represent the major challenge for development of stroke related medical gas treatments and point the direction for the future studies.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Contributor agreement
A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check
This paper has been checked twice with duplicationchecking software iThenticate.
Peer review
A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
REFERENCES
- 1.Zivin JA. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug Administration (FDA) Ann Neurol. 2009;66:6–10. doi: 10.1002/ana.21750. [DOI] [PubMed] [Google Scholar]
- 2.Versnick EJ, Do HM, Albers GW, Tong DC, Marks MP. Mechanical thrombectomy for acute stroke. AJNR Am J Neuroradiol. 2005;26:875–879. [PMC free article] [PubMed] [Google Scholar]
- 3.Xing C, Hayakawa K, Lo EH. Mechanisms, Imaging, and therapy in stroke recovery. Transl Stroke Res. 2017;8:1–2. doi: 10.1007/s12975-016-0503-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7:378–385. doi: 10.1111/j.1747-4949.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palzur E, Zaaroor M, Vlodavsky E, Milman F, Soustiel JF. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 2008;1221:126–133. doi: 10.1016/j.brainres.2008.04.078. [DOI] [PubMed] [Google Scholar]
- 6.Derwall M, Timper A, Kottmann K, Rossaint R, Fries M. Neuroprotective effects of the inhalational anesthetics isoflurane and xenon after cardiac arrest in pigs. Crit Care Med. 2008;36:S492–495. doi: 10.1097/ccm.0b013e31818a904a. [DOI] [PubMed] [Google Scholar]
- 7.Ramos Ramos V, Mesa Suárez P, Santotoribio JD, González García MÁ, Muñoz Hoyos A. Neuroprotective effect of sevoflurane in general anaesthesia. Med Clin (Barc) 2017;148:158–160. doi: 10.1016/j.medcli.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Yao C, Li Y, Shu S, et al. TASK channels contribute to neuroprotective action of inhalational anesthetics. Sci Rep. 2017;7:44203. doi: 10.1038/srep44203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng J, Lei C, Chen Y, et al. Neuroprotective gases-fantasy or reality for clinical use? Prog Neurobiol. 2014;115:210–245. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Bian H, Hu Q, Liang X, et al. Hyperbaric oxygen preconditioning attenuates hemorrhagic transformation through increasing PPARgamma in hyperglycemic MCAO rats. Exp Neurol. 2015;265:22–29. doi: 10.1016/j.expneurol.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chazalviel L, Haelewyn B, Degoulet M, et al. Hyperbaric oxygen increases tissue-plasminogen activator-induced thrombolysis in vitro, and reduces ischemic brain damage and edema in rats subjected to thromboembolic brain ischemia. Med Gas Res. 2016;6:64–69. doi: 10.4103/2045-9912.184713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamdzyk M, Malek M, Bratek E, et al. Hyperbaric oxygen and hyperbaric air preconditioning induces ischemic tolerance to transient forebrain ischemia in the gerbil. Brain Res. 2016;1648:257–265. doi: 10.1016/j.brainres.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Hao L, Guo X, Zou C, et al. Hyperbaric oxygen preconditioning ameliorates blood-brain barrier damage induced by hypoxia through modulation of tight junction proteins in an in vitro model. Croat Med. 2016;57:51–57. doi: 10.3325/cmj.2016.57.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue F, Huang JW, Ding PY, et al. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res. 2016;309:1–8. doi: 10.1016/j.bbr.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 15.Zhai X, Lin H, Chen Y, et al. Hyperbaric oxygen preconditioning ameliorates hypoxia-ischemia brain damage by activating Nrf2 expression in vivo and in vitro. Free Radic Res. 2016;50:454–466. doi: 10.3109/10715762.2015.1136411. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Peng H, Rowat A, et al. The effect of concentration and duration of normobaric oxygen in reducing caspase-3 and -9 expression in a rat-model of focal cerebral ischaemia. Brain Res. 2015;1618:205–211. doi: 10.1016/j.brainres.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Li H, Li G, Wang L. Effect of hyperbaric oxygen preconditioning on peri-hemorrhagic focal edema and aquaporin-4 expression. Exp Ther Med. 2015;10:699–704. doi: 10.3892/etm.2015.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Liu WC, Sun Y, et al. Normobaric hyperoxia extends neuro- and vaso-protection of n-acetylcysteine in transient focal ischemia. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9932-0. doi: 10.1007/s12035-016-9932-0. [DOI] [PubMed] [Google Scholar]
- 19.Cai L, Stevenson J, Peng C, et al. Adjuvant therapies using normobaric oxygen with hypothermia or ethanol for reducing hyperglycolysis in thromboembolic cerebral ischemia. Neuro-science. 2016;318:45–57. doi: 10.1016/j.neuroscience.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Chen F, Long Z, Yin J, Zuo Z, Li H. Isoflurane post-treatment improves outcome after an embolic stroke in rabbits. PLoS One. 2015;10:e0143931. doi: 10.1371/journal.pone.0143931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Z, Li C, Manuel ML, et al. Role of hydrogen sulfide in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS One. 2015;10:e0117982. doi: 10.1371/journal.pone.0117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadadha M, Vakili A, Bandegi AR. Effect of the inhibition of hydrogen sulfide synthesis on ischemic injury and oxidative stress biomarkers in a transient model of focal cerebral ischemia in rats. J Stroke Cerebrovasc Dis. 2015;24:2676–2684. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Wei X, Zhang B, Cheng L, et al. Hydrogen sulfide induces neuroprotection against experimental stroke in rats by down-regulation of AQP4 via activating PKC. Brain Res. 2015;1622:292–299. doi: 10.1016/j.brainres.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J. Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl Stroke Res. 2016;7:209–219. doi: 10.1007/s12975-016-0459-5. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi S, Nagatani K, Otani N, et al. Hydrogen improves neurological function through attenuation of blood-brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neurosci. 2015;16:22. doi: 10.1186/s12868-015-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han L, Tian R, Yan H, et al. Hydrogen-rich water protects against ischemic brain injury in rats by regulating calcium buffering proteins. Brain Res. 2015;1615:129–138. doi: 10.1016/j.brainres.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Haelewyn B, David HN, Blatteau JE, et al. Modulation by the noble gas helium of tissue plasminogen activator: effects in a rat model of thromboembolic stroke. Crit Care Med. 2016;44:e383–389. doi: 10.1097/CCM.0000000000001424. [DOI] [PubMed] [Google Scholar]
- 28.Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27:1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- 29.Avraham-Lubin BC, Dratviman-Storobinsky O, El SD, Hasanreisoglu M, Goldenberg-Cohen N. Neuroprotective effect of hyperbaric oxygen therapy on anterior ischemic optic neuropathy. Front Neurol. 2011;2:23. doi: 10.3389/fneur.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yiğit U, Erdenöz S, Uslu U, et al. An immunohistochemical analysis of the neuroprotective effects of memantine, hyperbaric oxygen therapy, and brimonidine after acute ischemia reperfusion injury. Mol Vis. 2011;17:1024–1033. [PMC free article] [PubMed] [Google Scholar]
- 31.Henninger N, Küppers-Tiedt L, Sicard KM, Günther A, Schneider D, Schwab S. Neuroprotective effect of hyperbaric oxygen therapy monitored by MR-imaging after embolic stroke in rats. Exp Neurol. 2006;201(2):316–323. doi: 10.1016/j.expneurol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Ji R, Wei R, Yin B, He F, Luo B. The efficacy of hyperbaric oxygen therapy on middle cerebral artery occlusion in animal studies: a meta-analysis. PLoS One. 2016;11:e0148324. doi: 10.1371/journal.pone.0148324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett MH, Weibel S, Wasiak J, Schnabel A, French C, Kranke P. Hyperbaric oxygen therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014:CD004954. doi: 10.1002/14651858.CD004954.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue F, Huang JW, Ding PY, et al. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res. 2016;309:1–8. doi: 10.1016/j.bbr.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 35.Yan L, Liang T, Cheng O. Hyperbaric oxygen therapy in China. Med Gas Res. 2015;5:3. doi: 10.1186/s13618-015-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazdeh M, Taher A, Torabian S, Seifirad S. Effects of normobaric hyperoxia in severe acute stroke: a randomized controlled clinical trial study. Acta Med Iran. 2015;53:676–680. [PubMed] [Google Scholar]
- 37.Ejaz S, Emmrich JV, Sitnikov SL, et al. Normobaric hyperoxia markedly reduces brain damage and sensorimotor deficits following brief focal ischaemia. Brain. 2016;139:751–764. doi: 10.1093/brain/awv391. [DOI] [PubMed] [Google Scholar]
- 38.Weaver J, Liu KJ. Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke? A critical review of the literature. Med Gas Res. 2015;5:11. doi: 10.1186/s13618-015-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain IH, Zazzeron L, Goli R, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352:54–61. doi: 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol. 2011;69:975–985. doi: 10.1002/ana.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai YW, Yang YR, Wang PS, Wang RY. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PLoS One. 2011;6:e24001. doi: 10.1371/journal.pone.0024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dempsey JA, Morgan BJ. Humans in hypoxia: a conspiracy of maladaptation?! Physiology (Bethesda) 2015;30:304–316. doi: 10.1152/physiol.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F, Long Z, Yin J, Zuo Z, Li H. Isoflurane post-treatment improves outcome after an embolic stroke in rabbits. PLoS One. 2015;10:e0143931. doi: 10.1371/journal.pone.0143931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Yin J, Wang S, et al. Effects of activin A and its downstream ERK1/2 in oxygen and glucose deprivation after isoflurane-induced postconditioning. Biomed Pharmacother. 2016;84:535–543. doi: 10.1016/j.biopha.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Yin J, Ge M, et al. Transforming growth-beta 1 contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury by regulating the c-Jun N-terminal kinase signaling pathway. Biomed Pharmacother. 2016;78:280–290. doi: 10.1016/j.biopha.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 46.Zwerus R, Absalom A. Update on anesthetic neuroprotection. Curr Opin Anaesthesiol. 2015;28:424–430. doi: 10.1097/ACO.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Y, Zheng J, Zhao T, Tang X, Hu N. Hydrogen sulfide alleviates uranium-induced acute hepatotoxicity in rats: Role of antioxidant and antiapoptotic signaling. Environ Toxicol. 2017;32(2):581–593. doi: 10.1002/tox.22261. [DOI] [PubMed] [Google Scholar]
- 48.Ivanciuc T, Sbrana E, Ansar M, et al. Hydrogen sulfide is an antiviral and antiinflammatory endogenous gasotransmitter in the airways. Role in respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2016;55:684–696. doi: 10.1165/rcmb.2015-0385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura H, Shibuya N, Kimura Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal. 2012;17:45–57. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 51.Wood KC, Gladwin MT. The hydrogen highway to reperfusion therapy. Nat Med. 2007;13:673–674. doi: 10.1038/nm0607-673. [DOI] [PubMed] [Google Scholar]
- 52.Zhuang Z, Sun XJ, Zhang X, et al. Nuclear factor-κB/Bcl-XL pathway is involved in the protective effect of hydrogen-rich saline on the brain following experimental subarachnoid hemorrhage in rabbits. J Neurosci Res. 2013;91:1599–1608. doi: 10.1002/jnr.23281. [DOI] [PubMed] [Google Scholar]
- 53.Itoh T, Hamada N, Terazawa R, et al. Molecular hydrogen inhibits lipopolysaccharide/interferon gamma-induced nitric oxide production through modulation of signal transduction in macrophages. Biomed Pharmacother. 2011;411:143–149. doi: 10.1016/j.bbrc.2011.06.116. [DOI] [PubMed] [Google Scholar]
- 54.Efrati S, Fishlev G, Bechor Y, et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients-randomized, prospective trial. PLoS One. 2013;8:e53716. doi: 10.1371/journal.pone.0053716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazdeh M, Taher A, Torabian S, Seifirad S. Effects of normobaric hyperoxia in severe acute stroke: a randomized controlled clinical trial study. Acta Med Iran. 2015;53:676–680. [PubMed] [Google Scholar]
- 56.Yan D, Shan J, Ze Y, Xiao-Yan Z, Xiao-Hua H. The effects of combined hyperbaric oxygen therapy on patients with post-stroke depression. J Phys Ther Sci. 2015;27:1295–1297. doi: 10.1589/jpts.27.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou BC, Liu LJ, Liu B. Neuroprotection of hyperbaric oxygen therapy in sub-acute traumatic brain injury: not by immediately improving cerebral oxygen saturation and oxygen partial pressure. Neural Regen Res. 2016;11:1445–1449. doi: 10.4103/1673-5374.191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li WA, Geng X, Ding Y. Stroke is a global epidemic: new developments in clinical and translational cerebrovascular diseases research. Neurol Res. 2017;39:475–476. doi: 10.1080/01616412.2017.1330307. [DOI] [PubMed] [Google Scholar]


