Abstract
Helium has been classified as a kind of inert gas that is not effortless to spark chemical reactions with other substances in the past decades. Nevertheless, the cognition of scientists has gradually changed accompanied with a variety of studies revealing the potential molecular mechanism underlying organ-protection induced by helium. Especially, as a non-anesthetic gas which is deficient of relevant cardiopulmonary side effects, helium conditioning is recognized as an emerging and promising approach to exert favorable effects by mimicking the cardioprotection of anesthetic gases or xenon. In this review we will summarize advances in the underlying biological mechanisms and clinical applicability with regards to the cardioprotective effects of helium.
Keywords: helium, pre-conditioning, post-conditioning, cardioprotection, mechanism
INTRODUCTION
Helium
Helium, a colorless, odorless, tasteless monatomic element, heads the noble gas series (neon, argon, krypton, xenon, and the radioactive element radon) in the periodic table.1 As a member of noble gases, it has outer shells completely filled with electrons, making it almost completely inert and thus less capable of forming covalent bonds with other elements.2,3 At the same time, helium is described as a “non-immobilizer”, which represents a gas that cannot only induce anesthesia but also possess other behavioral effects.4 Recent years have witnessed an increased interest in experimental and clinical investigations of helium, and it has been convincingly shown that helium is capable of exerting significant biologic effects in diversified basic experiments.5
Physical properties and its application
Helium is the second most abundant element in the known universe after hydrogen and has many unusual physical properties.6 For example, it is the lightest noble gas (molecular weight 4 g/mol) and its melting and boiling points are the lowest among all elements.7 Compared to oxygen (1.43 g/m3) and nitrogen (1.25 g/m3), helium has less density (0.179 g/m3).8 The absolute viscosity of helium is 201.8 mp (oxygen: 211.4 mp; ordinary air: 188.5 mp).9,10 The low density and high viscosity of helium just in line with the principle of Renault formula, this exactly is the cause that helium can be utilized to potentially benefit the problems associated with airway obstruction by reducing respiratory distress and improving respiratory efficiency.11 Studies within the past decade have increasingly demonstrated heliox inhalation (a mixture of helium and oxygen) could be a potential lung protective strategy in the clinical settings such as bronchiolitis, acute asthma exacerbations and chronic obstructive pulmonary disease.6 Furthermore, the volume of helium molecules is extremely small so that the diffusion rate is 2.5 times that of nitrogen, and its water-insoluble properties indicate that paralysis and toxicity are not easily caused via blood circulation even in high pressure environments in deep water. So we often employ helium-oxygen mixed gas (rather than nitrogen) in the proper proportion to effectively prevent deep water anesthesia, avoid decompression and increase the depth of diving when a diver needs to stay in the deep sea for a long time.12
Chemical properties and its application
The increasing evidences indicate that inert gases especially xenon and some volatile anesthetics can exert organ protection through influencing certain signaling pathways in diverse basic experiments.13 As a member of an inert gas, whether helium also has biochemical properties and thus produces a similar protective effect has received extensive attention. Furthermore, compared with xenon and other volatile anesthetics, helium can be easily and safely administered.13 It is essentially devoid of anesthetic properties and many studies have already revealed a stable hemodynamic profile when clinically applied.14 These properties would make helium a perfect alternative for organ-protection in the case of reversible or irreversible ischemia/reperfusion (I/R) damage in clinical scenes when anesthesia is not requisite.15 Findings from numerous experimental studies have demonstrated that helium acts as a protective agent against tissue injury in a variety of organs such as brain, heart, liver, and kidney damage, and helium’s cardioprotection has received most extensive attention.16,17 This review contains the existing knowledge about the cellular effects of helium, which may bring about new clinical strategies of salvage in myocardium I/R injury.
Myocardial I/R injury
Ischemic heart disease is the primary cause of morbidity because of its detrimental clinical consequences, such as acute myocardial infarction, heart failure, myocardial stunning and arrhythmias.18,19 Early reperfusion is the cornerstone in the treatment of myocardial damage but the flipside of the coin is reperfusion injury.20 Studies in the past have unraveled that therapeutic intervention with the purpose of reducing reperfusion-induced injury is beneficial at the time of opening the obstructed vessel.21 Naturally, it results in the discovery of pre- and post-conditioning. Cardioprotection induced by pre-conditioning covers two phases, an early phase (early pre-conditioning), lasting for 2–3 hours after the pre-conditioning stimulus,22 and a late phase (late pre-conditioning), reappearing 24 hours after the initial stimulus and lasting for 2–3 days.23 What has been proved is that short episodes of ischemia in advance of an ischemic event (ischemic pre-conditioning) generate cardioprotection, reduce infarct size and ease cell damage.24 Post-conditioning is achieved by conditioning cycles at the onset of reperfusion,25 the effectiveness and availability making it a promising clinical approach to alleviate I/R injury as well.26,27 According to the available research findings, several pathways contribute to the survival of myocardial cells via helium pre- and post-conditioning are as follows.
HEALTHY ANIMAL MODELS
Pre-conditioning by helium
Early pre-conditioning
Reperfusion injury salvage kinase (RISK) pathway: In 2007, Pagel et al.28 provided the first evidence that helium was able to exert protective effects by pre-conditioning in ischemic myocardium and the underlying mechanism involves the activation of the RISK pathway. The experiment showed that three 5-minute cycles of 70% helium significantly decreased infarct size among rabbits subjected to a 30-minute left anterior descending coronary artery occlusion and 3 hours reperfusion. Application of Wortmannin, PD 098059, and rapamycin, the selective inhibitors of phosphatidylinositol 3-kinase (PI3K), extracellular signal-regulated kinase 1/2 (Erk1/2), and 70-kDa ribosomal protein s6 kinase (p70s6K) were capable to abolish the cardioprotection induced by a brief, intermittent administration of helium. It further demonstrated that atractyloside, a selective opener of mitochondrial permeability transition pore (mPTP), could counteract the protection induced by helium pre-conditioning. It means that RISK pathway and its proposed putative mPTP end-effector have been intensely implicated in reduction of cell death during reperfusion.
Previous studies have revealed that activated pro-survival kinases from the RISK pathway regulate the transition state of the mPTP by inhibiting the activity of glycogen synthase kinase-3β (GSK-3β), preventing the degradation of apoptotic protein p53 and favorably affecting pro- versus anti-apoptotic protein balance.29 The GSK-3β or p53 inhibition-mediated protection effects occur in the downstream from PI3K.30,31 Employing SB 216763 and pifithrin-a, the selective inhibitors of GSK-3β and p53 respectively, lowered the threshold of helium pre-conditioning in vivo through an mPTP-dependent mechanism. Pagel et al.32 therefore demonstrated that the inhibition of GSK-3β and p53 favorably modulating mPTP and producing cardioprotection via RISK pathway in the helium-induced pre-conditioning.
G protein-coupled receptor ligands including δ1-opioid, adenosine, and bradykinin have been shown to activate pro- survival signaling and play significant roles in classical ischemic pre- and post-conditioning.33 Pagel and co-workers34 tested the hypotheses that morphine, a routinely applied opioid receptor in clinical practice, lowers the threshold of cardioprotection produced by helium in rabbits as well.
Mild intracellular acidosis: Persistent intracellular acidosis was demonstrated to reduce myocardial damage by inhibiting mPTP formation during early reperfusion.35,36 Cohen et al.37 proved that transient maintenance of myocardial acidosis contributed to myocardium protective action during ischemic post-conditioning by ultimately maintaining the close state of mPTP. We can accordingly make assumptions based on the discoveries that cardioprotection induced by ischemic post-conditioning is dependent on not only the activation of pro-survival signaling kinase pathways but also the preservation of intracellular acidosis during early reperfusion. In order to test the hypothesis that intracellular pH is involved in helium pre-conditioning during early reperfusion, Pagel et al.38 testified the decreases in myocardial infarct size induced by intermittent, repetitive exposures to helium were thoroughly abolished by transient metabolic alkalosis during early reperfusion. And the mPTP inhibitor cyclosporin A restored helium-induced reductions in infarct size in the presence of alkalosis. Current observations have indicated that helium pre-conditioning may protect the myocardium against ischemic injury by maintaining modest intracellular acidosis, preserving mPTP in its closed conformation and thereby attenuating myocardial necrosis.
Potassium channel: Previous investigation abundantly certified that reactive oxygen species (ROS) and mitochon-drial adenosine triphosphate potassium (KATP) channels play complementary roles during ischemic or anesthetic pre-conditioning,39,40 but the roles of ROS and mitochon-drial KATP channels in the process of helium preconditioning remain to be defined. Paul et al.41 verified that pretreatment with ROS scavengers N-acetylcysteine (NAC) and N-2 mercaptoproprionyl glycine (2-MPG) abolished helium-induced cardioprotection, suggesting that ROS participated in helium pre-conditioning in vivo. The research further indicated that the pretreatment with KATP channels blocker 5-hydroxydecanoate (5-HD) completely abolished decreases in myocardial infarct size produced by helium, implicating the underlying function of mitochondrial KATP channels in this process as well.41
In addition to activation of KATP channels, another kind of K+ channels: Ca2+-sensitive potassium (KCa) channels (mKCa) seems to be complicated in the pre-conditioning. Studies showed helium-induced pre-conditioning reduced infarct size accompanied with a significant decrease in the mitochondrial respiratory control index (state3/state4) by increasing state4 respiration. The K-channel antagonist iberiotoxin not only completely eliminates helium-induced infarct size reduction but also reduces helium-induced respiratory control index. From the data we conclude that the cardioprotection helium provided is associated with the activation of the mKCa channel and mild mitochondrial uncoupling.18 Recent data further points out: the activation of mKCa channel is mediated by protein kinase-A (PKA) in helium-induced pre-conditioning.42
Endothelial nitric oxide synthase (eNOS)-nitric oxide (NO): To our knowledge, eNOS acts as a downstream target of PI3K and AKT (protein kinase B), which can increase the formation of NO by phosphorylating Ser residues.43,44 As a major participant, NO plays a pivotal role via promoting the translocation of ε-isoform protein kinase C (PKC-ε) and direct activation of KATP in ischemia-induced or volatile anesthetic-induced cardioprotection.45,46 Paul et al.40 further provided the evidence that NO is involved in the helium-induced pre-conditioning and helium directly increases the production of NO in a NOS-dependent manner, rather than rely on I/R injury. Pretreatment with non-selective NOS inhibitor, N-nitro-L-arginine methyl ester (L-NAME), reduced the infarct size induced by helium. Neither inducible NOS (iNOS) antagonist aminoguanidine hydrochloride (AG) nor neuronal NOS (nNOS) inhibitor 7-nitroindazole (7-NI) had any effect on helium-induced cardioprotection. These studies suggest that eNOS can mediate helium preconditioning in rabbits but iNOS or nNOS cannot do it.
Late pre-conditioning
Huhn et al.47 demonstrated for the first time that helium can induce late pre-conditioning and reduce infarct size by approximately 40% compared with non-preconditioned myocardium. Late pre-conditioning was performed by administering 70%, 50%, 30% and 10% helium respectively for 15 minutes during 24 hours before I/R. The results showed that 70%, 50% and 30% helium significantly reduced infarct size from 55% in controls to 40%, 34%, and 37%. In contrast, no reduction in infarct size was observed at 10% helium. In other words, cardioprotection is maximal with administration of one cycle of 30% helium. Nevertheless, repetitive 30% helium inhalation subsequently 2–3 days before I/R did not further enhance the cardioprotective effects. The results showed that helium-mediated cardioprotection had a dose-dependent effect but not a time-dependent effect. The cyclooxygenase-2 (COX-2) inhibitor NS-398 completely eliminated 30% helium-mediated protection. It elucidated COX-2 is involved in helium-mediated late pre-conditioning.
Noteworthily, the above experimental studies simply indicated diversified kinases and their targets participated in helium-induce pre-conditioning by using their specific or non-specific blocking agents, but the actual expression and activity of these kinases remain to be measured and proved.48 How helium might affect these pro-survival kinases and enzymes and thus mediate cardioprotection is yet completely unknown.1
Post-conditioning by helium
Altered gene expression
Helium post-conditioning is the most clinically relevant and feasible form of conditioning, the investigation of which has becomes an area of scientist interest because ischemic events oftenoccur upon arrival of the patient in the hospital.49,50 In order to get more detailed mechanistic insights into the damage-ameliorating effects helium-induced during I/R, Oei et al.51 investigated helium post-conditioning by assessing cell damage and exploring the differential expression patterns of genes related to apoptosis, necrosis and autophagy following ischemia. The results revealed that 15 minutes of helium post-conditioning reduced the extent of I/R-induced cell damage and the beneficial effect was not observed under circumstance of 5 and 30 minutes of helium post-conditioning. Administration by 15 minutes of helium post-conditioning caused predominant up-regulation of genes involved in autophagy and inhibition of genes involved in apoptosis in comparison with I/R alone. It turned out that the reduction of cell damage that is at least partly induced by helium post-conditioning is mediated by the selective expression of genes that regulate programmed cell death.
Immune system
The underlying mechanisms of reperfusion-induced myocardial cell dysfunction are oxidative stress and an inflammatory burst.52,53 The innate immune system in I/R injury is a double-edged sword as a severe inflammatory burst is detrimental to the survival of cells but the inhibition of the innate immune system is accompanied with adverse consequences after myocardial infarction at the same time.54,55 Several processes occur during early reperfusion such as leukocyte activation and recruitment,56 cytokine and reactive oxygen species burst, and endothelial dysfunction are capable to influence cell viability.57
The influence of helium post-conditioning on infarct size and the I/R-induced immune response were explored by assessing the levels of protein and mRNA of pro-inflammatory cytokines. Rats inhaled 15, 30, or 60 minutes of 70% helium during reperfusion respectively. The results elucidated that 15 minutes of helium inhalation observably decrease infarct size from 43% to 21%, whereas 30 or 60 minutes of helium inhalation can hardly receive the similar profitable consequences. The protein levels of cytokine-induced neutrophil chemoattractant (CINC-3) and interleukin-1 beta (IL-1β) cytokine-induced were increased when employing 30 or 60 minutes of helium compared to control. The mRNA levels of CINC-3, IL-1β, interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) were all increased when exposed to 30 minutes of helium in the myocardial tissue not directly subjected to I/R. Above findings turned out the effectiveness of the helium post-conditioning was intimately dependent on the duration of helium administration.58 It is not clear that whether the higher levels of inflammatory cytokines is related to helium conditioning or just the causative of nature.
Caveolins
Caveolae, as the small flask-like invaginations of the cellular membrane, has been proven to be a subset of lipid rafts.33 And caveolins are structural proteins essential for the formation of caveolae. Caveolins contain three isoforms, including Cav-1, -2, -3, and a scaffolding domain (CSD), which plays a pivotal role in the regulation and localization of signaling molecules.59 The CSD is able to combined with several triggers, mediators of cardioprotective pathways known as RISK pathway proteins, such as PI3K, AKT, PKC isoforms, and ERK1/2.60 Caveolins are involved in mPTP transitioning through the binding of signaling molecules to the CSD.61 The three isoforms all are found in myocardial tissue62 and cardiac myocyte-specific over-expression of Cav-3 lead to the upregulation of survival kinases and therefore produced protection against I/R injury.63
In recent research, Flick et al.64 examined the role of the caveolin-associated RISK pathway in helium post-conditioning-induced cardioprotection in rat heart so as to explore the possible underlying mechanism. The findings indicated that: 1) changes in Cav-1 and Cav-3 localization only after 15 minutes of helium post-conditioning and no difference after 5 or 30 minutes; 2) 15 minutes of helium post-conditioning significantly increased accumulation of Cav-1 and Cav-3 in the membrane fraction of ischemic cardiac tissue but no difference in the mitochondrial fractions; 3) The serum analysis did not present any differences in the serum for Cav-1 levels, whereas Cav-3 showed increased amounts within 15 minutes of helium post-conditioning; 4) 15 minutes of helium post-conditioning activates RISK pathway kinases ERK1/2 and AKT. In conclusion the study points out that: 1) helium post-conditioning seems to induce protective effects shortly after the onset of reperfusion; 2) caveolins and RISK pathway might be crucially involved in helium post-conditioning mediated cardioprotection; 3) increased Cav-1 and -3 in the membrane fraction as well as the serum might indicate involvement of mitochondrial signaling.64 The results are contrast to another study that helium inhalation decreased caveolin-1 and caveolin-3 expressions after 24 hours in mice heart.65 One recent research suggested helium treatment induced secretion of Cav-1 and -3 into the serum,66 which is consistent with increased Cav-3 levels in the serum of current in vivo model in rats. Even though above studies employed different species and time periods, the consequences illuminated that secreted caveolin might potentially produce crucial protective effect on account of its availability in the whole body.
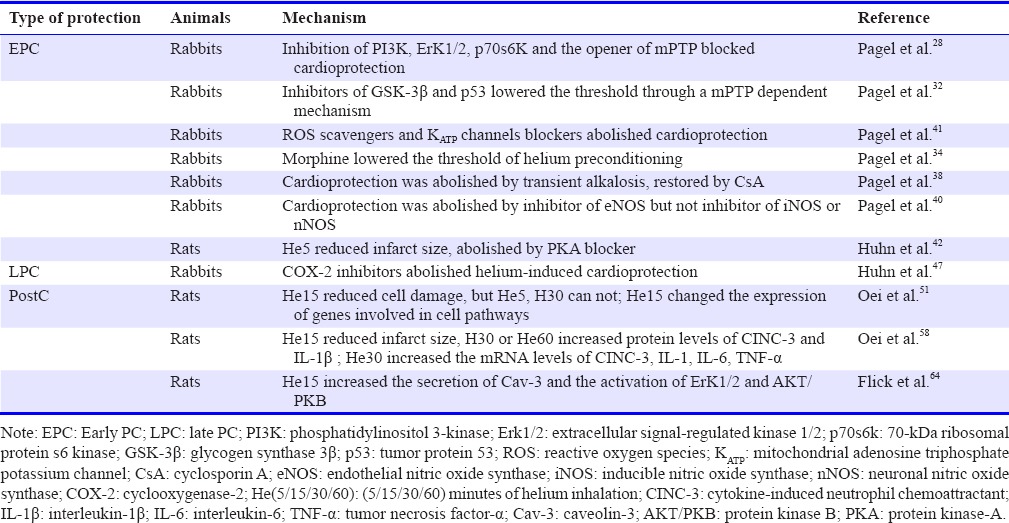
The experimental data for helium-induced cardioprotection are summarized in Table 1.
Table 1.
Summary of molecular mechanism of helium-induced pre-conditioning (PC) and post-conditioning (postC)
Disease animal models
All of above studies were carried out in healthy animals, whereas a majority of patients with heart disease are aged or suffer from multiple co-exiting chronic diseases such as hypertension and diabetes. The research in diseased animals is limited but still provides reliable guidance for us to convert basic experiments into clinical applications, the details are as follows.
Healthy Wistar and spontaneous hypertensive rats are both subjected to 25 minutes ischemia followed by 120 minutes reperfusion. The two kinds of rats are subjected to 70% helium inhalation for 15 minutes after index ischemia (PostC), or together with 15 minutes of helium 24 hours prior to ischemia (LPC + PostC), or a triple intervention with the addition of three repetitive, short cycles of 5 minutes helium inhalation before ischemia (EPC + LPC + PostC) respectively. We can conclude from the results that a triple intervention of helium conditioning could protect hypertensive myocardium against I/R injury, whereas a single intervention could not. The consequences showed that the existence of a “threshold” in hypertensive cardiac muscle, in which the combination of helium stimuli provide a more intense stimulus than each stimulus alone. No increased phosphorylation of GSK-β or PKC-ε were observed in these groups exposed to helium conditioning, indicating that helium-induced conditioning is not involved in GSK-β or PKC-ε pathway in diseased animals.49
The aging or diseased myocardium can not be protected by a single stimulus of helium conditioning was certified in the aged Wistar rats or pre-diabetic obese Zucker rats. The protective capability of helium pre-conditioning is entirely abolished in the obese Zucker rat, a extensively used animal model for pre-diabetic conditions of type 2 diabetes.50 An effect of helium on Erk1/2 and AKT phosphorylation was not detected and a depressed activity of GSK 3β was discovered in Zucker lean rats. Even through strengthening the stimulus of pre-conditioning could not overcome the threshold of reducing infarct size in the pre-diabetic heart. This may showed that only a combination of different conditioning stimuli at various time points could exert protection function in diseased myocardium. From previous data we can concluded that helium could induce mitochondrial uncoupling and generate pre-conditioning via the activation of mKCa channel. Nevertheless, above effects are eliminated in the senescent heart.42 The damage at the level of the mKCa channel or its upstream signaling molecules may explain the aging-related elimination of helium-induced pre-conditioning. Early research further indicated that the regulation of adenylylcyclase/PKA was a possible potential mechanism for the age-dependent loss of helium-induced cardioprotection.42
Clinical applications
The underlying mechanisms of cardioprotection helium-induced have been investigated extensively in animal experiments. However, mechanistic data from human studies are scarce.
In the latest clinical research, 125 patients undergoing coronary artery bypass grafting (CABG) surgery were allocated randomizedly to several groups include helium pre-conditioning via three cycles of helium inhalation for 5 minutes and subsequent 5 minutes inhalation of oxygen-enriched air, helium post-conditioning group receiving 15 minutes of helium inhalation before release of the aortic cross clamp, or the combination of both. The investigations indicated that the inability of helium conditioning to produce cardioprotection and no any statistically significant difference was observed with respect to the activation of p38 mitogen activated protein kinase (p38 MAPK), ERK 1/2 or levels of heat shock protein 27 (HSP27) and PKC-ε in human heart induced by helium pre-conditioning, post-conditioning or the combination of both. The result was in contradiction with the anterior experiments we demenstrated. Further more, helium pre- and post-conditioning did not affect postoperative troponin release at various time points postoperatively in patients undergoing CABG surgery.67
In an ealier study, the patients subjected to similar CABG surgery were administered helium inhalation (79%) for 5 minutes once for three sections prior to the start of cardiopulmonary bypass (He-Pre) or at the time when the perfusion was initiated (He-PostC). The result showed He-Pre or He-Post alone, and even the combination of both did not generate beneficial effects on the level of postoperatively troponin release.68
In one recent study investigating whether helium breathing in healthy volunteers affects the response capability of the human immune system in whole blood ex vivo, healthy male volunteers were administrated 30 minutes heliox (79% helium and 21% O2) or ordinary air respectively. Blood was withdrawn at various time points after helium breathing and subsequently received incubation with lipopolysaccharide (LPS), lipoteichoic acid (LTA), T-cell stimuli anti-CD3/anti-CD28 (TCS) or RPMI (as control) for different duration including 0, 2, 4 and 24 hours. The pro-inflammatory cytokines, TNF-α, IL-1β, IL-6, and chemokine, interleukin-8 (IL-8), were measured after stimulation with LPS and LTA. The study suggests that prolonged inhalation of helium did not produce any influence on the ability of the innate and early adaptive immune system to respond to immune stimuli. There were no differences among TNF-α, IL-1β, IL-6, IL-8, interferon-γ (IFN-γ) and interleukin-2 (IL-2) levels at different time points before and after helium inhalation in comparison to ordinary air inhalation.69 Consequently, helium can hardly exert influence on the responsiveness of immune system.
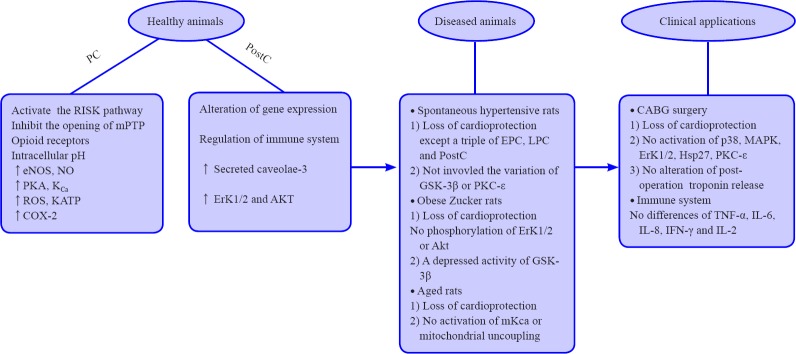
We systematically summarize the research advances obtained from a variety of basic experiments related to the helium-induced cardioprotection so as to get more comprehensive and concise knowledge. The relevant evidences are described as Figure 1.
Figure 1.
Research advances of helium-induced cardioprotection.
Note: PC: Pre-conditioning; EPC:early PC; LPC: late PC; PostC: post-conditioning; PI3K: phosphatidylinositol 3-kinase; Erk1/2: extracellular signal-regulated kinase 1/2; GSK-3β: glycogen synthase 3β; p38: tumor protein 38; MAPK: mitogen activated protein kinase; HSP: hot shock protein; PKC: protein Kinase C;ROS: reactive oxygen species; KATP: mitochondrial adenosine triphosphate potassium channel; CsA: cyclosporin A; eNOS: endothelial NO synthase; IL: interleukin; TNF-α: tumor necrosis factor-α; IFN: interferon.
Although above evidences have been sufficient to fully explain helium played a significant role in myocardial ischemic injury, whether helium inhalation would exert a negative influence on the human body caused more or less anxiety. For instance, when the body is completely enclosed in helium, helium with high thermal conductivity may cause heat loss and thus reduce metabolism, so the energy consumption of the body is lower in a helium-filled environment.70 What deserves our attention is that prolonging breathing by 75% of helium could induce hypothermia in rats.71 In addition, helium inhalation can caused distort voice because the density of helium is smaller than that of air and thus produce a higher resonance frequency to make the sound sharper.72 The above disadvantages may be a stumbling block for helium in the treatment of I/R injury, which makes people more hard to explore the most appropriate condition under which helium inhalation could minimize damage to the body.
CONCLUSION
From the above data we can conclude that multiple animal experiments suggest helium conditioning is associated with the inhibition of myocardial cell dysfunction and the reduction of myocardial I/R injury. The underlying mechanism of helium administration might involve the RISK pathway, the mPTP, the intracellular pH value, the caveolins and the immune system. The mechanisms by which these above-mentioned triggers, modulators and transducers generate favorable effects have not been completely elucidated, and abundant clinical researches are essential to prove their effectiveness and safety so as to translate the experimental data into clinical circumstances. As for how to alleviate the possible harm of helium inhalation on the organism, perhaps looking for the most suitable concentration, duration, timing and pause interval of helium inhalation is a good choice. In summary, helium is a promising alternative to protect the myocardium against I/R injury in the future investigations.
Footnotes
Funding: This work was supported by Suzhou Key Medical Center (No. Szzx201501), grants from the National Natural Science Foundation of China (No. 81571115, 81422013, and 81471196), Scientific Department of Jiangsu Province (No. BL2014045), Suzhou Government (No. SZS201413, SYS201608, and LCZX201601), Jiangsu Province (No. 16KJB320008, BK20160347, QNRC2016730).
Conflicts of interest
The authors declare that they have no competing interests.
Contributor agreement
A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check
This paper has been checked twice with duplicationchecking software iThenticate.
Peer review
A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewer
Wen-wu Liu, Second Military Medical University, China.
REFERENCES
- 1.Weber NC, Smit KF, Hollmann MW, Preckel B. Targets involved in cardioprotection by the non-anesthetic noble gas helium. Curr Drug Targets. 2015;16:786–792. doi: 10.2174/1389450116666150120104459. [DOI] [PubMed] [Google Scholar]
- 2.Preckel B, Schlack W. Inert gases as the future inhalational anaesthetics? Best Pract Res Clin Anaesthesiol. 2005;19:365–379. doi: 10.1016/j.bpa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Dong X, Oganov AR, Goncharov AF, et al. A stable compound of helium and sodium at high pressure. Nat Chem. 2017;9:440–445. doi: 10.1038/nchem.2716. [DOI] [PubMed] [Google Scholar]
- 4.Koblin DD, Fang Z, Eger EI, 2nd, et al. Minimum alveolar concentrations of noble gases, nitrogen, and sulfur hexafluoride in rats: helium and neon as nonimmobilizers (nonanesthetics) Anesth Analg. 1998;87:419–424. doi: 10.1097/00000539-199808000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Oei GT, Weber NC, Hollmann MW, Preckel B. Cellular effects of helium in different organs. Anesthesiology. 2010;112:1503–1510. doi: 10.1097/ALN.0b013e3181d9cb5e. [DOI] [PubMed] [Google Scholar]
- 6.Lique F, Kalugina Y, Chefdeville S, van de Meerakker SY, Costes M, Naulin C. Collisional excitation of O2 by H2: the validity of LTE models in interpreting O2 observations. Astron Astrophys. 2014;567:A22. [Google Scholar]
- 7.Schmidt G, Keesom WH. New measurements of liquid helium temperatures: I. The boiling point of helium. Physica. 1937;4:963–970. [Google Scholar]
- 8.Ulhôa CA, Larner L. Helium-oxigen (Heliox) mixture in airway obstruction. J Pediatr (Rio J) 2000;76:73–78. doi: 10.2223/jped.36. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran TE, Gamard S. Development of aerosol drug delivery with helium oxygen gas mixtures. J Aerosol Med. 2004;17:299–309. doi: 10.1089/jam.2004.17.299. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Cui C, Yang X, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke? Transl Stroke Res. 2017 doi: 10.1007/s12975-017-0520-z. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigham MT, Nowak JE, Wheeler DS. Therapeutic application of helium-oxygen and mechanical ventilation in a child with acute myelogenous leukemia and airway obstruction. Pediatr Emerg Care. 2009;25:469–472. doi: 10.1097/PEC.0b013e3181aba7de. [DOI] [PubMed] [Google Scholar]
- 12.Hu HJ, Fan DF, Lv Y, et al. Effects of simulated heliox diving at high altitudes on blood cells, liver functions and renal functions. Undersea Hyperb Med. 2013;40:329–337. [PubMed] [Google Scholar]
- 13.Uchino H, Suzuki M, Okita A, et al. Organ protective effects of volatile anesthetics and perioperative outcomes. Masui. 2012;61:478–495. [PubMed] [Google Scholar]
- 14.Gainnier M, Forel JM. Clinical review: use of helium-oxygen in critically ill patients. Crit Care. 2006;10:241. doi: 10.1186/cc5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucchinetti E, Wacker J, Maurer C, et al. Helium breathing provides modest antiinflammatory, but no endothelial protection against ischemia-reperfusion injury in humans in vivo. Anesth Analg. 2009;109:101–108. doi: 10.1213/ane.0b013e3181a27e4b. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Zhang L, Manaenko A, Ye Z, Liu W, Sun X. Helium preconditioning protects mouse liver against ischemia and reperfusion injury through the PI3K/Akt pathway. J Hepatol. 2014;61:1048–1055. doi: 10.1016/j.jhep.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Rizvi M, Jawad N, Li Y, Vizcaychipi MP, Maze M, Ma D. Effect of noble gases on oxygen and glucose deprived injury in human tubular kidney cells. Exp Biol Med (Maywood) 2010;235:886–891. doi: 10.1258/ebm.2010.009366. [DOI] [PubMed] [Google Scholar]
- 18.Heinen A, Huhn R, Smeele KM, et al. Helium-induced preconditioning in young and old rat heart: impact of mitochondrial Ca(2+) -sensitive potassium channel activation. Anesthesiology. 2008;109:830–836. doi: 10.1097/ALN.0b013e3181895aa0. [DOI] [PubMed] [Google Scholar]
- 19.Ahn JY, Tae HJ, Cho JH, et al. Activation of immediate-early response gene c-Fos protein in the rat paralimbic cortices after myocardial infarction. Neural Regen Res. 2015;10:1251–1257. doi: 10.4103/1673-5374.162757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern KB, Hanna JM, Young HN, et al. Importance of both early reperfusion and therapeutic hypothermia in limiting myocardial infarct size post-cardiac arrest in a porcine model. JACC Cardiovasc Interv. 2016;9:2403–2412. doi: 10.1016/j.jcin.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 21.DeFily DV, Chilian WM. Preconditioning protects coronary arteriolar endothelium from ischemia-reperfusion injury. Am J Physiol. 1993;265:H700–706. doi: 10.1152/ajpheart.1993.265.2.H700. [DOI] [PubMed] [Google Scholar]
- 22.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011;8:619–629. doi: 10.1038/nrcardio.2011.85. [DOI] [PubMed] [Google Scholar]
- 23.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Li X, Wang H, et al. Later phase cardioprotection of ischemic post-conditioning against ischemia/reperfusion injury depends on iNOS and PI3K-Akt pathway. Am J Transl Res. 2015;7:2603–2611. [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Xi L. Postconditioning of ischemic heart by intermittent ventricular pacing at the beginning of reperfusion: novel mechanisms and potential utilities in interventional cardiology settings. Am J Physiol Heart Circ Physiol. 2016;310:H1–3. doi: 10.1152/ajpheart.00835.2015. [DOI] [PubMed] [Google Scholar]
- 26.Lupi Herrera E, Gaspar J, Gonzalez Pacheco H, et al. Reperfusion and postconditioning in acute ST segment elevation myocardial infarction. A new paradigm for the treatment of acute myocardial infarction? From bench to bedside. Arch Cardiol Mex. 2006;76(Suppl 4):S76–101. [PubMed] [Google Scholar]
- 27.Sandu N, Schaller B. Postconditioning: a new or old option after ischemic stroke? Expert Rev Cardiovasc Ther. 2010;8:479–482. doi: 10.1586/erc.09.180. [DOI] [PubMed] [Google Scholar]
- 28.Pagel PS, Krolikowski JG, Shim YH, et al. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105:562–569. doi: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- 29.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 30.Juhaszova M, Zorov DB, Kim SH, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Pagel PS, Krolikowski JG, Pratt PF, Jr, et al. Inhibition of glycogen synthase kinase or the apoptotic protein p53 lowers the threshold of helium cardioprotection in vivo: the role of mitochondrial permeability transition. Anesth Analg. 2008;107:769–775. doi: 10.1213/ane.0b013e3181815b84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70:212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Pagel PS, Krolikowski JG, Amour J, Warltier DC, Weihrauch D. Morphine reduces the threshold of helium preconditioning against myocardial infarction: the role of opioid receptors in rabbits. J Cardiothorac Vasc Anesth. 2009;23:619–624. doi: 10.1053/j.jvca.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitakaze M, Weisfeldt ML, Marban E. Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts. J Clin Invest. 1988;82:920–927. doi: 10.1172/JCI113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitakaze M, Takashima S, Funaya H, et al. Temporary acidosis during reperfusion limits myocardial infarct size in dogs. Am J Physiol. 1997;272:H2071–2078. doi: 10.1152/ajpheart.1997.272.5.H2071. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 38.Pagel PS, Krolikowski JG. Transient metabolic alkalosis during early reperfusion abolishes helium preconditioning against myocardial infarction: restoration of cardioprotection by cyclosporin A in rabbits. Anesth Analg. 2009;108:1076–1082. doi: 10.1213/ane.0b013e318193e934. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–943. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Pagel PS, Krolikowski JG, Pratt PF, Jr, et al. The mechanism of helium-induced preconditioning: a direct role for nitric oxide in rabbits. Anesth Analg. 2008;107:762–768. doi: 10.1213/ane.0b013e3181815995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagel PS, Krolikowski JG, Pratt PF, Jr, et al. Reactive oxygen species and mitochondrial adenosine triphosphate-regulated potassium channels mediate helium-induced preconditioning against myocardial infarction in vivo. J Cardiothorac Vasc Anesth. 2008;22:554–559. doi: 10.1053/j.jvca.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huhn R, Weber NC, Preckel B, et al. Age-related loss of cardiac preconditioning: impact of protein kinase A. Exp Gerontol. 2012;47:116–121. doi: 10.1016/j.exger.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 45.Feng J, Fischer G, Lucchinetti E, et al. Infarct-remodeled myocardium is receptive to protection by isoflurane postconditioning: role of protein kinase B/Akt signaling. Anesthesiology. 2006;104:1004–1014. doi: 10.1097/00000542-200605000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Ping P, Takano H, Zhang J, et al. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res. 1999;84:587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- 47.Huhn R, Heinen A, Weber NC, et al. Helium-induced late preconditioning in the rat heart in vivo. Br J Anaesth. 2009;102:614–619. doi: 10.1093/bja/aep042. [DOI] [PubMed] [Google Scholar]
- 48.Oei GT, Weber NC, Hollmann MW, Preckel B. Cellular effects of helium in different organs. Anesthesiology. 2010;112:1503–1510. doi: 10.1097/ALN.0b013e3181d9cb5e. [DOI] [PubMed] [Google Scholar]
- 49.Oei GT, Huhn R, Heinen A, et al. Helium-induced cardioprotection of healthy and hypertensive rat myocardium in vivo. Eur J Pharmacol. 2012;684:125–131. doi: 10.1016/j.ejphar.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 50.Huhn R, Heinen A, Weber NC, et al. Helium-induced early preconditioning and postconditioning are abolished in obese Zucker rats in vivo. J Pharmacol Exp Ther. 2009;329:600–607. doi: 10.1124/jpet.108.149971. [DOI] [PubMed] [Google Scholar]
- 51.Oei GT, Heger M, van Golen RF, et al. Reduction of cardiac cell death after helium postconditioning in rats: transcriptional analysis of cell death and survival pathways. Mol Med. 2015;20:516–526. doi: 10.2119/molmed.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girn HR, Ahilathirunayagam S, Mavor AI, Homer-Vanniasinkam S. Reperfusion syndrome: cellular mechanisms of microvascular dysfunction and potential therapeutic strategies. Vasc Endovascular Surg. 2007;41:277–293. doi: 10.1177/1538574407304510. [DOI] [PubMed] [Google Scholar]
- 53.Leichtweis S, Ji LL. Glutathione deficiency intensifies ischaemia-reperfusion induced cardiac dysfunction and oxidative stress. Acta Physiol Scand. 2001;172:1–10. doi: 10.1046/j.1365-201X.2001.00820.x. [DOI] [PubMed] [Google Scholar]
- 54.Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–283. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 55.Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 56.Ritter LS, Wilson DS, Williams SK, Copeland JG, McDonagh PF. Early in reperfusion following myocardial ischemia, leukocyte activation is necessary for venular adhesion but not capillary retention. Microcirculation. 1995;2:315–327. doi: 10.3109/10739689509148276. [DOI] [PubMed] [Google Scholar]
- 57.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8:292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 58.Oei GT, Aslami H, Kerindongo RP, et al. Prolonged helium postconditioning protocols during early reperfusion do not induce cardioprotection in the rat heart in vivo: role of infiammatory cytokines. J Immunol Res 2015. 2015 doi: 10.1155/2015/216798. 216798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Fridolfsson HN, Roth DM, Insel PA, Patel HH. Regulation of intracellular signaling and function by caveolin. FASEB J. 2014;28:3823–3831. doi: 10.1096/fj.14-252320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel HH, Head BP, Petersen HN, et al. Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol. 2006;291:H344–350. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 63.Horikawa YT, Patel HH, Tsutsumi YM, et al. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;44:123–130. doi: 10.1016/j.yjmcc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flick M, Albrecht M, Oei GT, et al. Helium postconditioning regulates expression of caveolin-1 and -3 and induces RISK pathway activation after ischaemia/reperfusion in cardiac tissue of rats. Eur J Pharmacol. 2016;791:718–725. doi: 10.1016/j.ejphar.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Weber NC, van de Vondervoort D, Niesman IR, et al. Effects of noble gas conditioning on Caveolin expression in the rat heart in vivo. FASEB J. 2012;26:1114–17. [Google Scholar]
- 66.Weber NC, Schilling JM, Finley JC, et al. Helium inhalation induces caveolin secretion to blood. FASEB J. 2013;27:1089–3. [Google Scholar]
- 67.Smit KF, Brevoord D, De Hert S, et al. Effect of helium pre- or postconditioning on signal transduction kinases in patients undergoing coronary artery bypass graft surgery. J Transl Med. 2016;14:294. doi: 10.1186/s12967-016-1045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smit KF, Brevoord D, de Hert SG, Hollmann MW, Weber NC. Helium induced pre and postconditioning in patients subjected to coronary artery bypass graft (CABG) surgery: 4AP15. Eur J Anaesthesiol. 2012;29:53. [Google Scholar]
- 69.Oei GT, Smit KF, vd Vondervoort D, et al. Effects of helium and air inhalation on the innate and early adaptive immune system in healthy volunteers ex vivo. J Transl Med. 2012;10:201. doi: 10.1186/1479-5876-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singer D. Why 37 degrees C? Evolutionary fundamentals of thermoregulation. Anaesthesist. 2007;56:899–902. doi: 10.1007/s00101-007-1220-y. 904-896. [DOI] [PubMed] [Google Scholar]
- 71.David HN, Haelewyn B, Chazalviel L, et al. Post-ischemic helium provides neuroprotection in rats subjected to middle cerebral artery occlusion-induced ischemia by producing hypothermia. J Cereb Blood Flow Metab. 2009;29:1159–1165. doi: 10.1038/jcbfm.2009.40. [DOI] [PubMed] [Google Scholar]
- 72.Montgomery C. Why does inhaling helium make one’s voice sound strange? Sci Am. 2004;291:122. [PubMed] [Google Scholar]