Abstract
Hydrogen sulfide (H2S) has been recognized and studied for nearly 300 years, but past researches mainly focus on its toxicity effect. During the past two decades, the majority of researches have reported that H2S is a novel endogenous gaseous signal molecule in organisms, and play an important role in various systems and diseases. H2S is mainly produced by three enzymes, including cystathionine β-synthase, cystathionine γ-lyase and 3-mercaptopyruvate sulfurtransferase along with cysteine aminotransferase. H2S had been firstly reported as a neuromodulator in the brain, because of its essential role in the facilitating hippocampal long-term potentiation at physiological concentration. It is subsequently reported that H2S may have relevance to neurologic disorders through antioxidative, anti-inflammatory, anti-apoptotic and additional effects. Recent basic medical studies and preclinical studies on neurologic diseases have demonstrated that the administration of H2S at physiological or pharmacological levels attenuates brain injury. However, the neuroprotective effect of H2S is concentration-dependent, only a comparatively low dose of H2S can provide beneficial effect. Herein, we review the neuroprotevtive role of H2S therapy in brain diseases from its mechanism to clinical application in animal and human subjects, and therefore provide the potential strategies for further clinical treatment.
Keywords: hydrogen sulfide, gaseous signal molecule, neuroprotection, antioxidation, anti-inflammation, anti-apoptosis, therapy, brain diseases
INTRODUCTION
Hydrogen sulfide (H2S), a gas that smells like rotten eggs, was firstly described in 1731. Since then, most researches about H2S have been devoted to its toxic effects with little attention paid to its physiological function.1 H2S therapy has been an intense subject of interest following the discovery of an endogenous sulfide in mammalian brain by Warenycia et al.2 in 1989. In 1996, Abe et al.3 demonstrated that H2S, as a neuromodulator, facilitates the induction of hippocampal long-term potentiation (LTP) by enhancing the activity of N-methyl D-aspartate (NMDA) receptors. In 2009, Mustafa et al.4 demonstrated a mode of action for H2S, suggesting that it physiologically modifies cysteine (Cys) in a large number of proteins by S-sulfhydration. In the same year, Ishigami et al.5 showed that H2S is released from bound sulfur, an intracellular store of sulfur, in the presence of physiologic concentrations of endogenous reducing substances glutathione (GSH) and Cys.
Currently, H2S has been recognized as the third endogenous gaseous signal molecule in organisms, following nitric oxide (NO) and carbon monoxide (CO).6 Understanding of H2S biological effect and its mechanism has been deepened, especially the physiopathologic significance of H2S in various diseases such as neurological diseases, cardiovascular diseases, hematologic diseases, urological diseases, and so on.7,8 Based on previous studies and literatures, we summarize recent progresses of experimental and clinical researches related to endogenous H2S, including its formation, metabolism, modulation and mechanism, as well as the role of endogenous H2S therapy in various brain diseases.
PRODUCTION, METABOLISM AND MODULATION
H2S is endogenously generated in mammalian cells via enzymatic and nonenzymatic pathways, although the nonenzymatic pathway is less vital in the production of H2S.9 On the one hand, there are at least three enzymes in the organisms: β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST). H2S can be produced from L-Cys by CBS and CSE through the transsulfuration pathway.10 In addition, H2S also can be produced by 3-MST through the Cys catabolism pathway.11 The Cys aminotransferase (CAT) catalyzes the transamination of Cys to the 3-mercaptopyruvate, a substrate of 3-MST to produce pyruvate and sulfane sulfur, which may liberate H2S in the presence of reductants such as dithiothreitol and GSH.12 Both CBS and CSE are classified as the pyridoxal-5’-phosphate (PLP)-dependent enzymes and use either Cys or Cys together with homocysteine (Hcy) as their principal substrates, while 3-MST is non-PLP dependent enzyme. The distribution of the above enzymes of endogenous H2S generation is different in different tissues: CBS is mainly expressed in the nervous system, while the cardiovascular system only expresses CSE. Both CBS and CSE are expressed in the liver, ileum, kidney and pancreas. Meanwhile, the 3-MST, a class of zinc dependent enzymes, is active in erythrocytes and heart cells.13 On the other hand, endogenous H2S can be produced non-enzymatically and generated either from glucose via glycolysis (> 90%) or from phosphogluconate via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (< 10%).14,15
The metabolism and modulation of endogenous H2S are unclear.16 In general, there are two possible forms of H2S in vivo: one third in the form of gas and two-thirds in the form of sodium bisulfide (NaHS). It is reported that the catabolism of H2S may involve chemical reactions, including oxidation to sulfate,17 methylation to methanethiol and dimethyl sulfide, and reactions with Cys-containing proteins.18,19 The metabolites are excreted mainly from the kidneys, partly from the intestine, and exhaled slightly from the lungs. Eto et al.19,20 proposed that there are three pathways to regulate the generation of endogenous H2S: (1) rapid regulation pathway: Ca2+/calmodulin-mediated signal transduction pathway; (2) slow regulation pathway: regulation of testosterone and S-adenosyl-L-methionine (a CBS activator); (3) basic level regulation pathway linked to age and gender.
MECHANISMS
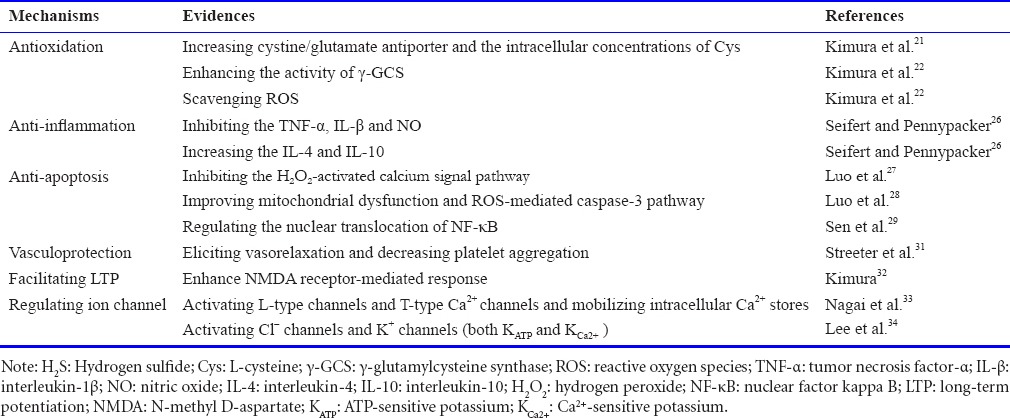
Antioxidation
Kimura et al.21 firstly revealed that the protective effect of H2S on neurons against oxidative stress by increasing the substrate for the production of the antioxidant GSH, including the cystine/glutamate antiporter and the intracellular concentrations of Cys. It has been subsequently reported at the cellular level that H2S also is able to enhance the activity of the γ-glutamylcysteine synthase (γ-GCS), a rate-limiting enzyme, which regulates the generation of GSH.22 In addition, H2S produced in mitochondria, the major organelle that releases reactive oxygen species (ROS) causing toxic effects and ultimately leading to cell death, also may directly suppress oxidative stress through scavenging ROS.22 These findings offer evidences for the powerful anti-anti-oxidative role of H2S.
Anti-inflammation
Thus far, there is no consistent conclusion on the problem whether H2S is an pro-inflammatory factor or an anti-inflammatory factor.23 Neuroinflammation can result from nerve injury in a process mediated by inflammatory cells and cytokines.24 H2S plays a protective role in inflammation by inhibiting lipopolysaccharide-stimulated tumor necrosis factor-α (TNF-α), the proinflammatory cytokine interleukin-1β (IL-β) and NO release in astrocytes and microglial cells.25 Meanwhile, H2S can increase the release of anti-inflammatory cytokines, such as interleukin-4 (IL-4) or interleukin-10 (IL-10).26 As a result, H2S may play an anti-inflammatory role in the central nervous system (CNS).
Anti-apoptosis
According to previous studies, a great deal of evidences described that H2S may exert its anti-apoptotic role by inhibiting oxidative stress.27,28 Pretreatment with NaHS (H2S donor) could significantly suppress hypoxia-induced mouse hippocampal neuronal apoptosis via inhibition of the hydrogen peroxide (H2O2)-activated calcium signal pathway.27 In addition, H2S can improve mitochondrial dysfunction and inhibit an ROS-mediated caspase-3 pathway in the model of oxygen-glucose deprivation/reoxygenation (OGD/R)-induced neuronal apoptosis.28 Besides, H2S could confer its anti-apoptotic effect through regulating nuclear translocation of nuclear factor kappa B (NF-κB), a transcription factor, which is translocated into the nucleus to activate several anti-apoptotic genes.29
Additional mechanisms
The underlying mechanisms of H2S is reflected in other aspects, including acting as a vasculoprotective factor, facilitating hippocampal LTP and regulating ion channel function.30 Specifically, it has been revealed that H2S has vasculoprotective properties in endothelial cells and vascular smooth muscle cells, such as eliciting vasorelaxation and decreasing platelet aggregation.31 Physiological concentrations of H2S may selectively enhance NMDA receptor-mediated response, which has an essential role in the induction of hippocampal LTP.32 H2S possibly activates plasma membrane voltage-gated channels (L-type and T-type Ca2+ channels) and mobilized intracellular Ca2+ stores.33,34 In addition, endogenous H2S was found to activate chloride (Cl–) channels and potassium (K+) channels (ATP-sensitive K channels (KATP) and KCa2+), which may provide neuroprotective effects.35,36
Collectively, the underlying mechanisms of H2S have been investigated in a large number of studies, and the evidence is summarized in Table 1.
Table 1.
Summary of experimental evidences for the mechanisms of H2S
ROLES OF H2S IN BRAIN DISEASES
Traumatic brain injury (TBI)
TBI is defined as a serious public health problem that disrupts the normal function of the brain and can be caused by a bump, blow or jolt to the head, rapid acceleration and deceleration of the calvarium, or a penetrating head injury.37 There are certain experiments have shown that TBI usually led to brain edema, tissue loss, neurocognitive impairments, and dysfunction of the CNS.38 Severe traumatic brain injury is a leading cause of increased long-term mortality and reduced life expectancy in the word, and trauma-induced changes in neuronal receptor composition render cells vulnerable to secondary injury.39 Furthermore, activation of inflammatory reaction and production of ROS are two momentous elements in the early and secondary TBI-induced neuropathology.40,41
When TBI occurs in mice, endogenous H2S in mouse brain cortex and hippocampus exhibits dynamic decrease, in parallel with CBS mRNA and protein expression in the brain.42 Then, pretreatment with H2S donor (NaHS, administered intraperitoneally) attenuates TBI-induced lesion volume, suggesting that H2S is an important neuromodulator in the model of TBI.42 Other studies have revealed the neuroprotective effects of H2S on controlled cortical impact injury in rats: neurologic dysfunction is improved, endogenous antioxidant enzymatic (superoxide dismutase (SOD) and catalase) activities increase and the levels of oxidative products (malondialdehyde (MDA) and 8-iso-prostaglandin F2a) decrease, the blood-brain barrier (BBB) permeability increases and the brain edema is attenuated. Furthermore, the KATP channel blocker 5-hydroxydecanoate further proves that mitochondrial adenosine triphosphateesensitive potassium (mitoKATP) channels are activated and oxidative stress is reduced following exogenous H2S therapy.43
Stroke
As the society ages rapidly, stroke has become the devastating disease second only to ischemia myocardial as a cause of disability and death worldwide, and also become a major threat to human health and life.44 Although a great deal of factors can lead to the stroke, its main causes include cerebral vasospasm, obstacles in cerebral blood circulation, and the rupture of cerebral vessels.45 A stroke is usually defined as one of two types: ischemic stroke caused by a blockage in an artery and hemorrhagic stroke caused by a tear in the arterial wall that produces bleeding into or around the brain. Either in the early brain injury (EBI) phase or in the late repair stage, the key factors of stroke pathobiology are oxidative stress and immunity.46,47
One study on rats revealed that H2S provides potent nerve protection against a severe cerebral injury induced by transient middle cerebral artery occlusion.48 It is regarded as the evidence that declination of the post-ischemic cerebral edema and the infarct volume as well as the improvement of behavior function after treatment with H2S donor (NaHS). In the same experiment, researchers also demonstrated that H2S could act as an antioxidant and significantly increase SOD activity in brain tissues. In contrast, the MDA content was selectively reduced and the mRNA levels of p47phox and gp91phox subunits of NADPH oxidase were up-regulated. Meanwhile, the expression of the anti-inflammatory cytokine IL-10 and the anti-apoptotic marker Bcl-2 was markedly induced in NaHS-tread group compared with ischemia/reperfusion (I/R) group.49 In conclusion, H2S has potent neuroprotective effect in the model of cerebral I/R through its anti-oxidative, anti-inflammatory, and anti-apoptotic effects. There is a point we have to say, however, that different concentration of H2S may result in different outcomes, and even get the opposite conclusion. It means that the neuroprotective effect of H2S is concentration-dependent in the model of I/R, only a comparatively low dose of H2S can provide beneficial effect.50
The hemorrhagic stroke is generally divided into subarachnoid hemorrhage and intracerebral hemorrhage, two major types with high morbidity and mortality.51 According to previous study, hemorrhagic strokes are typically more dangerous than ischemic strokes.52 In a rat model of subarachnoid hemorrhage, treatment with NaHS attenuates EBI in vivo, including brain edema, BBB disruption, brain cell apoptosis, inflammatory response, and cerebral vasospasm. Further more, H2S protects neurons and endothelial function via functioning as an antioxidant and antiapoptotic mediator in vitro.53 In conclusion, H2S may improve EBI and secondary brain injury in the subarachnoid hemorrhage model. In another study, treatment with NaHS reduced tissue plasminogen activator-induced the hemorrhagic transformation following ischemic stroke possibly by inhibiting the Akt-vascular endothelial growth factor-metalloproteinase 9 cascade.54 However, it is unclear whether H2S has potential application value in brain injury induced by intracerebral hemorrhage.
Neurodegenerative diseases
Neurodegenerative disease is an umbrella term for a range of conditions that primarily affect the neurons in human brain, which are incurable and debilitating conditions that result in progressive degeneration and death of nerve cells. In general, there are two main types of neurodegenerative diseases: impacting mental functioning (called dementias) and affecting movement (called ataxias).
Alzheimer’s disease (AD), a form of dementia, is the most common progressive neurodegenerative disease, which may cause a series of clinical symptoms, such as memory impairment, logagnosia, personality changes and other neuropsychiatric symptoms. As previously described, AD usually damages neurons through activated neuroinflammation, oxidative stress and neuron apoptosis.55 Admittedly, Hcy, a potential risk factor for AD, has harmful effects on cognitive function. Recent study have demonstrated that H2S improved Hcy-induced cognitive dysfunction, which may play a benificial role through inhibiting reactive aldehydes accumulation, preserving glutathione homeostasis, and upregulating aldehyde-dehydrogenase 2 activity and expression in the hippocampus of Hcy-exposed rats.56 Moreover, the beta-amyloid peptides (Aβ) cascade theory is regarded as a major pathogenese that may induce AD through oxidative stress and the change of synapsis.57 However, H2S can reverse Aβ-induced cognitive deficits via attenuating the production of Aβ and suppressing the down-regulation of CBS and 3-MST.58 In addition, one study found that the progression of AD can be deterred through treatment with H2S donors or spa-waters rich in H2S content targeting multiple pathophysiological mechanismsappropriate.59 In that study, a significant decrease in TNF-α and Bcl-2 expression increased, resulting in a attenuation of hippocampus morphological alterations and improved the ability to spatial learning and memory.59 In other AD models, the cytotoxic lipid oxidation product 4-hydroxynonenal was scavenged with H2S therapy, which provides a novel hope against AD through the neuroprotection effects of H2S.60
Parkinson’s disease (PD) is an age-related neurodegenerative disease histopathologically characterized by progressive degeneration of dopaminergic neurons in substantia nigra of midbrain and formation of the Louis bodies in cytoplasm of residual neurons.61 At present, clinical treatment of PD is levodopa (L-DOPA) replacement therapy to improve symptoms, but it not only may induce side effects like dyskinesia, also can not obstruct the development of PD. According to previous studies, plasma Hcy levels are significantly elevated in PD when patients are treated with L-DOPA group compared to other groups.62 Furthermore, recent studies demonstrated that treatment with NaHS is able to significantly reduce the loss of substantia nigra neurons and slow down the development of motor dysfunction in 6-hydroxydopamine hydrobromide-induced and rotenone-induced PD models.63 Moreover, inhalation of H2S prevented the movement disorder from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced PD.64 Therefore, H2S is expected to provide new ideas for the pathogenesis and clinical treatment of PD.
In order to facilitate simple comprehension, we systematically analyzed the latest researches concerned with the use of H2S in the above brain diseases and the relevant conclusions are described through illustration (Figure 1).
Figure 1.
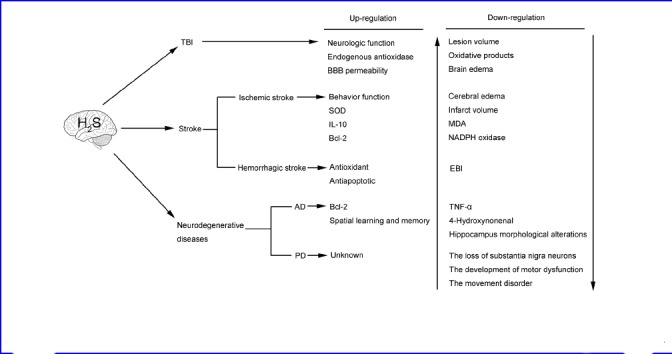
The application of H2S therapy in brain diseases.
Notes: H2S: hydrogen sulfide; TBI: traumatic brain injury; BBB: blood-brain barrier; SOD: superoxide dismutase; IL-10: interleukin-10; AD: Alzheimer's disease; PD: Parkinson's disease; MDA: malondialdehyde; TNF-α: tumor necrosis factor-α; EBI: early brain injury; NADPH: nicotinamide adenine dinucleotide phosphate.
CLINICAL STUDIES
Until now, no direct clinical studies have confirmed the neuroprotection of H2S in brain disease. However, it is reported that there is a close touch between the plasma H2S level and the long-term clinical outcome in stroke patients. A growing number of evidence has suggested that hyperhomocysteinaemia is a risk factor for stroke, although several meta-analyses have not came to an agreement.65,66,67 In another study, results indicated that increased plasma Cys in patients with acute stroke may show an increase in the production of H2S in the brain, thus leading to poor clinical outcomes.68 Generally speaking, indirect evidence proves that it is no doubt that H2S exerts neuroprotective effects in clinical trials and has a close association with brain injury caused by acute stroke, but its underlying mechanisms are needed to be further studied.
CONCLUSION
As endogenous gas signal molecules, NO, CO and H2S have extensive tissue distribution and diverse bioeffects. Over the past two decades, H2S has been proved to be the third gas signal molecule that plays an important role in physiology and pathology. Furthermore, a unique gas signal network may come into being among the three gaseous systems, which are not only independent of each other but also jointly participate in the regulation and control of diseases. Increasing studies have shown that H2S has been regarded as a neuromodulator in the brain, rather than the previously described toxic effects. It is noteworthy that only the appropriate dose of H2S may provide neuroprotective effects, because H2S is toxic to the body when it is higher than the physiological dose. However, it is poor that our understanding of the underlying mechanisms of H2S actions in the CNS. And there are still many controversies that are needed to further explore. H2S therapy has only entered a preliminary stage whether in basic medical research or preclinical research. Along with the deepening of research, we firmly believe that the clinical application of H2S therapy will become a potent treatment regimen in the near future.
Footnotes
Funding: This work was supported by Suzhou Key Medical Center (No. Szzx201501), grants from the National Natural Science Foundation of China (No. 81571115, 81422013, and 81471196), Scientific Department of Jiangsu Province (No. BL2014045), the Project of Invigorating Health Care through Science, Technology and Education, Suzhou Government (No. SZS201413, SYS201608, and LCZX201601), Jiangsu Province (No. 16KJB320008).
Conflicts of interest
The authors declare that they have no competing interests.
Contributor agreement
A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check
This paper has been checked twice with duplicationchecking software iThenticate.
Peer review
A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewer
Wen-wu Liu, Second Military Medical University, China.
REFERENCES
- 1.Kimura H, Nagai Y, Umemura K, Kimura Y. Physiological roles of hydrogen sulfide: synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid Redox Signal. 2005;7:795–803. doi: 10.1089/ars.2005.7.795. [DOI] [PubMed] [Google Scholar]
- 2.Warenycia MW, Goodwin LR, Benishin CG, et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 3.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustafa AK, Gadalla MM, Sen N, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 6.Huang YM, Cheng Y, Jiang R. Hydrogen sulfide and penile erection. Zhonghua Nan Ke Xue. 2012;18:823–826. [PubMed] [Google Scholar]
- 7.Chan SJ, Wong PT. Hydrogen sulfide in stroke: Protective or deleterious? Neurochem Int. 2017;105:1–10. doi: 10.1016/j.neuint.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Li XJ, Li CK, Wei LY, et al. Hydrogen sulfide intervention in focal cerebral ischemia/reperfusion injury in rats. Neural Regen Res. 2015;10:932–937. doi: 10.4103/1673-5374.158353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Wang J, Li H, Xue M, Ji A, Li Y. Role of Hydrogen sulfide in ischemia-reperfusion injury. Oxid Med Cell Longev 2015. 2015 doi: 10.1155/2015/186908. 186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci. 2008;29:84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15:405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- 14.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26(3):243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 17.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Bian JS. Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci. 2014;5:876–883. doi: 10.1021/cn500185g. [DOI] [PubMed] [Google Scholar]
- 19.Eto K, Kimura H. The production of hydrogen sulfide is regulated by testosterone and S-adenosyl-L-methionine in mouse brain. J Neurochem. 2005;93:1633. doi: 10.1111/j.1471-4159.2005.03283.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Bhatia M, Moore PK. Hydrogen sulphide-a novel mediator of inflammation? Curr Opin Pharmacol. 2006;6:125–129. doi: 10.1016/j.coph.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Doursout MF, Schurdell MS, Young LM, et al. Inflammatory cells and cytokines in the olfactory bulb of a rat model of neuroinflammation; insights into neurodegeneration? J Interferon Cytokine Res. 2013;33:376–383. doi: 10.1089/jir.2012.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JF, Li Y, Song JN, Pang HG. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int. 2014;64:37–47. doi: 10.1016/j.neuint.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Transl Stroke Res. 2014;5:543–553. doi: 10.1007/s12975-014-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Y, Liu X, Zheng Q, et al. Hydrogen sulfide prevents hypoxia-induced apoptosis via inhibition of an H2O2-activated calcium signaling pathway in mouse hippocampal neurons. Biochem Biophys Res Commun. 2012;425:473–477. doi: 10.1016/j.bbrc.2012.07.131. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y, Yang X, Zhao S, et al. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem Int. 2013;63:826–831. doi: 10.1016/j.neuint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Sen N, Paul BD, Gadalla MM, et al. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streeter EY, Badoer E, Woodman OL, Hart JL. Effect of type 1 diabetes on the production and vasoactivity of hydrogen sulfide in rat middle cerebral arteries. Physiol Rep. 2013;1:e00111. doi: 10.1002/phy2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streeter E, Ng HH, Hart JL. Hydrogen sulfide as a vasculoprotective factor. Med Gas Res. 2013;3:9. doi: 10.1186/2045-9912-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 33.Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- 34.Lee SW, Hu YS, Hu LF, et al. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia. 2006;54:116–124. doi: 10.1002/glia.20362. [DOI] [PubMed] [Google Scholar]
- 35.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 36.Dawe GS, Han SP, Bian JS, Moore PK. Hydrogen sulphide in the hypothalamus causes an ATP-sensitive K+ channel-dependent decrease in blood pressure in freely moving rats. Neuroscience. 2008;152:169–177. doi: 10.1016/j.neuroscience.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Nizamutdinov D, Shapiro LA. Overview of traumatic brain injury: an immunological context. Brain Sci. 2017;7 doi: 10.3390/brainsci7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chua KS, Ng YS, Yap SG, Bok CW. A brief review of traumatic brain injury rehabilitation. Ann Acad Med Singapore. 2007;36:31–42. [PubMed] [Google Scholar]
- 39.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27:1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 40.Arvin B, Neville LF, Barone FC, Feuerstein GZ. Brain injury and inflammation. A putative role of TNF alpha. Ann N Y Acad Sci. 1995;765:62–71. doi: 10.1111/j.1749-6632.1995.tb16561.x. discussion 98-99. [DOI] [PubMed] [Google Scholar]
- 41.Zhang YP, Cai J, Shields LB, Liu N, Xu XM, Shields CB. Traumatic brain injury using mouse models. Transl Stroke Res. 2014;5:454–471. doi: 10.1007/s12975-014-0327-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Shan H, Wang T, et al. Dynamic change of hydrogen sulfide after traumatic brain injury and its effect in mice. Neurochem Res. 2013;38:714–725. doi: 10.1007/s11064-013-0969-4. [DOI] [PubMed] [Google Scholar]
- 43.Jiang X, Huang Y, Lin W, Gao D, Fei Z. Protective effects of hydrogen sulfide in a rat model of traumatic brain injury via activation of mitochondrial adenosine triphosphate-sensitive potassium channels and reduction of oxidative stress. J Surg Res. 2013;184:e27–35. doi: 10.1016/j.jss.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 44.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann S, Shields NJ, Balle T, Chebib M, Clarkson AN. Innate immunity and inflammation post-stroke: an a7-nicotinic agonist perspective. Int J Mol Sci. 2015;16:29029–29046. doi: 10.3390/ijms161226141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gheibi S, Aboutaleb N, Khaksari M, et al. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci. 2014;54:264–270. doi: 10.1007/s12031-014-0284-9. [DOI] [PubMed] [Google Scholar]
- 49.Yin J, Tu C, Zhao J, et al. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain Res. 2013;1491:188–196. doi: 10.1016/j.brainres.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 50.Ren C, Du A, Li D, Sui J, Mayhan WG, Zhao H. Dynamic change of hydrogen sulfide during global cerebral ischemia-reperfusion and its effect in rats. Brain Res. 2010;1345:197–205. doi: 10.1016/j.brainres.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Shinohara Y. Hemorrhagic stroke syndromes: clinical manifestations of intracerebral and subarachnoid hemorrhage. Handb Clin Neurol. 2009;93:577–594. doi: 10.1016/S0072-9752(08)93028-1. [DOI] [PubMed] [Google Scholar]
- 52.Leithäuser B, Jung F, Park JW. Oral anticoagulation for prevention of cardioembolic stroke in patients with atrial fibrillation: Focussing the elderly. Appl Cardiopulm Pathophysiol. 2009;13:307–317. [Google Scholar]
- 53.Cui Y, Duan X, Li H, et al. Hydrogen sulfide ameliorates early brain injury following subarachnoid hemorrhage in rats. Mol Neurobiol. 2016;53:3646–3657. doi: 10.1007/s12035-015-9304-1. [DOI] [PubMed] [Google Scholar]
- 54.Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J. Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl stroke res. 2016;7:209–219. doi: 10.1007/s12975-016-0459-5. [DOI] [PubMed] [Google Scholar]
- 55.Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: possible role of hydrogen sulfide. J Alzheimers Dis. 2011;24(Suppl 2):173–182. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- 56.Li M, Zhang P, Wei HJ, et al. Hydrogen Sulfide Ameliorates Homocysteine-induced cognitive dysfunction by inhibition of reactive aldehydes involving upregulation of ALDH2? Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyw103. doi: 10.1093/ijnp/pyw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Deng Y, Liu H, Yin C, Li X, Gong Q. Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: a novel mechanism mediated by the activation of Nrf2. Pharmacol Biochem Behav. 2016;150-151:207–216. doi: 10.1016/j.pbb.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Giuliani D, Ottani A, Zaffe D, et al. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol Learn Mem. 2013;104:82–91. doi: 10.1016/j.nlm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Schreier SM, Muellner MK, Steinkellner H, et al. Hydrogen sulfide scavenges the cytotoxic lipid oxidation product 4-HNE. Neurotox Res. 2010;17:249–256. doi: 10.1007/s12640-009-9099-9. [DOI] [PubMed] [Google Scholar]
- 61.Kida K, Ichinose F. Hydrogen sulfide and neuroinflammation. Handb Exp Pharmacol. 2015;230:181–189. doi: 10.1007/978-3-319-18144-8_9. [DOI] [PubMed] [Google Scholar]
- 62.Zoccolella S, Lamberti P, Armenise E, et al. Plasma homocysteine levels in Parkinson’s disease: role of antiparkinsonian medications. Parkinsonism Relat Disord. 2005;11:131–133. doi: 10.1016/j.parkreldis.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Xue X, Bian JS. Neuroprotective effects of hydrogen sulfide in Parkinson’s disease animal models: methods and protocols. Methods Enzymol. 2015;554:169–186. doi: 10.1016/bs.mie.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Kida K, Yamada M, Tokuda K, et al. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxid Redox Signal. 2011;15:343–352. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassan A, Hunt BJ, O’Sullivan M, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2004;127:212–219. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]
- 66.Abbate R, Sofi F, Brogi D, Marcucci R. Emerging risk factors for ischemic stroke. Neurol Sci. 2003;24(Suppl 1):S11–12. doi: 10.1007/s100720300027. [DOI] [PubMed] [Google Scholar]
- 67.Kim NK, Choi BO, Jung WS, Choi YJ, Choi KG. Hyperhomocysteinemia as an independent risk factor for silent brain infarction. Neurology. 2003;61:1595–1599. doi: 10.1212/01.wnl.0000096010.98989.49. [DOI] [PubMed] [Google Scholar]
- 68.Wong PT, Qu K, Chimon GN, et al. High plasma cyst(e)ine level may indicate poor clinical outcome in patients with acute stroke: possible involvement of hydrogen sulfide. J Neuropathol Exp Neurol. 2006;65:109–115. doi: 10.1097/01.jnen.0000199571.96472.c7. [DOI] [PubMed] [Google Scholar]


