Abstract
Horseshoe kidney is the most common congenital renal fusion anomaly with an incidence of 1 in 400–600 individuals. The most common type is fusion at the lower poles seen in greater than 90% of the cases, with the rest depicting fusion at the upper poles, resulting in an inverted horseshoe kidney. Embryologically, there are two theories hypothesizing the genesis of horseshoe kidney – mechanical fusion theory and teratogenic event theory. As an entity, horseshoe kidney is an association of two anatomic anomalies, namely, ectopia and malrotation. It is also associated with other anomalies including vascular, calyceal, and ureteral anomalies. Horseshoe kidney is prone to a number of complications due to its abnormal position as well as due to associated vascular and ureteral anomalies. Complications associated with horseshoe kidney include pelviureteric junction obstruction, renal stones, infection, tumors, and trauma. It can also be associated with abnormalities of cardiovascular, central nervous, musculoskeletal and genitourinary systems, as well as chromosomal abnormalities. Conventional imaging modalities (plain films, intravenous urogram) as well as advanced cross-sectional imaging modalities (ultrasound, computed tomography, and magnetic resonance imaging) play an important role in the evaluation of horseshoe kidney. This article briefly describes the embryology and anatomy of the horseshoe kidney, enumerates appropriate imaging modalities used for its evaluation, and reviews cross-sectional imaging features of associated complications.
Keywords: Complications, horseshoe kidney, multimodality
Introduction
Horseshoe kidney is the most common renal fusion anomaly with an incidence of 1 in 400–600 and male predominance (M:F = 2:1).[1] Increased incidence has been reported in identical twins and siblings.[2] Abnormal ascent and malrotation of the kidneys underlie the pathophysiology of this condition. At least one-third of the patients with horseshoe kidney are asymptomatic and horseshoe kidney is discovered as an incidental imaging finding.[3] The rest of the patients present with symptoms secondary to pelviureteric junction (PUJ) obstruction, infection, and stones. Other less possible presentations are malignancy and trauma. Horseshoe kidney is prone to develop PUJ obstruction, infection, and stone formation due to the abnormal location and orientation of the kidneys and calyces as well as abnormal course and insertion of the ureters. Associated vascular anomalies also play an auxiliary role in the pathogenesis of ureteral obstruction. The teratogenic event theory hypothesizes the increased risk of development of malignancies such as renal cell carcinoma (RCC), Wilms tumor, and carcinoids in horseshoe kidney. It is also prone to trauma due to superficial midline location of isthmus combined with absence of ribcage protection.[4] Cross-sectional imaging modalities play an important role in the diagnosis of horseshoe kidney and associated complications and aid in surgical planning and follow-up of these patients. This aim of this article is to review anatomy of horseshoe kidney with associated ureteral and vascular anomalies, embryology of horseshoe kidney, and associated common and uncommon complications, with special emphasis on the conventional as well as cross sectional imaging features.
Embryology of Horseshoe Kidney
The theory of mechanical fusion suggests that the metanephric blastema of the two kidneys come in contact in the fetal pelvis during the 4th week of embryogenesis (CRL = 5–12 mm). It may be a consequence of abnormal flexion or growth of fetal spine and pelvic organs. At this stage, due to lack of renal capsule, the blastema of the immature kidneys fuse at the point of contact resulting in the formation of fibrous isthmus. The normal kidneys ascend from the pelvis during the 7th–8th week of life with rotation taking place around the same time, so that the renal pelvis turns from anterior to medial aspect.[3,5,6] As the horseshoe kidney ascends, the isthmus is trapped under the inferior mesenteric artery (IMA), arresting further ascent and rotation, resulting in lower location of the kidneys with anteriorly facing pelvis.[7] This explains the ectopia as well as malrotation components of horseshoe kidney. Approximately 98–99% of the horseshoe kidneys are located at or around the origin of IMA from the aorta and the rest in the pelvis.[8] The other theory proposes that development of horseshoe kidney results from abnormal migration of posterior nephrogenic cells resulting in the formation of parenchymal isthmus.[3,5,6] This theory explains the greater than normal risk of carcinogenesis in horseshoe kidney including increased risk of development of carcinoid and Wilms tumor.[6]
Anatomy of Horseshoe Kidney
Horseshoe kidney can lie anywhere from the pelvis to mid-abdomen with most common location of isthmus at L3 to L5 level beneath the origin of IMA.[7,9] Horseshoe kidney may result due to horizontal fusion of the two renal moieties at the midline or lateral fusion on either side of the midline. Fusion in the midline leads to the formation of U-shaped or inverted U-shaped horseshoe kidney depending on the fusion at the lower or upper poles, respectively.[5] Lateral fusion results in formation of L-shaped horseshoe kidney, with ipsilateral moiety in vertical and other moiety in a horizontal orientation.[10] The isthmus lies anterior to the IVC and aorta in most cases [Figure 1C] but may also run posterior or even between the great vessels.[9]
Figure 1(A-C).
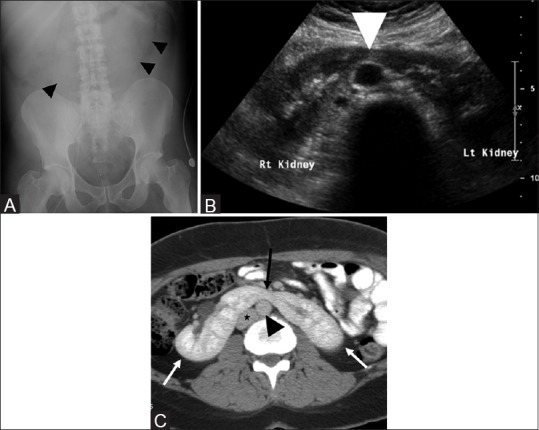
Imaging appearance of normal horseshoe kidney. Abdominal radiograph (A) showing the medially directed renal outlines on both sides (black arrowheads) of midline. Ultrasound image in transverse plane (B) shows the midline isthmus (white arrowhead) connecting the lower poles of a horseshoe kidney. Contrast-enhanced axial CT (C) showing the two kidneys (white arrows) with midline isthmus (black arrow) in front of the aorta (black arrowhead) and inferior vena cava (asterisk)
Apart from abnormal location and orientation of the horseshoe kidney, associated calyceal, ureteral, and vascular abnormalities are also noted. Owing to incomplete medial rotation, the calyces point more towards the spine or downwards or both.[11,12] There is abnormal high insertion of the ureter into the renal pelvis. The ureters course medially over the isthmus and then laterally inferiorly.[12] Single renal artery is present in one-third of the cases while various combinations of single and multiple hilar and isthmic vessels arising from the abdominal aorta, iliac arteries, or IMA are present in rest of the patients.[13,14] In more than two-thirds of the cases, the isthmus receives independent blood supply through branches arising from the abdominal aorta.[15]
Imaging Evaluation
The lower poles of both moieties in a horseshoe kidney point in a more medial direction than expected and can be incidentally detected on plain film or intravenous urogram (IVU) [Figure 1A].[16] However, plain films are very insensitive for the detection of horseshoe kidney. IVU and computed tomography (CT) excretory urogram demonstrate the abnormal orientation of calyces and high insertion as well as the abnormal course of the ureters.[17] Ultrasonography (US) may help in the direct visualization of the isthmus and also demonstrate the abnormal location and orientation of the horseshoe kidney [Figure 1B].[18] However, US is not sensitive in patients with large body habitus or in cases of horseshoe kidney with fibrous isthmus [Figure 2]. In addition, it is operator dependent and detection rate varies depending on operator skill and experience. Moreover, if unsuspected, the isthmus may mimic a midline mass on US [Figure 3]. Horseshoe kidney can be incidentally detected on nuclear medicine studies carried out for other indications. Technetium99m bone scan imaging in adults can outline the abnormal axis and location of horseshoe kidney secondary to uptake and excretion of radionuclide by functioning renal tissue. The isthmus may also be identified as a band across midline if it comprises functional renal parenchyma.[19] Similarly, horseshoe kidney can be identified in the pediatric age group on Technetium 99 Dimercaptosuccinic acid (DMSA) renal scan for evaluation of renal cortical scarring. Contrast-enhanced CT is the modality of choice for evaluation of the horseshoe kidney and relation to surrounding structures [Figure 1C]. It also plays an important role in the evaluation of potential complications and surgical planning. MRI can be used with similar advantages and without the risk of radiation, however, some complications including stones and trauma are better evaluated with CT. Finally, computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are both useful for the depiction of vascular anatomy of horseshoe kidney.
Figure 2.
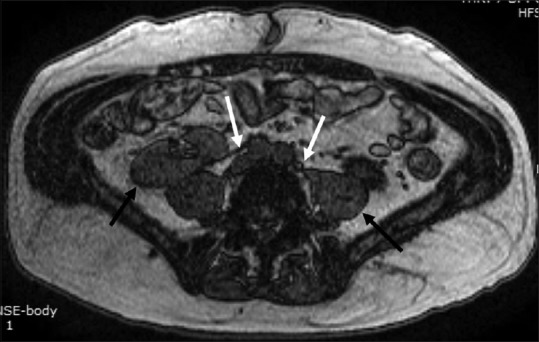
Horseshoe kidney with fibrous isthmus. Axial T1W image showing the thin hypointense fibrous isthmus (white arrow) connecting the lower poles of both kidneys
Figure 3(A and B).
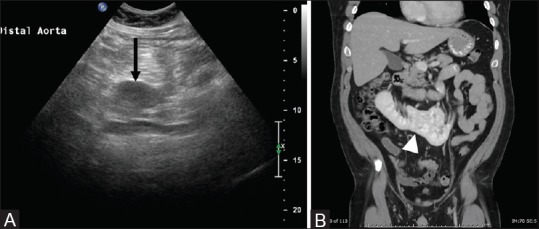
Isthmus of horseshoe kidney simulating a mass on ultrasound: Ultrasound in transverse plane (A) shows a well-defined hypoechoic mass like structure (black arrow) in the midline, confirmed as an isthmus (white arrowhead) of a horseshoe kidney on coronal contrast-enhanced CT (B)
Associated Abnormalities
Horseshoe kidney has been reported to be associated with cardiovascular, central nervous system, musculoskeletal, genitourinary, and chromosomal abnormalities.[3] Higher association of up to 78% has been found in stillborn fetuses and infants and incidences of up to 28.5% and 3.5% in children and adults. This suggests that some of these anomalies are incompatible with life leading to death in-utero or in early infancy. Cardiovascular abnormalities include ventricular septal defects whereas neurological abnormalities include encephalocele, myelomeningocele, and spina bifida.[6,20,21,22] Osseous abnormalities include kyphosis, scoliosis, hemeivertebra, and micrognathia.[21] Genitourinary abnormalities include septate vagina, bicornuate uterus, hypospadias, undescended testis, adult polycystic kidney disease, as well as supernumerary kidneys.[6] Patients with Turner syndrome have a horseshoe kidney in as high as 60% while the incidence is 20% in Down's syndrome.[23] Additional associated chromosomal anomalies include Edward and Patau syndrome (Trisomy 18 and Trisomy 13 respectively), as well as oral-cranial-digital syndrome.[9,23]
Complications Associated with Horseshoe Kidney
Pelviureteric junction obstruction
The most frequent complication associated with horseshoe kidney is pelviureteric junction (PUJ) obstruction and is seen in approximately one-third of the patients.[20] It may be bilateral.[20] The pathogenesis of PUJ obstruction lies behind the abnormal high insertion of the ureters into the renal pelvis leading to delayed pelvic emptying and stasis. The ureters pass over the isthmus along their downward course, which may also contribute to the obstruction.[24] One of the less consistent but possible causes of ureteral obstruction is abnormal origin and course of renal arteries from aorta, common iliac arteries or IMA causing ureteral indentation and obstruction along their course.
Imaging features of PUJ obstruction are somewhat different and unique from that observed in those of normal kidneys. The typical imaging features can be seen on intravenous urography (IVU) or CT excretory urogram and include dilated renal pelvis with high riding ureter [Figure 4]. CT is a better imaging tool because it not only delineates the anatomy of horseshoe kidney but also its relation to surrounding structures, cortical thickness, and any associated vascular abnormalities. All the above information is of utmost importance if surgery or radiological intervention is planned. In some cases, the pattern of PUJ obstruction may be intermittent and may require the use of diuresis radioisotope renal scan to differentiate between dilatation and obstruction. Treatment options include palliative interventions such as image-guided percutaneous nephrostomy or antegrade ureteric stenting, until more definitive surgery can be performed. Surgical treatment options include open ureteroplasty or laparoscopic dismembered pyeloplasty.[25,26] The basic principle is PUJ reconstruction with resection of any strictured segment and corrective insertion of pelvis into the most dependent part of kidney.[27] This helps to promote pelvic emptying as well as takes care of any structural obstruction including strictures. Midline transperitoneal approach can be used if surgical reconstruction is planned on both sides.[28] Surgical division of isthmus has been performed in the past to bring the kidneys and their drainages as close to physiological as possible, but is rarely performed now due to increased risk of complications including bleeding and renal infarction.[29]
Figure 4.
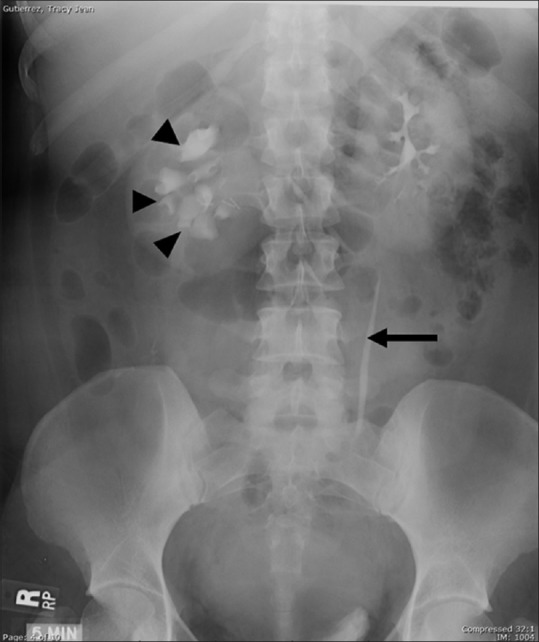
Pelviureteric junction obstruction of right renal moiety of a horseshoe kidney: Delayed IVU image shows abnormally oriented and dilated right-sided calyces (arrowheads) due to incomplete medial rotation. The right ureter is not visualized suggesting PUJ obstruction. The left pelvicalyceal system and ureter (arrow) are normal
PUJ obstruction in fetal life can lead to the formation of multicystic dysplastic kidney characterized by small size kidney with multiple cysts and absence of renal parenchyma. The process when bilateral is incompatible with life.[30] Multicystic dysplastic kidney can be detected on prenatal sonography. In 50% of the cases, it is associated with abnormalities including PUJ obstruction, reflux and scarring in other kidney.[31]
Renal stones
Renal stones are one of the frequent complications of horseshoe kidney and seen in 16–60% of the cases. The promoting factors for stone formation are stasis and infection. Stasis results from PUJ obstruction, reflux, and delayed emptying secondary to abnormal calyceal orientation.[32] In few cases, ureteral obstruction due to its course over the isthmus can also be a contributing factor.[6] Renal stones are multiple and bilateral with staghorn stones noted in many cases. Renal stones can be detected and followed up on plain films. IVU, though used rarely nowadays, can better localize stones and complications including obstruction and hydronephrosis. Noncontrast CT is the the investigation of choice for evaluation of renal stones and associated complications including obstruction [Figure 5]. Ureteral stones are better visualized on CT [Figure 5B]. There is increased risk of development of xanthogranulomatous pyelonephritis in horseshoe kidney with staghorn calculus. It is a chronic granulomatous infection characterized by the presence of lipid laden foamy macrophages on microscopy. It is a locally invasive and destructive process, hence the name pseudotumor.[33] The characteristic US features are large size kidney with calyceal enlargement that may be misdiagnosed as pyonephrosis. CT also demonstrates renal enlargement with perinephric fat stranding. The diagnostic finding is a staghorn calculus with contracted renal pelvis and calyceal enlargement.[34] Calyceal enlargement is not due to hydronephrosis but secondary to the chronic inflammatory infiltrate.
Figure 5(A and B).
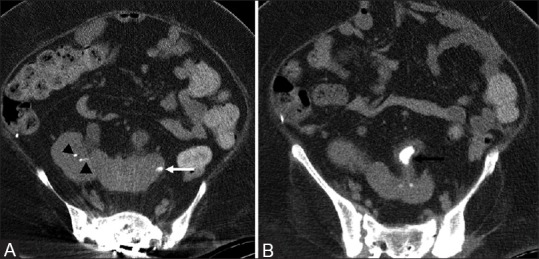
Horseshoe kidney with stone formation: Axial non-contrast CT images (A and B) show tiny calculi in the right kidney (black arrowheads) and large calculus in the left upper ureter (black arrow) of a horseshoe kidney. Note that the left upper ureter is thickened. There is focus of cortical calcification in left kidney (white arrow), which is a sequelae of prior infection
Treatment options for renal stones include extracorporeal shockwave lithotripsy (ESWL) and open surgery.[35] Minimally invasive procedure such as percutaneous nephrostomy (PCN) can be used as a palliative measure in hydronephrosis and pyonephrosis for decompression of the collecting system and symptomatic relief.[33] PCN can also serve as a technique for tract formation for percutaneous stone extraction and can be done under US or fluoroscopy guidance. The lower pole renal puncture approach utilized in normal kidneys is avoided in horseshoe kidney and more superior and lateral approach is rather preferred. The reason for the altered approach is the more medial orientation of the lower poles of horseshoe kidney resulting in technically difficult lower pole puncture.
Infection
Horseshoe kidney is predisposed to infection owing to a combination of factors including stasis, reflux, and stone formation. It is observed in approximately one-third of the patients and is the most common cause of death in these patients.[5,20] Stasis and stone formation occur as a result of a number of factors as described above. The most common route of infection is ascending infection secondary to vesico-ureteric reflux (VUR).[3]
VUR is seen in 50% of the patients with horseshoe kidney. The gold standard test for diagnosis of VUR is micturating cystourethrogram (MCU). It is a dynamic assessment of the nature and grade of VUR. Radionuclide cystography is a nuclear imaging equivalent of MCU. It involves instillation of 500 ml of saline mixed with technetium-99 labelled radionuclide. Indirect radionuclide cystography involves intravenous administration of technetium-99 labelled radionuclide followed by a renogram. After 20 minutes, the patient is asked to micturate in front of a gamma camera whenever there is an urge to pass urine. Any amount of radiolabelled urine in the ureters at the end of micturition is suggestive of reflux.
IVU gives information about the functional status of the kidneys and the structural assessment of the urinary tracts. Secondary signs of reflux such as calyceal clubbing, cortical scarring, and urinary tract dilatation can be seen on IVU, however, it does not evaluate the dynamic nature of reflux. Similarly, CT and MRI are excellent imaging modalities for the evaluation of secondary signs and complications of reflux, but lack dynamic assessment of refluxing urine. On CT and MR, the affected kidney appears bulky with focal or diffuse perinephric fat stranding. On contrast-enhanced CT, striated nephrogram characterized by alternating areas of enhancing and nonenhancing renal cortex can be seen. If the infection is not controlled, it can lead to the formation of a renal abscess, which is seen as focal fluid collection with thick enhancing walls and associated perinephric fat stranding [Figure 6]. Renal infection in diabetic patients can progress to emphysematous pyelonephritis, which is a bacterial infection by lactose forming bacteria, including Escherichia coli, Klebsiella, and Aerobacter as the most common causative agents.[36] Contrast-enhanced CT is the modality of choice and shows intrarenal and perinephric foci of air in addition to features of pyelonephritis and renal abscesses [Figure 7]. It is a potentially life threatening condition with a mortality rate as high as 40–90%.[36] PCN can serve as a temporary stabilizing measure until the antibiotics take effect. PCN contributes to drainage of renal abscess as well as decompression of the obstructed collecting system [Figure 7].
Figure 6.
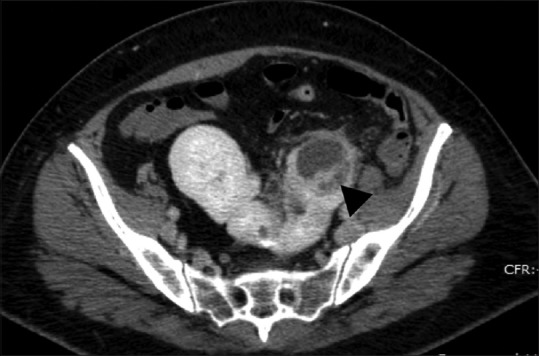
Pyelonephritis with abscess in a horseshoe kidney: Contrast-enhanced axial CT shows a thick walled and septated low density fluid collection in the left moiety of the horseshoe kidney (black arrowhead). There is associated perinephric fat stranding
Figure 7(A-D).
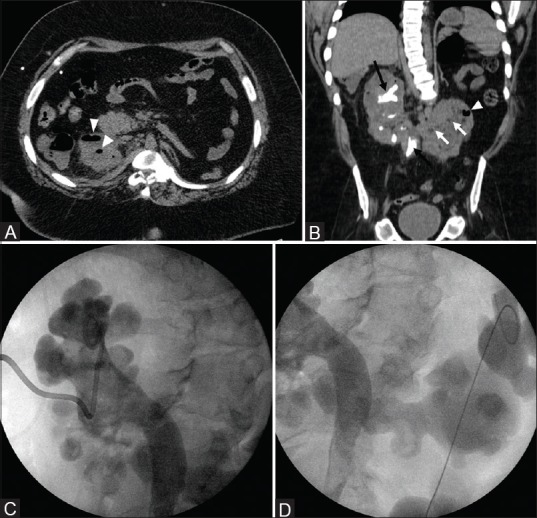
Emphysematous pyelonephritis in a horseshoe kidney: Axial and coronal CT (A and B) show multiple foci of air in both moieties of a horseshoe kidney (white arrowheads). Both renal moieties are bulky with multiple tiny intraparenchymal hypodensities (white arrows) representing microabscesses. Also note the staghorn calculi in the upper pole of right kidney and right upper ureter (black arrows) with few small calculi in the lower pole of right kidney. This patient was managed with right percutaneous nephrostomy (C) and left nephroureteral stent placement (D). Note bilateral hydronephrosis with percutaneous nephrostomy catheter
Genitourinary tuberculosis (TB) is the most common site of extrapulmonary and extranodal TB.[37] CT urography is the modality of choice for the assessment of renal involvement. Hypodense nodule, miliary nodules, renal abscess, and calcification are the imaging features on CT. CT urography is excellent for the evaluation of the collecting system and shows urothelial thickening and enhancement, calyceal stricture, caliectasis, as well as hydronephrosis. In chronic cases, there is parenchymal thinning and cortical scarring with small calcified kidney representing the irreversible end stage of infection.
Tumors
There is an increased risk of benign and malignant tumors in horseshoe kidney. The tumors with increased risk include renal cell carcinoma (RCC), transitional cell carcinoma (TCC), Wilm's tumor, carcinoid, squamous cell carcinoma (SCC), and oncocytoma. RCC is the most common malignant tumor in horseshoe kidney and accounts for 45% of all tumors.[38] However, some published studies in the past suggest that although RCC is the most common tumor in horseshoe kidney; there is no increased risk of RCC in horseshoe kidney and is the same as in the general population.[38] There is a 3-4-fold increase in the incidence of TCC in horseshoe kidney, which accounts for approximately 28% of all tumors.[38] The elevated risk is explained by an increased incidence of chronic stasis, obstruction, infection, and stone formation in horseshoe kidney, which are predisposing factors for the development of TCC. The risk of Wilm's tumor and carcinoid is also increased in horseshoe kidney and is explained by the hypothesis of teratogenic event in the embryological development of horseshoe kidney.[39,40] This also explains the increased risk of development of these tumors in the isthmus. There is almost two-fold increased risk in the incidence Wilm's tumor, particularly in children.[40,41] The increased risk of Wilm's tumor in the isthmic location, accounting for almost half all Wilm's tumors, is explained by the teratogenic event involving abnormal proliferation of metanephric blastema to form isthmus.[42,43] Though carcinoid tumors are rare in horseshoe kidney, however, there is still a significant 62-fold higher risk than that of the general population.[44,45] Similar to Wilm's tumor, carcinoid also has a predilection for the isthmus of horseshoe kidney because the presence of neuroendocrine cells within the metanephric blastema that forms the isthmus during the embryological development of horseshoe kidney.[44,46] However, carcinoid tumors in horseshoe kidney have a relatively benign course.[44] Other relatively rare malignant tumors such as SCC and benign tumors such as oncocytoma and angiomyolipoma also have an increased risk in horseshoe kidney.[38] Oncocytoma can be diagnosed on cross-sectional imaging due to the presence of the characteristic nonenhancing central scar.[6] Primary renal sarcoma is relatively rare with an incidence of 1%.[47] Primary leiomyosarcoma has also been reported in horseshoe kidney and may arise from renal capsule, smooth muscle fibers of renal pelvis and blood vessels.[48]
Contrast-enhanced CT is the imaging modality of choice for the preoperative evaluation of tumors in horseshoe kidney and information should be given about the relationship of the tumor to surrounding structures including local invasion, distant metastasis, presence of functional or fibrous tissue in isthmus, vascular, and collecting system anatomy [Figure 8]. Interventional radiology plays an important role in the treatment of smaller tumors. The smaller tumors in horseshoe kidney can be treated by minimally invasive techniques such as radiofrequency ablation or cryoablation avoiding the need for surgery. It also has a role in the treatment of larger tumors in the form of vascular embolization reducing their size and vascularity leading to symptomatic relief or as a preoperative measure.
Figure 8(A and B).
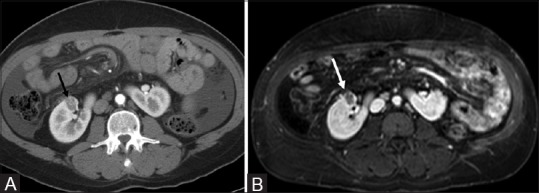
Renal cell carcinoma in a horseshoe kidney: Axial contrast enhanced CT (A) and T1W fat saturated (B) images show a heterogeneously enhancing mass in the anterior interpolar region of the right moiety of a horseshoe kidney. This proved to be a RCC and was treated with cryoablation
Trauma
Horseshoe kidney is predisposed to trauma due to the presence of risk factors as well as the absence of normal protective mechanism seen in normal kidneys. Predisposing factors include low and relatively superficial location of the isthmus in front of the vertebral column across the midline [Figure 9A].[46] PUJ obstruction seen more commonly in horseshoe kidney increases the renal size due to hydronephrosis and is also a risk factor for trauma. Protective effect of ribs seen in normal kidneys is absent in cases of horseshoe kidney. Contrast-enhanced CT is modality of choice in cases of renal trauma. It is helpful in the evaluation of intrarenal and perinephric hematoma, grading of renal injuries, renal infarction, renal vascular as well as collecting system injuries [Figure 9B]. CT not only guides surgical management in these cases but also assists in patient triage depending on injury grading.
Figure 9(A and B).
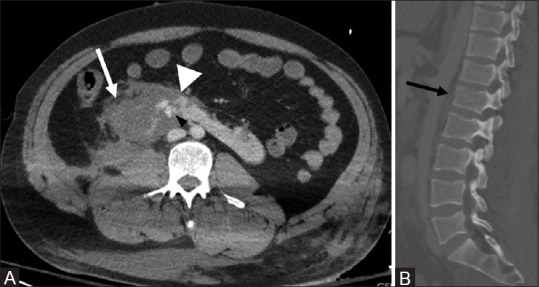
Trauma in a horseshoe kidney: Axial CT (A) shows laceration of the right renal moiety and isthmus (white arrowhead) with an associated hematoma (white arrow). Hyperdense areas (black arrowhead) in the left moiety represent active extravasation of contrast. Sagittal CT image (B) shows a fracture of vertebral body of L1 (black arrow)
Conclusion
Horseshoe kidney is the most common renal fusion anomaly. It is predisposed to a number of complications by virtue of its ectopic position, malrotation, and associated vascular and ureteral anomalies. Knowledge of these consortium of anomalies, associated complications, and their imaging features plays an important role in timely diagnosis as well as in guiding management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yoshinaga K, Kodama K, Tanii I, Toshimori K. Morphological study of a horseshoe kidney with special reference to the vascular system. Anat Sci Int. 2002;77:134–9. doi: 10.1046/j.0022-7722.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 2.Gleason PE KS. Ectopic kidneys and renal fusion anomalies. AUA Update Serie. 1995:268–71. [Google Scholar]
- 3.Cascio S, Sweeney B, Granata C, Piaggio G, Jasonni V, Puri P. Vesicoureteral reflux and ureteropelvic junction obstruction in children with horseshoe kidney: Treatment and outcome. J Urol. 2002;167:2566–8. [PubMed] [Google Scholar]
- 4.Murphy JT, Borman KR, Dawidson I. Renal autotransplantation after horseshoe kidney injury: A case report and literature review. J Trauma. 1996;40:840–4. doi: 10.1097/00005373-199605000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Boatman DL, Cornell SH, Kolln CP. The arterial supply of horseshoe kidneys. The Am J Roentgenol Radium Ther Nucl Med. 1971;113:447–51. doi: 10.2214/ajr.113.3.447. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien J, Buckley O, Doody O, Ward E, Persaud T, Torreggiani W. Imaging of horseshoe kidneys and their complications. J Med Imaging Radiat Oncol. 2008;52:216–26. doi: 10.1111/j.1440-1673.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 7.TW S. Langman's medical embryology. 11th ed. Baltimore: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 8.Je BK, Kim HK, Horn PS. Incidence and Spectrum of Renal Complications and Extrarenal Diseases and Syndromes in 380 Children and Young Adults With Horseshoe Kidney. AJR Am J Roentgenol. 2015;205:1306–14. doi: 10.2214/AJR.15.14625. [DOI] [PubMed] [Google Scholar]
- 9.Decter RM. Renal duplication and fusion anomalies. Pediatr Clin North Am. 1997;44:1323–41. doi: 10.1016/s0031-3955(05)70559-9. [DOI] [PubMed] [Google Scholar]
- 10.Oktem H, Gozil R, Calguner E, Bahcelioglu M, Mutlu S, Kurkcuoglu A, et al. Morphometric study of a horseshoe kidney. Med Princ Pract. 2008;17:80–3. doi: 10.1159/000109596. [DOI] [PubMed] [Google Scholar]
- 11.Dajani AM. Horseshoe kidney: A review of twenty-nine cases. Br J Urol. 1966;38:388–402. doi: 10.1111/j.1464-410x.1966.tb09725.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta M, Pandey AK, Goyal N. Horseshoe kidney-A case report. Nepal Med Coll J. 2007;9:63–6. [PubMed] [Google Scholar]
- 13.Davidovic LB, Kostic DM, Jakovljevic NS, Perisic M, Cinara IS, Cvetkovic SD, et al. Abdominal aortic surgery and horseshoe kidney. Ann Vasc Surg. 2004;18:725–8. doi: 10.1007/s10016-004-0076-8. [DOI] [PubMed] [Google Scholar]
- 14.Gay SB, Armistead JP, Weber ME, Williamson BR. Left infrarenal region: Anatomic variants, pathologic conditions, and diagnostic pitfalls. Radiographics. 1991;11:549–70. doi: 10.1148/radiographics.11.4.1887111. [DOI] [PubMed] [Google Scholar]
- 15.Ferko A, Krajina A, Jon B, Lesko M, Voboril Z. Juxtarenal aortic aneurysm associated with a horseshoe kidney. Transfemoral endoluminal repair. Arch Surg. 1997;132:316–7. doi: 10.1001/archsurg.1997.01430270102021. [DOI] [PubMed] [Google Scholar]
- 16.Segura JW, Kelalis PP, Burke EC. Horseshoe kidney in children. J Urol. 1972;108:333–6. doi: 10.1016/s0022-5347(17)60732-8. [DOI] [PubMed] [Google Scholar]
- 17.BW S. Ectopic kidneys and renal fusion anomalies. AUA Update Series. 1987:2–5. [Google Scholar]
- 18.Banerjee B, Brett I. Ultrasound diagnosis of horseshoe kidney. Br J Radiol. 1991;64:898–900. doi: 10.1259/0007-1285-64-766-898. [DOI] [PubMed] [Google Scholar]
- 19.Kao PF, Sheih CP, Tsui KH, Tsai MF, Tzen KY. The 99mTc-DMSA renal scan and 99mTc-DTPA diuretic renogram in children and adolescents with incidental diagnosis of horseshoe kidney. Nucl Med Commun. 2003;24:525–30. doi: 10.1097/00006231-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Fernbach SK, Davis TM. The abnormal renal axis in children with spina bifida and gibbus deformity-the pseudohorseshoe kidney. J Urol. 1986;136:1258–60. doi: 10.1016/s0022-5347(17)45307-9. [DOI] [PubMed] [Google Scholar]
- 21.Pitts WR, Jr, Muecke EC. Horseshoe kidneys: A 40-year experience. J Urol. 1975;113:743–6. doi: 10.1016/s0022-5347(17)59571-3. [DOI] [PubMed] [Google Scholar]
- 22.Mandell GA, Maloney K, Sherman NH, Filmer B. The renal axes in spina bifida: Issues of confusion and fusion. Abdom Imaging. 1996;21:541–5. doi: 10.1007/s002619900122. [DOI] [PubMed] [Google Scholar]
- 23.Araki K, Matsumoto K, Shiraishi T, Ogura H, Kurashige T, Kitamura I. Turner's syndrome with agenesis of the corpus callosum, Hashimoto's thyroiditis and horseshoe kidney. Acta Paediatr Japonica. 1987;29:622–6. doi: 10.1111/j.1442-200x.1987.tb02251.x. [DOI] [PubMed] [Google Scholar]
- 24.Dewan PA, Clark S, Condron S, Henning P. Point of technique: Ureterocalycostomy in the management of pelvi-ureteric junction obstruction in the horseshoe kidney. BJU Int. 1999;84:366–8. doi: 10.1046/j.1464-410x.1999.00087.x. [DOI] [PubMed] [Google Scholar]
- 25.Friedland GW dP, Nino-Murcia M, Cohen R, Rifkin MD. Clinical Urography Philadelphia. PA: W. B. Saunders; 1990. [Google Scholar]
- 26.Bellman GC, Yamaguchi R. Special considerations in endopyelotomy in a horseshoe kidney. Urology. 1996;47:582–5. doi: 10.1016/S0090-4295(99)80501-9. [DOI] [PubMed] [Google Scholar]
- 27.Kausik S, Segura JW. Surgical management of ureteropelvic junction obstruction in adults. Int Braz J Urol. 2003;29:3–10. doi: 10.1590/s1677-55382003000100002. [DOI] [PubMed] [Google Scholar]
- 28.Grainger R, Murphy DM, Lane V. Horseshoe kidney-a review of the presentation, associated congenital anomalies and complications in 73 patients. Irish Med J. 1983;76:315–7. [PubMed] [Google Scholar]
- 29.Schuster T, Dietz HG, Schutz S. Anderson-Hynes pyeloplasty in horseshoe kidney in children: Is it effective without symphysiotomy? Pediatr Surg Int. 1999;15:230–3. doi: 10.1007/s003830050563. [DOI] [PubMed] [Google Scholar]
- 30.Merrot T, Lumenta DB, Tercier S, Morisson-Lacombes G, Guys JM, Alessandrini P. Multicystic dysplastic kidney with ipsilateral abnormalities of genitourinary tract: Experience in children. Urology. 2006;67:603–7. doi: 10.1016/j.urology.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 31.Belk RA, Thomas DF, Mueller RF, Godbole P, Markham AF, Weston MJ. A family study and the natural history of prenatally detected unilateral multicystic dysplastic kidney. J Urol. 2002;167:666–9. doi: 10.1016/S0022-5347(01)69120-1. [DOI] [PubMed] [Google Scholar]
- 32.Shokeir AA, El-Nahas AR, Shoma AM, Eraky I, El-Kenawy M, Mokhtar A, et al. Percutaneous nephrolithotomy in treatment of large stones within horseshoe kidneys. Urology. 2004;64:426–9. doi: 10.1016/j.urology.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Lorentzen M, Nielsen HO. Xanthogranulomatous pyelonephritis. Scand J Urol Nephrol. 1980;14:193–200. doi: 10.3109/00365598009179560. [DOI] [PubMed] [Google Scholar]
- 34.Craig WD, Wagner BJ, Travis MD. Pyelonephritis: Radiologic-pathologic review. Radiographics. 2008;28:255–77. doi: 10.1148/rg.281075171. [DOI] [PubMed] [Google Scholar]
- 35.Lampel A, Hohenfellner M, Schultz-Lampel D, Lazica M, Bohnen K, Thurof JW. Urolithiasis in horseshoe kidneys: Therapeutic management. Urology. 1996;47:182–6. doi: 10.1016/s0090-4295(99)80412-9. [DOI] [PubMed] [Google Scholar]
- 36.d'Escrivan T, Duval A, Faure K. Severe emphysematous pyelonephritis on a horseshoe kidney. Med Mal Infect. 2006;36:432–3. doi: 10.1016/j.medmal.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Tirumani SH, Ojili V, Gunabushanam G, Shanbhogue AK, Nagar A, Fasih N, et al. Imaging of tuberculosis of the abdominal viscera: Beyond the intestines. J Clin Imaging Sci. 2013;3:17. doi: 10.4103/2156-7514.111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fazio L, Razvi H, Chin JL. Malignancy in horseshoe kidneys: Review and discussion of surgical implications. Can J Urol. 2003;10:1899–904. [PubMed] [Google Scholar]
- 39.Talpallikar MC, Sawant V, Hirugade S, Borwankar SS, Sanghani H. Wilms' tumor arising in a horseshoe kidney. Pediatr Surg Int. 2001;17:465–6. doi: 10.1007/s003830000472. [DOI] [PubMed] [Google Scholar]
- 40.Neville H, Ritchey ML, Shamberger RC, Haase G, Perlman S, Yoshioka T. The occurrence of Wilms tumor in horseshoe kidneys: A report from the National Wilms Tumor Study Group (NWTSG) J Pediatr Surg. 2002;37:1134–7. doi: 10.1053/jpsu.2002.34458. [DOI] [PubMed] [Google Scholar]
- 41.Sawicz-Birkowska K, Apoznanski W, Kantorowicz-Szymik S, Urbanowicz W, Szydelko T. Malignant tumours in a horseshoe kidney in children: A diagnostic dilemma. Eur J Pediatr Surg. 2005;15:48–52. doi: 10.1055/s-2004-830552. [DOI] [PubMed] [Google Scholar]
- 42.Huang EY, Mascarenhas L, Mahour GH. Wilms' tumor and horseshoe kidneys: A case report and review of the literature. J Pediatr Surg. 2004;39:207–12. doi: 10.1016/j.jpedsurg.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Hohenfellner M, Schultz-Lampel D, Lampel A, Steinbach F, Cramer BM, Thuroff JW. Tumor in the horseshoe kidney: Clinical implications and review of embryogenesis. J Urol. 1992;147:1098–102. doi: 10.1016/s0022-5347(17)37486-4. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan B, Truong LD, Saleh G, Sirbasku DM, Slawin KM. Horseshoe kidney is associated with an increased relative risk of primary renal carcinoid tumor. J Urol. 1997;157:2059–66. [PubMed] [Google Scholar]
- 45.Begin LR, Guy L, Jacobson SA, Aprikian AG. Renal carcinoid and horseshoe kidney: A frequent association of two rare entities-A case report and review of the literature. J Surg Oncol. 1998;68:113–9. doi: 10.1002/(sici)1096-9098(199806)68:2<113::aid-jso8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Isobe H, Takashima H, Higashi N, Murakami Y, Fujita K, Hanazawa K, et al. Primary carcinoid tumor in a horseshoe kidney. Int J Urol. 2000;7:184–8. doi: 10.1046/j.1442-2042.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 47.Moazzam M, Ather MH, Hussainy AS. Leiomyosarcoma presenting as a spontaneously ruptured renal tumor-case report. BMC Urol. 2002;2:13. doi: 10.1186/1471-2490-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moudouni SM, En-Nia I, Rioux-Leclerq N, Guille F, Lobel B. Leiomyosarcoma of the renal pelvis. Scand J Urol Nephrol. 2001;35:425–7. doi: 10.1080/003655901753224530. [DOI] [PubMed] [Google Scholar]


