Abstract
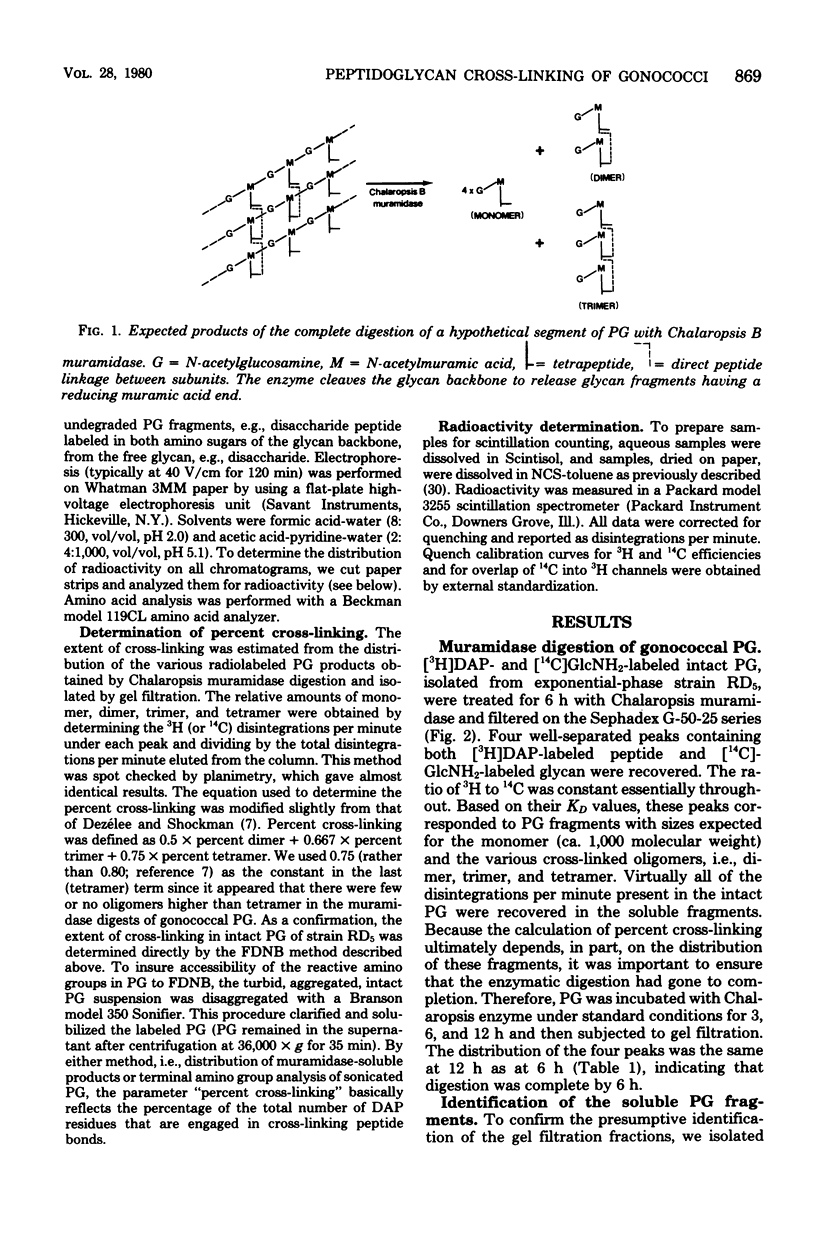
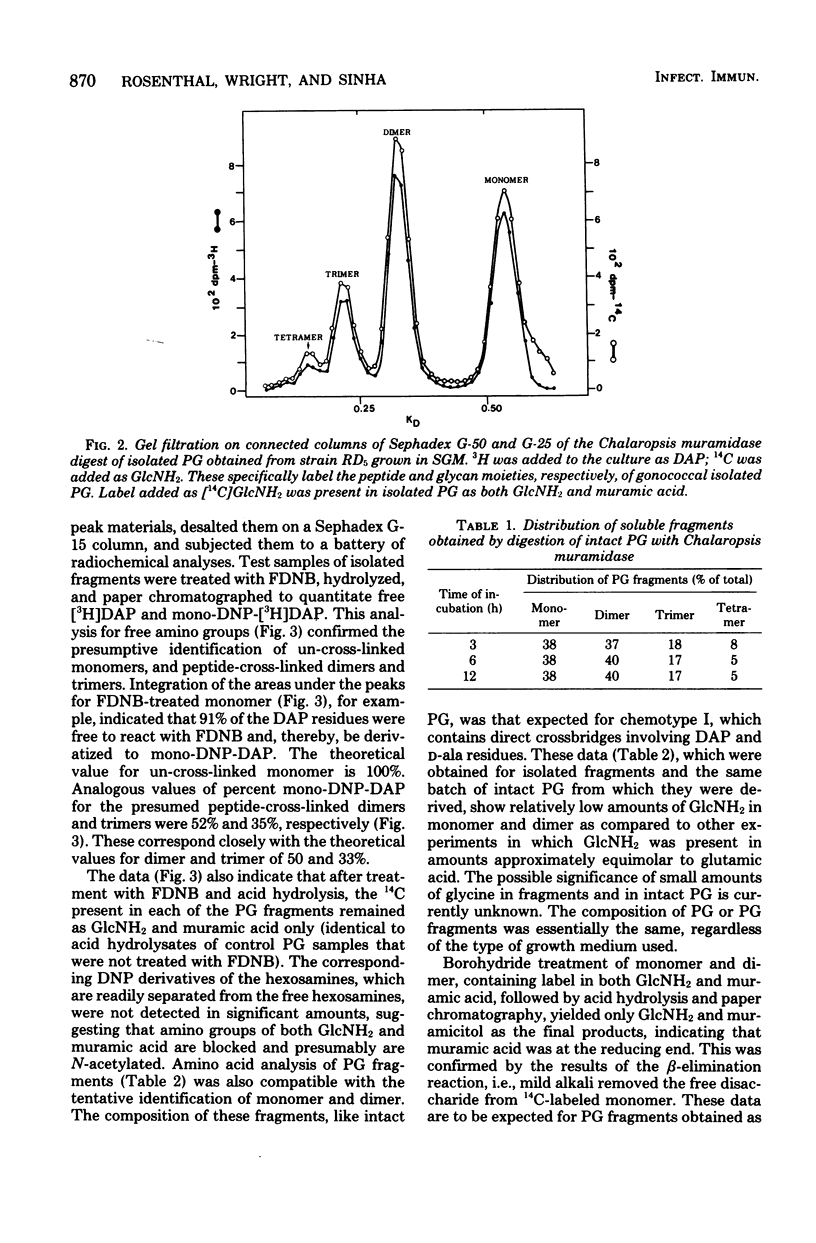
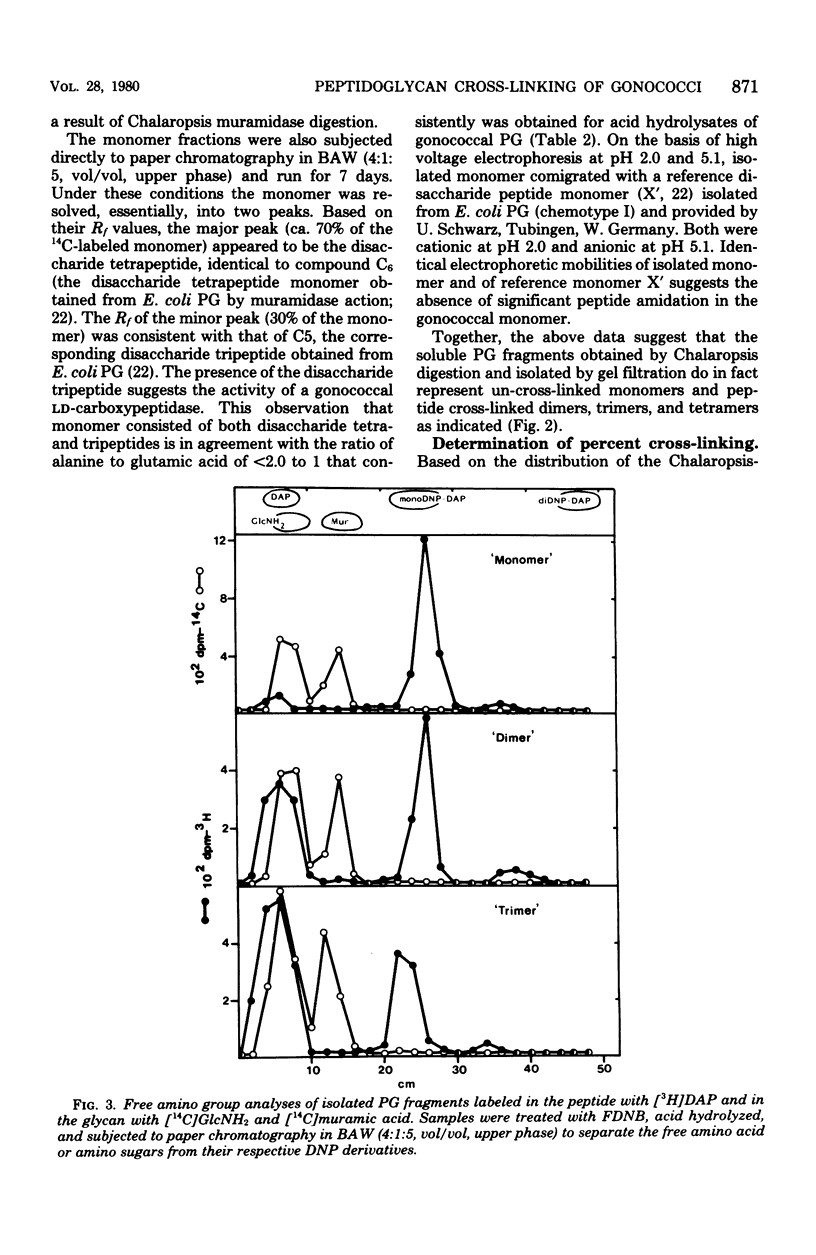
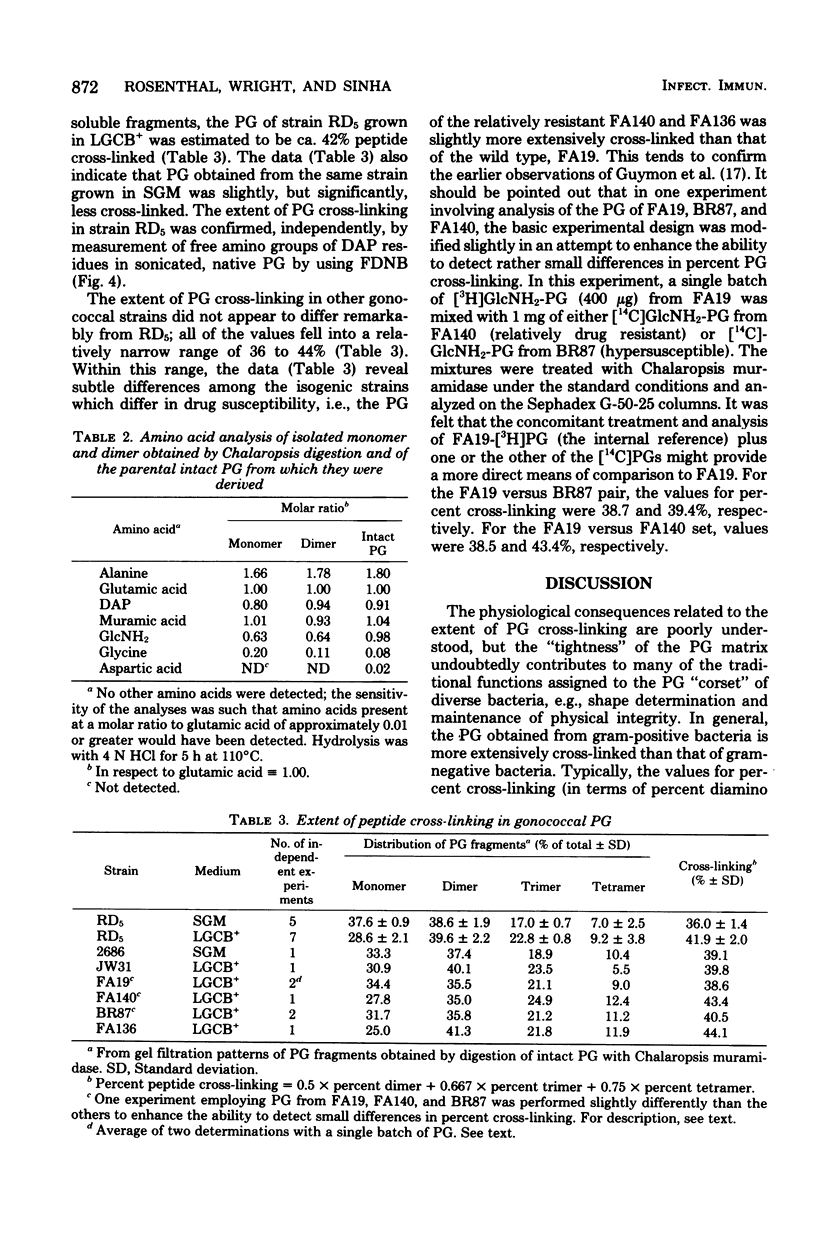
The extent of peptide cross-linking in peptidoglycan (PG) isolated from various strains of Neisseria gonorrhoeae was examined. Purified PG, specifically labeled in the peptide moiety with [3H]diaminopimelic acid (DAP) and labeled in the glycan with [14C]glucosamine and [14C]muramic acid, was digested completely with Chalaropsis B muramidase. Gel filtration of the digest on connected columns of Sephadex G-50 and G-25 revealed four well-defined peaks corresponding to soluble PG fragments and containing a constant ratio of 3H to 14C. On the basis of (i) KD values, (ii) amino acid composition, (iii) free amino group analysis of [3H]DAP residues, (iv) borohydride reduction, (v) the β-elimination reaction, (vi) high-voltage electrophoresis, and (vii) paper chromatography in various solvents, the PG fragments were identified as un-cross-linked disaccharide peptide monomer, typical of chemotype I PG, and the corresponding peptide cross-linked dimers, trimers, and tetramers. The percent cross-linking of PG basically reflects the percentage of DAP residues that are involved in peptide cross-linking bonds. This value was estimated from the distribution of labeled fragments that resulted from the enzymatic digestion of PG and was confirmed by the analysis of free amino groups in [3H]DAP of intact PG. Although there were subtle, strain- and medium-dependent differences in percent cross-linking, these values varied only over a relatively narrow range (36 to 44%). The percent cross-linking of PG in the prototype strain, RD5, grown in a standard gonococcal medium (LGCB+) was 41.0 ± 2.0%. This is a relatively high degree of peptide cross-linking for a gram-negative bacterium. We also confirmed previous observations that the extent of PG cross-linking among isogenic gonococci was higher in strains, e.g., FA140 and FA136, carrying loci that govern increased resistance to multiple drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Ellouz F., Petit J. F., Lederer E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem Biophys Res Commun. 1974 Feb 4;56(3):561–567. doi: 10.1016/0006-291x(74)90640-8. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Bloom G. D. Murein and the outer penetration barrier of Escherichia coli K-12, Proteus mirabilis, and Pseudomonas aeruginosa. J Bacteriol. 1972 Dec;112(3):1364–1374. doi: 10.1128/jb.112.3.1364-1374.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M. Structure of the walls of Lactobacillus acidophilus strain 63 AM Gasser. Biochemistry. 1970 Jul 21;9(15):2935–2943. doi: 10.1021/bi00817a001. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée P., Shockman G. D. Studies of the formation of peptide cross-links in the cell wall peptidoglycan of Streptococcus faecalis. J Biol Chem. 1975 Sep 10;250(17):6806–6816. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Dziarski R., Dziarski A. Mitogenic activity of staphylococcal peptidoglycan. Infect Immun. 1979 Mar;23(3):706–710. doi: 10.1128/iai.23.3.706-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmros T., Burman L. G., Bloom G. D. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 May;126(2):969–976. doi: 10.1128/jb.126.2.969-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rayman M. K., Costerton J. W., MacLeod R. A. Isolation, characterization, and ultrastructure of the peptidoglycan layer of a marine pseudomonad. J Bacteriol. 1972 Feb;109(2):895–905. doi: 10.1128/jb.109.2.895-905.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Fazio M., Tomasz A. Effect of benzylpenicillin on the synthesis and structure of the cell envelope of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978 Mar;13(3):514–526. doi: 10.1128/aac.13.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov A., Sveen K. Induction of leukochemotaxis by peptidoglycan of Staphylococcus aureus. Acta Pathol Microbiol Scand B. 1978 Dec;86B(6):375–378. doi: 10.1111/j.1699-0463.1978.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975 May;122(2):385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Mechanism of autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1186–1193. doi: 10.1128/jb.126.3.1186-1193.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann H. D. On the peptidoglycan of the cell walls of Pseudomonas aeruginosa. Eur J Biochem. 1972 Dec 18;31(3):456–463. doi: 10.1111/j.1432-1033.1972.tb02552.x. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. Y., White D. Myxospore formation in Myxococcus xanthus: chemical changes in the cell wall during cellular morphogenesis. J Bacteriol. 1972 Nov;112(2):849–855. doi: 10.1128/jb.112.2.849-855.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Iwata S., Suginaka H., Namba K., Kotani S. Chemical structure of the peptidoglycan of Vibrio parahaemolyticus A55 with special reference to the extent of interpeptide cross-linking. Biken J. 1976 Dec;19(4):139–150. [PubMed] [Google Scholar]
- Katz W., Martin H. H. Peptide crosslinkage in cell wall murein of Proteus mirabilis and its penicillin-induced unstable L-form. Biochem Biophys Res Commun. 1970 May 22;39(4):744–749. doi: 10.1016/0006-291x(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S. Preparation of arthritogenic hydrosoluble peptidoglycans from both arthritogenic and non-arthritogenic bacterial cell walls. Infect Immun. 1977 Jun;16(3):861–866. doi: 10.1128/iai.16.3.861-866.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. P., Fleck J., Mock M., Ghuysen J. M. The wall peptidoglycans of Neisseria perflava, Moraxella glucidolytica, Pseudomonas alcaligenes and Proteus vulgaris strain P18. Eur J Biochem. 1973 Oct 5;38(2):301–306. doi: 10.1111/j.1432-1033.1973.tb03062.x. [DOI] [PubMed] [Google Scholar]
- Pearce S. M., Kaethler A. H. Extent of cross-linking of germ cell wall of a variant of Bacillus cereus. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1251–1256. doi: 10.1016/s0006-291x(77)80114-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Shockman G. D. Characterization of the presumed peptide cross-links in the soluble peptidoglycan fragments synthesized by protoplasts of Streptococcus faecalis. J Bacteriol. 1975 Oct;124(1):410–418. doi: 10.1128/jb.124.1.410-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Hammes W. P., Kandler O. Effect of endogenous and exogenous factors on the primary structures of bacterial peptidoglycan. Adv Microb Physiol. 1976;13:245–292. doi: 10.1016/s0065-2911(08)60040-5. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEBE I. EXTENT OF CROSS LINKAGE IN THE MUREIN SACCULUS OF ESCHERICHIA COLI B CELL WALL. Biochim Biophys Acta. 1965 Mar 1;101:124–126. doi: 10.1016/0926-6534(65)90038-2. [DOI] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: penicillin enhancement of peptidoglycan hydrolysis. Infect Immun. 1977 Dec;18(3):717–725. doi: 10.1128/iai.18.3.717-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: relationship between autolysis in buffer and the hydrolysis of peptidoglycan. Infect Immun. 1977 Oct;18(1):210–219. doi: 10.1128/iai.18.1.210-219.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Katz W., Martin H. H. Murein (peptidoglycan) structure of Vibrio fetus. Comparison of a venereal and an intestinal strain. Biochim Biophys Acta. 1971 Jul 20;244(1):58–64. doi: 10.1016/0304-4165(71)90120-6. [DOI] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



