Abstract
Despite years of research, treatment of traumatic brain injury (TBI) remains challenging. Considerable data exists that some volatile anesthetics might be neuroprotective. However, several studies have also revealed a rather neurotoxic profile of anesthetics. In this study, we investigated the effects of argon 50%, desflurane 6% and their combination in an in vitro TBI model with incubation times similar to narcotic time slots in a daily clinical routine. Organotypic hippocampal brain slices of 5- to 7-day-old mice were cultivated for 14 days before TBI was performed. Slices were eventually incubated for 2 hours in an atmosphere containing no anesthetic gas, argon 50% or desflurane 6% or both. Trauma intensity was evaluated via fluorescent imagery. Our results show that neither argon 50% nor desflurane 6% nor their combination could significantly reduce the trauma intensity in comparison to the standard atmosphere. However, in comparison to desflurane 6%, argon 50% displayed a rather neuroprotective profile within the first 2 hours after a focal mechanical trauma (P = 0.015). A 2-hour incubation in an atmosphere containing both gases, argon 50% and desflurane 6%, did not result in significant effects in comparison to the argon 50% group or the desflurane 6% group. Our findings demonstrate that within a 2-hour incubation time neither argon nor desflurane could affect propidium iodide-detectable cell death in an in vitro TBI model in comparison to the standard atmosphere, although cell death was less with argon 50% than with desflurane 6%. The results show that within this short time period processes concerning the development of secondary injury are already taking place and may be manipulated by argon.
Keywords: argon, desflurane, traumatic brain injury, neuroprotection, organotypic hippocampal brain slices, in vitro model, secondary injury, propidium iodide
INTRODUCTION
It has been estimated that over 1,600,000 patients annually sustain a traumatic brain injury (TBI) in the European Union. Of these patients, 70,000 die and 100,000 suffer from disabilities. The majority of victims are children and young adults.1 Worldwide, TBI is the main cause of death and disability among the young.2 This also entails massive economic implications as demonstrated by Finkelstein et al.,3 who consider the lifetime costs of TBI in the United States to amount to $60 billion a year (total lifetime cost of TBI in 2000). Gustavsson et al.4 and Olesen et al.5 scrutinized the economic cost of brain disorders in Europe in 2010. Total annual cost for TBI was estimated to be €33 billion (direct health care costs, direct non-medical costs and indirect costs). Until now, treatment of TBI remains difficult due to the multitude of trauma mechanisms and the limited knowledge of pathological pathways.6,7,8
In the past few years, several studies have investigated the potentially protective and toxic effects of volatile anesthetics on the developing brain. On the one hand, they have revealed mounting evidence of potentially detrimental effects of inhaled anesthetics on the developing brain.9,10,11,12,13 On the other hand, considerable data exists that especially specific noble gases might act in a neuroprotective manner in models of hypoxic ischemia and TBI.6,9,14,15,16,17
While argon 50% has been shown to be effective in a TBI model involving incubation periods of 72 hours, desflurane 6% displayed a rather neurotoxic profile in a similarly conducted study.6,18 However, the amount of data available regarding argon’s and desflurane’s effects on cells of the developing brain, which take incubation periods similar to narcotic time slots in a daily clinical routine into account, are sparse. Therefore, we tested the effects of argon in an in vitro model of TBI in the developing brain after a clinically relevant incubation time of 2 hours and compared the results to those of desflurane. We also explored how argon’s potentially neuroprotective effects would interfere with desflurane’s rather neurotoxic impact.
MATERIALS AND METHODS
Ethics statement
This study was approved by the local Institutional Ethical Review Committee and the animal protection representative at the Institute of Animal Research at the RWTH Aachen University Hospital according to the German animal protection law (TierSchG §4, III).
Organotypic hippocampal slices
The organotypic hippocampal slices (OHBSs) were obtained from pups of 5- to 7-day-old mice (C57BL/6N, Charles River Laboratories, Sulzfeld, Germany) and cultured as previously described (Additional Table 1 (81.4KB, pdf) ).6,7,18,19,20,21,22
Different media used for culturing of the slices
The cell cultures were incubated for 14 days with the growth medium (GM; Additional Table 1 (81.4KB, pdf) ) being changed 24 hours after preparation and thereafter regularly every 3 days.
TBI protocol
After the incubation period, the GM was exchanged by experimental medium (EM; Additional Table 1 (81.4KB, pdf) ) containing propidium iodide (PI). Eventually, baseline fluorescence imaging took place. Slices were then randomly assigned to four non-trauma and four trauma groups in which no intervention (standard atmosphere) and the three respective atmospheric interventions took place (Additional Table 2 (50.6KB, pdf) ).
Groups according to the protocol
In the TBI groups, TBI was performed as described below and EM was exchanged followed by the incubation of the tissue cultures at 37°C in the respective atmosphere for 2 hours. In the non-trauma groups, EM was exchanged as well before the slices were incubated.
TBI was induced with an apparatus in a similar way to the procedure described by other researchers.6,7,18,23 A round metal stylus positioned 7 mm above the hippocampus slice was dropped onto the slice positioned at a marked spot. After the incubation period, final fluorescence images were taken.
Microscopy and assessment of cell death
PI, a dyeing agent that rapidly enters cells with damaged membranes and binds to their DNA, was used to assess the proportion of dead cells.24 Baseline and final fluorescence images were captured with a fluorescence microscope and MetaVue software (MetaVue, Molecular Devices, Sunnyvale, CA, USA).
The generated images were analyzed with Image J software (National Institutes of Health, Bethesda, MD, USA). A histogram of the red channel was generated for each image, showing the sum of all pixels sharing the same gray scale value from 0 to 255. As previous studies have shown, values below a threshold of 100 are caused by background fluorescence.6,21,22 That is why only the values of pixels above this threshold were summed up to evaluate the extent of cell injury. The extent of cell injury amounts to the integrated area under the histogram curve exceeding the threshold as demonstrated in Figure 1. The damage of a slice was thus represented in the sum of pixels of its image.
Figure 1.
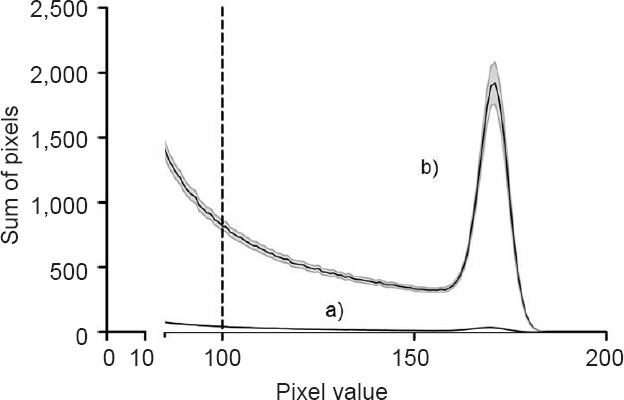
Histogram of traumatized versus non-traumatized organotypic hippocampal brain slices.
Note: This figure shows the difference in histograms of traumatized in comparison to non-traumatized slices in the no-intervention groups. Curve a) presents the histogram of non-traumatized slices and curve b) presents the histogram of traumatized slices. Standard error of the mean in length means standard error of the mean. The black line equals the mean value and the grey lines show the scanning electron microscope. The values of pixels above the established threshold (grey scale value of 100) were summed up to evaluate the extent of cell injury. The extent of cell injury amounts to the integrated area under the histogram curve exceeding the threshold. This figure is also part of another publication of ours (unpublished).
Statistical analysis
In each group of slices, the mean value ± standard error of the mean of the sum of pixels was calculated using SPSS 22.0 (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA). The trauma no intervention (standard atmosphere) group served as a reference value, its mean value being normalized to unity.
One-way analysis of variance (ANOVA) was applied to investigate whether the administration of gases resulted in an effect in the trauma and non-trauma groups. In a next step, student’s t-test was used post hoc to determine the exact differences in between the different groups. P values < 0.05 were considered statistically significant.
In order to enhance comparability and reproducibility of our study we defined necessary checkpoints and developed specific inclusion and exclusion criteria which are presented and discussed in a different paper (unpublished). In short, pre-damaged slices were identified and restrained from entering into the experiment, and unrealistic outliers were detected and eventually excluded as they might distort the final outcome: First, if baseline image histograms already presented more than 1,000 pixels, they were sorted out as they were considered to be pre-damaged. We observed specific morphological peculiarities, such as “holes”, “frayed margins” or “emigrated cells”. As we did know why they occurred, we still included these images if the threshold was met. Some slices presented with a prominent margin. If a margin amounted to more than 250 pixels, this was regarded as a margin stemming from preparation errors and the pixels of the margin were not taken into consideration. In the non-trauma groups, if the number of pixels after incubation was lower than before, it was concluded that the picture taken was not sharp enough and the slices were excluded. Within the course of the experiment we observed that trauma intensities varied over time. Thus, we saw the necessity to define a scope of trauma strength. A boxplot analysis was performed for the trauma no intervention group (group 5; Additional Table 2 (50.6KB, pdf) ) and extreme outliers were identified (n = 2) and excluded. The new mean value of this group plus 2× the standard deviation served as an upper threshold for all the other trauma groups. In non-trauma groups there was no reason for a set scope as no manual action was taken and no considerable differences between groups were expected. In these groups, a boxplot analysis was performed separately for each group and outliers were excluded. We subjectively excluded 11 slices that were too small to be considered as the hippocampus and 7 slices that were not clearly hit by the pin. 61% of all 1,459 slices met the proposed inclusion criteria.
RESULTS
Our results demonstrate that the trauma induced significant PI-detectable cell death (P < 0.001, no-intervention trauma vs. no-intervention non-trauma group; Figure 2). We analyzed a total of 93 slices in the trauma standard atmosphere group, 136 slices in the trauma argon 50% group, 60 slices in the trauma argon 50%/desflurane 6% group and 52 slices in the trauma desflurane 6% group. In the non-trauma groups analogously, 171 slices in the standard atmosphere group, 166 slices in the argon 50% group, 92 slices in the argon 50%/desflurane 6% group and 118 slices in the desflurane 6% group were taken into consideration.
Figure 2.
Trauma intensity in traumatized vs. non-traumatized organotypic hippocampal brain slices.
Note: Slices were subjected to a focal trauma and incubated in the respective atmosphere for 2 hours. In the non-trauma groups no traumatic brain injury was performed. After 2 hours, fluorescence images were taken and the trauma intensity was quantified as described under microscopy and assessment of cell death. The trauma induced resulted in a significant increase of trauma intensity in comparison to the slices not subjected to a trauma (*P < 0.001, no-intervention trauma group vs. no-intervention non-trauma group).
Within the four trauma groups, one-way ANOVA did not show a significant effect (P = 0.07). Student’s t-test revealed no differences of PI-detectable cell death except for the trauma desflurane 6% group vs. the trauma argon 50% group (P = 0.015). The difference between the trauma desflurane 6% group and the trauma standard atmosphere group was not statistically significant (P = 0.058; Figure 3). A 2-hour incubation in an atmosphere containing argon 50% and desflurane 6% did not result in a significant effect in comparison to the other groups (argon 50%/desflurane 6% vs. standard atmosphere: P = 0.324; argon 50%/desflurane 6% vs. argon 50%: P = 0.144; argon 50%/desflurane 6% vs. desflurane 6%: P = 0.304).
Figure 3.
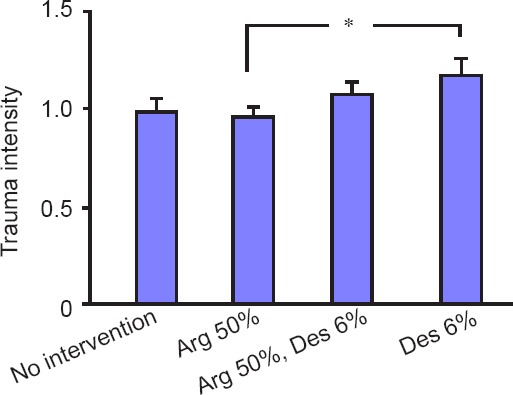
Trauma intensity of traumatized organotypic hippocampal brain slices incubated for 2 hours with standard atmosphere or argon (Arg) 50% or desflurane (Des) 6% or both.
Note: 93 slices in the trauma standard atmosphere group, 136 slices in the trauma Arg 50% group, 60 slices in the trauma Arg 50%/Des 6% group and 52 slices in the trauma Des 6% group were considered. One-way analysis of variance did not show a significant difference within the trauma groups (P = 0.070). The difference between the trauma Arg 50% group and trauma Des 6% group was significant (*P = 0.015). The difference between the trauma standard atmosphere group and the trauma Des 6% group was not significant (P = 0.058). The combination of Arg 50% and Des 6% did not result in significant differences in comparison to the other groups.
Within the four non-trauma groups, one-way ANOVA showed no significant effect either (P = 0.056). Differences determined by student’s t-test showed a slightly significant difference between the non-trauma argon 50% group and the non-trauma desflurane 6% group (P = 0.047). The difference between the non-trauma desflurane 6% group and the non-trauma standard atmosphere group was statistically not significant (P = 0.075; Figure 4). In the non-trauma groups an incubation in an atmosphere containing argon 50% and desflurane 6% did not result in a significant effect in comparison to the other groups (argon 50%/desflurane 6% vs. standard atmosphere: P = 0.229; argon 50%/desflurane 6% vs, argon 50%: P = 0.139; argon 50%/desflurane 6% vs. desflurane 6%: P = 0.372).
Figure 4.
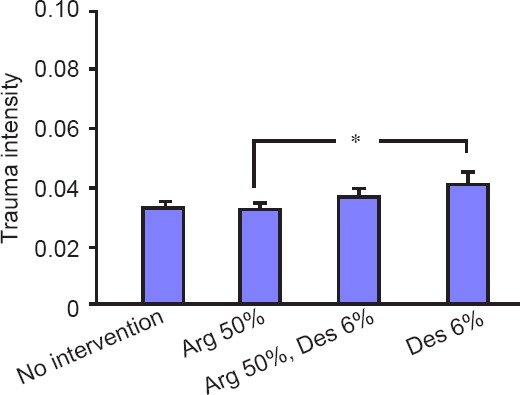
Trauma intensity of non-traumatized organotypic hippocampal brain slices incubated for 2 hours with standard atmosphere or argon (Arg) 50% or desflurane (Des) 6% or both.
Note: In the non-trauma groups, 171 slices in the standard atmosphere group, 166 slices in the Arg 50% group, 92 slices in the Arg 50%/Des 6% group and 118 in the Des 6% group were analyzed. One-way analysis of variance did not show a significant difference within the non-trauma groups (P = 0.056). The difference between the non-trauma Arg 50% group and the non-trauma Des 6% was slightly significant (*P = 0.047). The difference between the non-trauma standard atmosphere group and the non-trauma Des 6% group was not significant (P = 0.075). The combination of Arg 50% and Des 6% did not result in significant differences in comparison to the other groups.
DISCUSSION
We investigated the effects of a 2-hour exposure of argon and desflurane alone as well as combined in an in vitro model of TBI on hippocampus brain slices. Our results show that there is no difference in trauma intensity when comparing groups of different gas administrations to the standard atmosphere group. However, when comparing argon 50% to desflurane 6% administration, a neuroprotective effect of argon 50% could be demonstrated. The combination of argon 50% and desflurane 6% did not result in significant differences in comparison to the argon 50% group or the desflurane 6% group.
Previously, in vitro studies were conducted with incubation periods of up to 72 hours assuming that the neuroprotective effect of gases unfolds when the secondary injury emerges: Loetscher et al.6 found that argon significantly reduces cell damage in an in vitro model of TBI or oxygen-glucose deprivation (OGD) when applying argon 25%, 50% and 74% for 72 hours after inducing a trauma. Within the TBI set-up, the largest impact was seen in the group exposed to argon 50%. Harris et al.17 confirmed a neuroprotective effect of argon 50% in a similar TBI model. At 24, 48 and 72 hours, argon 50% reduced the advancement of secondary injury. It was also shown that the injury followed by a mechanical trauma on mice hippocampal slices developed to 32 ± 4% of total injury by one hour and to 51 ± 8% by 6 hours (total injury present at 72 hours).17 This underlines the importance of the initial time slot right after a trauma with regards to the development of secondary injury.
Surprisingly, there is hardly any data to be found on in vitro studies investigating the effect of gases in combination with a shorter exposure time in the first critical hours after a mechanical trauma takes place.
In a study similar to ours, Krings et al.18 showed that incubation for 2 hours with desflurane 2% and 4% and 6% after performing a trauma resulted in no difference between the groups. However, an incubation time of 24 hours showed a significant increase of trauma intensity in the desflurane 4% and 6% groups compared to the control group. This development was preeminent in the desflurane 6% group.
In our study, we chose an incubation period of only 2 hours. Thus, we aimed at investigating cell injury in the early and critical time period after a trauma. Furthermore, this study design provided a more adequate simulation of the clinical setting. To date, it is not clear how even short periods of anesthesia affect the developing brain of the young. Jevtovi-Todoro25 pointed to a “therapeutic dilemma” explaining that anesthetics on the one hand have displayed a neuroprotective profile during focal and global brain ischemia but on the other hand also have induced developmental neuroapoptosis. It is of far reaching interest to explore potential neuroprotective and injurious side effects of anesthetics.9,10,13
Our results suggest that within an incubation period of 2 hours neither argon nor desflurane could display a definite neuroprotective or neuro-harming profile in a hippocampal TBI model compared to the standard atmosphere. However, when comparing argon 50% to desflurane 6%, a tendency towards neuro-protectivity related to argon was observed suggesting that argon’s neuroprotective impact as demonstrated by Loetscher and Harris,6,17 may start to take place within the first 2 hours. Our findings regarding the effect of desflurane are consistent with Krings et al.‘s18 result that a 2-hour incubation period does not significantly affect the development of secondary injury in comparison to the control group.
Loetscher et al.6 confirmed a neuroprotective effect even if argon administration set for a total of 72 hours was delayed by 2 or 3 hours. Trauma intensity that developed within the third hour was significantly higher in comparison to a 0 and 2 hours delay.6 This could hint at the importance of the third hour after a mechanical trauma took place or at an exponential development of emerging secondary injury. Considering our results and Harris et al.‘s17 findings, it would be appropriate to test argon’s and desflurane’s impact for 3–6 hours.
Hypothesizing that there were differences in the two gases’ neuroprotective impact, our study also aimed at scrutinizing the effect when administering argon and desflurane together. Neither argon nor desflurane seemed to dominate the overall effect as no significant differences were seen among the groups.
A lot of information on in vitro studies originates from OGD models, e.g., it was shown that “postischemic” argon reduces lactic dehydrogenase levels in such a set-up.26 As for desflurane, it was found that preconditioning generated a neuroprotective effect.27 A protocol on cortical cells demonstrated a neuroprotective effect when applying desflurane before and throughout OGD.28
Up until now argon’s and desflurane’s mechanisms of action are not fully understood.14,29,30,31 Action at GABAA receptors is discussed to be a possible neuroprotective component; however, it remains unclear whether argon’s neuro-protectivity evolves along these lines.14,29,32,33,34 In a systematic review on argon and its effects as a neuroprotectant, it is summarized that impact on further signaling as Bcl-2 involvement, ERK 1/2-signaling and argon’s oxygen-like properties might play a decisive role whereas N-methyl-D-aspartic acid (NMDA)-receptor signaling or action at potassium channels seem to be less relevant.14,17,26,35,36,37,38,39
Although we could not confirm a neuroprotective effect of desflurane in our TBI-model, several researchers could demonstrate a positive impact. Wang et al.27 suggested that involvement of excitatory amino acid transporters may be one of the mechanisms responsible for the positive preconditioning effect of desflurane. Recently, it was also shown that desflurane caused an increase of excitatory amino-acid carrier 1 activity in Xenopus oocytes suggesting that this may be a mechanism for the potential neuroprotective effect.40 Schallner et al.41 could show that whereas isoflurane postexposure aggravated neuronal cell injury in an in vitro model in pre-injured SH-SY5Y cells and in an in vivo retinal ischemia-reperfusion injury model through activation of p75NTR and NF-κB, desflurane postexposure, which was tested in vitro, did not increase neuronal cell injury. Desflurane postexposure reduced NF-κB DNA-binding activity and was not associated with an increase in NF-κB-dependent gene expression.41 In their review, Deng et al.28,42,43,44 explained that possible mechanisms besides an improved cerebral blood flow and oxygen supply might implicate reduction of sympathetic activity, inhibition of neuronal apoptosis and preservation of mitochondrial function.
To our knowledge, there is no data to be found on argon or desflurane in an in vivo TBI model. However, neuroprotection by argon could be confirmed in several other in vivo models warranting further investigation in a TBI protocol. Interestingly, argon administration in in vivo models already seems to result in significantly positive effects in a shorter time period: Brücken et al.45 showed that a single 1-hour application of argon 70% in rodents subjected to cardiac arrest led to significantly better results in the neurological deficit score and a significant reduction in the neuronal damage index in the CA3/4 region of the hippocampus and in the neocortex. In an in vivo rat model of transient middle cerebral artery occlusion, treatment with argon 50% for 60 minutes resulted in a significant reduction in infarct size and composite adverse outcomes.46 Recently, it was also shown that argon improved mortality and quantity of vital hippocampal neurons in rats after experimental subarachnoid hemorrhage.47
As for desflurane, the neuroprotective influence of preconditioning demonstrated in in vitro experiments could be transferred to an in vivo setting. 9-day-old mice undergoing 3 hours of preconditioning with desflurane, isoflurane or sevoflurane 24 hours before being exposed to 60 minutes of hypoxia-ischemia performed better in certain behavioral tests. Histologically, preconditioning did not significantly affect injury scores.48 Moreover, post-ischemic desflurane administration in rats subjected to incomplete cerebral ischemia was investigated and it was found that, when evaluating injury in the early recovery period, histopathological injury and ischemic neurons could thus be reduced.49 On the other hand, a study comparing apoptotic cell death in neocortical neurons of neonatal mice exposed to desflurane, isoflurane or sevoflurane for 6 hours, revealed a similar neurotoxic profile for all three anesthetics.50
LIMITATIONS
In general, in vitro experiments can only to some extent imitate the in-vivo conditions. Vital parameters such as blood pressure or oxygenation cannot be considered. Furthermore, it is impossible to evaluate whether effects seen histologically in one area of the brain are of relevance to the living organism, i.e., with regards to performance of cognition. However, OHBSs provide a useful tool to simulate the in vivo state enabling investigations into mechanisms of brain synapses.51,52,53,54,55,56 The model we worked with has successfully been used in different settings to testing other gases or substances and incubation times.6,7,18,19,21,22 As OHBSs are very sensitive and can be influenced by many factors, variation in an OHBS TBI model should be taken into consideration. To our knowledge, other researchers have not described how exactly they dealt with i.e., pre-damage. The eligibility criteria mentioned above are discussed in detail in a different paper (unpublished). On the one hand, the criteria present a first approach to cope with the variation in such a TBI model as we could objectively set the same requirements for all groups. On the other hand, several slices were sorted out as they did not meet the inclusion criteria. In order to avoid subjective choices, we recommend setting a defined size prior to the experiment that has to be met concerning the hippocampi and the area of TBI. The morphological peculiarities should be scrutinized to determine whether electrophysiological consequences are entailed.
CONCLUSION
Our findings demonstrate that within a 2-hour incubation time neither argon nor desflurane could affect PI-detectable cell death in an in vitro TBI model in comparison to the standard atmosphere, although cell death was less with argon than with desflurane. These results demonstrate that within this short time period processes concerning the development of secondary injury have already taken place and may be manipulated by argon. Taking into consideration that previous in vivo studies investigating other diseases than TBI have shown that shorter exposure periods result in significantly different outcomes, further investigation into the first hours after TBI is warranted.
Acknowledgments
We thank Prof. Joachim Weis, head of the Department of Neuropathology, RWTH Aachen University Hospital, Aachen, Germany and his team for support in the culturing of the slices.
Footnotes
Conflicts of interest
None declared.
Research ethics
The study protocol was approved by the local Institutional Ethical Review Committee at Aachen, Germany. The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE). All efforts were made to minimize the number and suffering of the animals used in the experiments. The paper was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Contributor agreement
A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check
This paper has been checked twice with duplication-checking software iThenticate.
Peer review
A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewer
Mancuso Cesare, Università Cattolica del Sacro Cuore, Italy.
Additional file
Additional Table 1 (81.4KB, pdf) : Different media used for culturing of the slices.
Additional Table 2 (50.6KB, pdf) : Groups according to the protocol.
REFERENCES
- 1.TBI care. Evidence-based Diagnostic and Treatment Planning for Traumatic Brain Injuries. [2016-08-12]. http://www.tbicare.eu/5 .
- 2.World Health Organization. Neurological Disorders: Public Health Challenges. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 3.Finkelstein E, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. New York: Oxford University Press; 2006. [Google Scholar]
- 4.Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 6.Loetscher PD, Rossaint J, Rossaint R, et al. Argon: neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury. Crit Care. 2009;13:R206. doi: 10.1186/cc8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn M, Maze M, Franks NP. The neuroprotective effects of xenon and helium in an in vitro model of traumatic brain injury. Crit Care Med. 2008;36:588–595. doi: 10.1097/01.CCM.0B013E3181611F8A6. [DOI] [PubMed] [Google Scholar]
- 8.Kochanek PM, Bramlett HM, Dixon CE, et al. Approach to modeling, therapy evaluation, drug selection, and biomarker assessments for a multicenter pre-clinical drug screening consortium for acute therapies in severe traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2016;33:513–522. doi: 10.1089/neu.2015.4113. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Rossaint R, Sanders RD, Coburn M. Toxic and protective effects of inhaled anaesthetics on the developing animal brain: systematic review and update of recent experimental work. Eur J Anaesthesiol. 2014;31:669–677. doi: 10.1097/EJA.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 10.Jevtovic-Todorovic V, Absalom AR, Blomgren K, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–151. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiao S, Zuo Z. A double-edged sword: volatile anesthetic effects on the neonatal brain. Brain Sci. 2014;4:273–294. doi: 10.3390/brainsci4020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 14.Höllig A, Schug A, Fahlenkamp AV, Rossaint R, Coburn M. Argon: systematic review on neuro- and organoprotective properties of an “inert” gas. Int J Mol Sci. 2014;15:18175–18196. doi: 10.3390/ijms151018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowrangi DS, Tang J, Zhang JH. Argon gas: a potential neuroprotectant and promising medical therapy. Med Gas Res. 2014;4:3. doi: 10.1186/2045-9912-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jawad N, Rizvi M, Gu J, et al. Neuroprotection (and lack of neuroprotection) afforded by a series of noble gases in an in vitro model of neuronal injury. Neurosci Lett. 2009;460:232–236. doi: 10.1016/j.neulet.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 17.Harris K, Armstrong SP, Campos-Pires R, Kiru L, Franks NP, Dickinson R. Neuroprotection against traumatic brain injury by xenon, but not argon, is mediated by inhibition at the N-methyl-D-aspartate receptor glycine site. Anesthesiology. 2013;119:1137–1148. doi: 10.1097/ALN.0b013e3182a2a265. [DOI] [PubMed] [Google Scholar]
- 18.Krings M, Höllig A, Liu J, Grüsser L, Rossaint R, Coburn M. Desflurane impairs outcome of organotypic hippocampal slices in an in vitro model of traumatic brain injury. Med Gas Res. 2016;6:3–9. doi: 10.4103/2045-9912.179338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossaint J, Rossaint R, Weis J, Fries M, Rex S, Coburn M. Propofol: neuroprotection in an in vitro model of traumatic brain injury. Crit Care. 2009;13:R61. doi: 10.1186/cc7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 21.Roehl AB, Hein M, Loetscher PD, et al. Neuroprotective properties of levosimendan in an in vitro model of traumatic brain injury. BMC Neurol. 2010;10:97. doi: 10.1186/1471-2377-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoeler M, Loetscher PD, Rossaint R, et al. Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury. BMC Neurol. 2012;12:20. doi: 10.1186/1471-2377-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamchik Y, Frantseva MV, Weisspapir M, Carlen PL, Perez Velazquez JL. Methods to induce primary and secondary traumatic damage in organotypic hippocampal slice cultures. Brain Res Brain Res Protoc. 2000;5:153–158. doi: 10.1016/s1385-299x(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 24.Macklis JD, Madison RD. Progressive incorporation of propidium iodide in cultured mouse neurons correlates with declining electrophysiological status: a fluorescence scale of membrane integrity. J Neurosci Methods. 1990;31:43–46. doi: 10.1016/0165-0270(90)90007-3. [DOI] [PubMed] [Google Scholar]
- 25.Jevtovic-Todorovic V. General anesthetics and the developing brain: friends or foes? J Neurosurg Anesthesiol. 2005;17:204–206. doi: 10.1097/01.ana.0000178111.26972.16. [DOI] [PubMed] [Google Scholar]
- 26.David HN, Haelewyn B, Degoulet M, Colomb DG, Jr, Risso JJ, Abraini JH. Ex vivo and in vivo neuroprotection induced by argon when given after an excitotoxic or ischemic insult. PLoS One. 2012;7:e30934. doi: 10.1371/journal.pone.0030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Jin Lee J, Jung HH, Zuo Z. Pretreatment with volatile anesthetics, but not with the nonimmobilizer 1,2-dichlorohexa-fluorocyclobutane, reduced cell injury in rat cerebellar slices after an in vitro simulated ischemia. Brain Res. 2007;1152:201–208. doi: 10.1016/j.brainres.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise-Faberowski L, Raizada MK, Sumners C. Desflurane and sevoflurane attenuate oxygen and glucose deprivation-induced neuronal cell death. J Neurosurg Anesthesiol. 2003;15:193–199. doi: 10.1097/00008506-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Coburn M, Sanders RD, Ma D, et al. Argon: the ‘lazy’ noble gas with organoprotective properties. Eur J Anaesthesiol. 2012;29:549–551. doi: 10.1097/EJA.0b013e328357bfdd. [DOI] [PubMed] [Google Scholar]
- 30.Dart RC, Caravati EM, McGuigan MA, et al. Medical Toxicology. Philadeplphia, PA, USA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 31.Jorch G, Kluge S, Markewitz A, Putensen C, Quintel M, Sybrecht GW. DIVI Jahrbuch. Berlin, Germany: MWV Medizinisch Wissenschaftliche Verlagsgesellschaft mbH & Co. KG; 2015. [Google Scholar]
- 32.Bickler PE, Warner DS, Stratmann G, Schuyler JA. gamma-Aminobutyric acid-A receptors contribute to isoflurane neuroprotection in organotypic hippocampal cultures. Anesth Analg. 2003;97:564–571. doi: 10.1213/01.ANE.0000068880.82739.7B. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz-Bloom RD, Miller KA, Evenson DA, Crain BJ, Nadler JV. Benzodiazepines protect hippocampal neurons from degeneration after transient cerebral ischemia: an ultrastructural study. Neuroscience. 2000;98:471–484. doi: 10.1016/s0306-4522(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 34.Abraini JH, Kriem B, Balon N, Rostain JC, Risso JJ. Gamma-aminobutyric acid neuropharmacological investigations on narcosis produced by nitrogen, argon, or nitrous oxide. Anesth Analg. 2003;96:746–749. doi: 10.1213/01.ANE.0000050282.14291.38. [DOI] [PubMed] [Google Scholar]
- 35.Fahlenkamp AV, Rossaint R, Haase H, et al. The noble gas argon modifies extracellular signal-regulated kinase 1/2 signaling in neurons and glial cells. Eur J Pharmacol. 2012;674:104–111. doi: 10.1016/j.ejphar.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang L, Yang T, Zhao H, et al. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit Care Med. 2012;40:1724–1730. doi: 10.1097/CCM.0b013e3182452164. [DOI] [PubMed] [Google Scholar]
- 37.Brücken A, Kurnaz P, Bleilevens C, et al. Dose dependent neuroprotection of the noble gas argon after cardiac arrest in rats is not mediated by K(ATP)-channel opening. Resuscitation. 2014;85:826–832. doi: 10.1016/j.resuscitation.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Soviet’s first Nobelist. New Scientist. New Science Publications. 1974:732–734. [Google Scholar]
- 39.David HN, Haelewyn B, Risso JJ, Abraini JH. Modulation by the noble gas argon of the catalytic and thrombolytic efficiency of tissue plasminogen activator. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:91–95. doi: 10.1007/s00210-012-0809-0. [DOI] [PubMed] [Google Scholar]
- 40.Park SJ, Shin HJ, Gu BW, et al. Desflurane increased the activity of excitatory amino-acid carrier 1 (EAAC1) expressed in Xenopus oocytes. Eur J Pharmacol. 2015;757:84–89. doi: 10.1016/j.ejphar.2015.03.058. [DOI] [PubMed] [Google Scholar]
- 41.Schallner N, Ulbrich F, Engelstaedter H, et al. Isoflurane but not sevoflurane or desflurane aggravates injury to neurons in vitro and in vivo via p75NTR-NF-kB activation. Anesth Analg. 2014;119:1429–1441. doi: 10.1213/ANE.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 42.Deng J, Lei C, Chen Y, et al. Neuroprotective gases-fantasy or reality for clinical use? Prog Neurobiol. 2014;115:210–245. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B, Wei X, Cui X, Zhou H, Ding W, Li W. Desflurane affords greater protection than halothane in the function of mitochondria against forebrain ischemia reperfusion injury in rats. Anesth Analg. 2008;106:1242–1249. doi: 10.1213/ane.0b013e318164f2a5. [DOI] [PubMed] [Google Scholar]
- 44.Engelhard K, Werner C, Reeker W, et al. Desflurane and isoflurane improve neurological outcome after incomplete cerebral ischaemia in rats. Br J Anaesth. 1999;83:415–421. doi: 10.1093/bja/83.3.415. [DOI] [PubMed] [Google Scholar]
- 45.Brücken A, Cizen A, Fera C, et al. Argon reduces neurohistopathological damage and preserves functional recovery after cardiac arrest in rats. Br J Anaesth. 2013;110(Suppl 1):i106–112. doi: 10.1093/bja/aes509. [DOI] [PubMed] [Google Scholar]
- 46.Ryang YM, Fahlenkamp AV, Rossaint R, et al. Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit Care Med. 2011;39:1448–1453. doi: 10.1097/CCM.0b013e31821209be. [DOI] [PubMed] [Google Scholar]
- 47.Höllig A, Weinandy A, Liu J, Clusmann H, Rossaint R, Coburn M. Beneficial properties of argon after experimental subarachnoid hemorrhage: early treatment reduces mortality and influences hippocampal protein expression. Crit Care Med. 2016;44:e520–529. doi: 10.1097/CCM.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 48.McAuliffe JJ, Loepke AW, Miles L, Joseph B, Hughes E, Vorhees CV. Desflurane, isoflurane, and sevoflurane provide limited neuroprotection against neonatal hypoxia-ischemia in a delayed preconditioning paradigm. Anesthesiology. 2009;111:533–546. doi: 10.1097/ALN.0b013e3181b060d3. [DOI] [PubMed] [Google Scholar]
- 49.Erdem AF, Cesur M, Alici HA, et al. Effects of sevoflurane and desflurane in CA1 after incomplete cerebral ischemia in rats. Saudi Med J. 2005;26:1424–1428. [PubMed] [Google Scholar]
- 50.Istaphanous GK, Howard J, Nan X, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 51.Bahr BA, Kessler M, Rivera S, et al. Stable maintenance of glutamate receptors and other synaptic components in long-term hippocampal slices. Hippocampus. 1995;5:425–439. doi: 10.1002/hipo.450050505. [DOI] [PubMed] [Google Scholar]
- 52.Morrison B, 3rd, Saatman KE, Meaney DF, McIntosh TK. In vitro central nervous system models of mechanically induced trauma: a review. J Neurotrauma. 1998;15:911–928. doi: 10.1089/neu.1998.15.911. [DOI] [PubMed] [Google Scholar]
- 53.Noraberg J, Poulsen FR, Blaabjerg M, et al. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- 54.Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Han X, Wang J. Organotypic Hippocampal Slices as Models for Stroke and Traumatic Brain Injury. Mol Neurobiol. 2016;53:4226–4237. doi: 10.1007/s12035-015-9362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol. 2007;5:19–33. doi: 10.2174/157015907780077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Different media used for culturing of the slices
Groups according to the protocol



