Abstract
Aims:
To assess the severity of acute pancreatitis (AP) using computed tomography (CT) severity index (CTSI) and modified CT severity index (MCTSI), to correlate with clinical outcome measures, and to assess concordance with severity grading, as per the revised Atlanta classification (RAC).
Materials and Methods:
In this prospective study approved by the Institutional Review Board (November 2014 to March 2016), sixty patients with AP (as per the RAC definition) underwent contrast-enhanced computed tomography (CECT) 5–11 days (median 6 days) after symptom onset. Two radiologists, blinded to clinical parameters, independently assessed CTSI and MCTSI (differences were resolved by consensus). Clinical outcome parameters included duration of stay in the hospital and intensive care unit (ICU), presence of persistent organ failure (OF), evidence of infection, need for intervention, and mortality.
Results:
We included 60 cases [36 males, age range 19–65 (mean 37) years]. As per the RAC, 26 patients had mild AP, 12 moderately severe, and 22 severe AP. According to CTSI and MCTSI, mild, moderate, and severe cases were 27 (45%), 19 (31.7%), 14 (23.3%) and 24 (40%), 10 (16.7%), 26 (43.3%), respectively. MCTSI was concordant with the RAC grading in 54 (90.0%), CTSI was concordant in 47 (78.3%), and both were concordant in 43 (71.7%) cases. Area under the receiver-operating characteristic (ROC) curves (AUROC) was compared by the Hanley and McNeil method. Both CTSI and MCTSI were significantly associated with outcome parameters (P < 0.001), except duration of ICU stay. Sensitivity, specificity, positive predictive value (PPV), and accuracy of CTSI for detecting moderate/severe disease were 97.1%, 100%, 100%, and 98.3% respectively, and of MCTSI were 100%, 92.3%, 94.4%, and 96.7% respectively.
Conclusion:
Both CTSI and MCTSI showed significant correlation with clinical outcome parameters, and good concordance with RAC grading of severity. MCTSI showed a higher sensitivity but lower specificity than CTSI in differentiating mild from moderate/severe AP.
Keywords: Acute pancreatitis, contrast-enhanced computed tomography, CT Severity Index, interstitial edematous pancreatitis, modified CT severity index, necrotizing pancreatitis, organ failure, revised Atlanta classification
Introduction
Acute pancreatitis (AP) is a complex disease with a variable clinical course. The majority of patients with mild disease recover completely, where as approximately 15–20% of patients develop clinically severe AP with local and systemic complications; and mortality in this group may reach 20–30%.[1,2] Identification of patients with clinically severe AP is important as these patients may benefit from transfer to a specialized or intensive care unit (ICU), where they can receive aggressive fluid resuscitation and be closely monitored for the development of organ failure (OF). Severity stratification is important during the initial work-up of cases, and a number of clinical and/or laboratory as well as computed tomography (CT) prognostic scoring systems are in use.[3,4,5,6,7,8,9,10,11,12,13,14] Two commonly used CT scoring systems –CT severity index (CTSI), designed by Balthazar et al.,[8] and modified CT severity index (MCTSI), proposed by Mortele et al.[9]–require the use of intravenous (IV) contrast agents to determine the presence and extent of pancreatic necrosis, as well as inflammatory changes and local and/or extrapancreatic complications.
In 1992, the Atlanta classification was proposed, which divided AP into two groups, i.e., mild and severe.[15] In 2012, this classification was revised with an aim to standardize and clarify various terminologies related to AP, as a review of previous studies revealed a number of inconsistencies and variations in interpretation of definitions.[16] This revised Atlanta classification (RAC) classified “severity” of AP as mild, moderately severe, and severe, and clearly defined the various types of collections.[17,18,19,20]
Wide availability and excellent spatial resolution of contrast-enhanced CT (CECT) make it the most commonly used imaging modality in AP for diagnosis, severity assessment, and in morphological classification, which is the basis of RAC. CT imaging helps in the delineation of pancreatic and/or peripancreatic necrosis, inflammatory changes, morphology of fluid collections to assess the presence of drainable fluid before intervention, and monitoring of treatment response through follow-up studies.
Recent literature highlights the judicious use of CECT in AP patients.[18] Majority of the patients do not require a CT for diagnosis of AP. CECT is not indicated initially in patients who are clinically stable and show rapid improvement. However, CECT should be performed in patients who develop or are likely to develop severe AP or complications related to AP. The ideal time for performing CT scan is at least after 72 hours from the onset of symptoms.[17] RAC recommendations state that CECT should be done after 5 to 7 days of onset of symptoms for detecting necrosis, which is easily underestimated by immediate CT.[18]
Severity assessment of AP has been described by using various CT scoring systems,[8,9,10,11] the most commonly used on a CECT are CTSI and MCTSI. There is a lack of comparative studies between these two radiologic scoring systems and the severity grading according to the RAC. Therefore, the aim of our study was to assess the severity of AP using CTSI and MCTSI, to correlate with the clinical outcome measures, and to assess concordance with severity assessment as per the RAC.
Materials and Methods
Study design and patient selection
In this prospective study, we evaluated 74 (adult) patients with a diagnosis of AP, defined as per the RAC[17,18,21] who underwent a CT abdomen between November 2014 and March 2016. Institutional review board approval and ethical clearance for this study were obtained (IRB number: LHMC/ECHR/2014/504). After taking an informed consent from each patient, detailed demographic data, clinical history and examination, and laboratory data were reviewed, along with the CT findings. Of the total of 74 patients, 13 cases with imaging findings suggestive of acute on chronic pancreatitis were excluded. One patient of AP was excluded for undergoing an unenhanced CT scan. The final study group consisted of 60 patients (36 males, 24 females; mean age 36.6 years; age range 19–65 years) who underwent CECT abdomen. CT was performed at least 5 days after onset of pain, and within 12 days in all cases (median of 6 days; range of 5–11 days). The clinical assessment was performed by the treating clinicians and recorded in the patients' files. A surgeon with 17 years' experience collaborated on the study.
Imaging technique
In all 60 patients, CECT scan was performed on a 40 slice CT scanner (Philips Brilliance). Non-ionic, iodinated contrast (Iopomide–Ultravist 370), 70–100ml contrast material (at a dose of 1.5 ml/kg) was administered intravenously by using a pressure injector at the rate of 3 ml/s, followed by a saline chase of 20 ml normal saline at a rate of 2.5 ml/s. Post-contrast scanning was done in porto-venous phase (70s), and the scans were obtained in the cranio-caudal direction from the domes of the diaphragm to the level of pubic symphysis in the supine position. Scan parameters used were as follows: 120 kVp, 200 mA/slice. Axial CT sections were taken at a collimation of 40 × 0.625 and a pitch of 0.9, and were reconstructed at 3 mm thickness, increment of −1.5 mm.
Image analysis
Two radiologists (with 14 years and 31 years experience), who were blinded to the patient outcome parameters, reviewed all imaging studies and recorded all pancreatic, peripancreatic findings, local, as well as extrapancreatic complications. They independently scored the severity grading of all patients, and any differences between the two readers were subsequently resolved by consensus to obtain a consensus score. We used two CT scoring systems –CTSI, developed by Balthazar et al.,[8] and MCTSI, developed by Mortele et al.[9] Total score in both the CT scoring systems is 10 points. The morphologic severity of AP according to CTSI was categorized as mild (0–3 points), moderate (4–6 points), or severe (7–10 points); similarly, according to MCTSI as mild (0–2 points), moderate (4–6 points), or severe (8–10 points). Both scores were calculated during the same interpretation session.
Definitions
OF was defined as a score of 2 or more in one or more of the three organ systems (respiratory, renal, and cardiovascular) of the modified Marshall score, and a score of 2 or more in ≥2 system defined multiorgan failure.[22] OF was further categorized as transient (duration <48 h) or persistent (duration >48 h). As per the RAC, we defined three degrees of severity of AP: mild AP –no OF and absence of local complications; moderately severe (moderate) AP–transient OF <48h or local complications or exacerbation of comorbid disease in the absence of persistent OF; and severe AP–persistent OF >48 h. We categorized AP on the basis of imaging as acute interstitial edematous pancreatitis (IEP) and acute necrotizing pancreatitis (NP). Pancreatic necrosis on CECT was defined as visualization of one or more areas of nonenhancing pancreatic parenchyma. Peripancreatic necrosis was demonstrated as extrapancreatic areas of non-enhancement containing nonliquefied components (seen as heterogeneous areas of increased attenuation).[17,18,20]
Local complications were defined as per the RAC, either the presence of acute collections [acute peripancreatic fluid collection (APFC) or acute necrotic collection (ANC)] or vascular complications (splenic/portal vein thrombosis or arterial pseudoaneurysm). An acute peripancreatic fluid collection was defined as a fluid collection with no definable wall in the peripancreatic region, seen in patients with acute IEP, confined by the normal peripancreatic fascial planes and limited to anatomic boundaries of retroperitoneum. Collections containing variable amounts of fluid as well as solid (necrotic) material secondary to pancreatic and/or peripancreatic necrosis were defined as acute necrotic collections.[17,18,19,20]
Evidence of infection was defined as follows–clinical evidence of infection, i.e., development of fever and/or leukocytosis during hospital stay, and radiological evidence of infection, i.e., presence of gas within pancreas or peripancreatic tissue, in the absence of intervention. Intervention was defined as percutaneous catheter drainage or surgical necrosectomy.
Clinical outcome parameters
Clinical follow-up data for all patients were collected until discharge or demise. Clinical outcome parameters were noted in terms of duration of hospital stay, duration of ICU stay, occurrence of persistent organ failure, evidence of infection, need for intervention, and mortality. Clinical outcome parameters were compared with severity grading according to CTSI and MCTSI scores in all the cases. Furthermore, both the CT severity scores were compared with grading of severity as per the RAC (mild/moderately severe/severe AP).
Statistical analysis
Normally distributed variables were expressed as mean ± standard deviation (SD) and continuous variables with skewed distribution as median (range). The qualitative data were expressed as number (percentage). Comparison of categorical variables was done using the Fisher's exact test or Chi-square test. A P value of 0.05 was considered as statistically significant. Receiver operating characteristic (ROC) curves were used to assess the performance of the various scores (except duration of hospital stay that was calculated by Pearson's correlation coefficient). Interobserver agreement between the two observers for assessing the severity of pancreatitis (mild, moderate or severe) was calculated for both CTSI and MCTSI scores using the Cohen's kappa statistic. The different diagnostic measures such as sensitivity, specificity, and predictive values were reported with respect to the clinical assessment of severity (for the purpose of differentiating “mild” disease from “moderate/severe” disease). The comparison of the area under the ROC curves (AUROC) for CTSI and MCTSI was performed by the Hanley and McNeil method. The data was analyzed using IBM SPSS Statistics software (version 20.0, Chicago, IL, USA) and Medcalc software (version 15.11.4, MedCalc Software, Ostend, Belgium).
Results and Observations
Demographic characteristics, imaging findings, complications, and outcomes
The most common clinical presentation was epigastric pain in 47 (78.3%) patients, followed by vomiting in 46 (76.7%). Chronic alcohol abuse was the most common cause of AP (n = 30, 50.0%), followed by gallstone disease (n = 15, 31%). The details of demographic characteristics, etiologies, imaging findings, local complications, and outcomes are shown in Table 1. Two types of acute pancreatitis were identified on CECT– IEP in 46.7% (28/60) and NP in 50% (30/60). Two patients with (mild) AP showed no CT abnormality (radiologically normal). Acute collections were noted in 53.3% of the cases overall; however, were much more common in cases of NP (29/30, 96.7% showed acute necrotic collections) rather than IEP (3/28, 10.7% showed acute peripancreatic fluid collections). Out of the 30 patients with NP, 29 patients had both pancreatic parenchymal and peripancreatic necrosis. OF was noted in 25 (41.7%) cases, of which 21 (35%) had persistent OF. In 25 patients, there was evidence of 34 episodes of OF. Renal system was the most common system involved followed by cardiovascular and respiratory systems.
Table 1.
Demographic characteristics, imaging findings, complications, and outcomes in patients with acute pancreatitis (n=60)
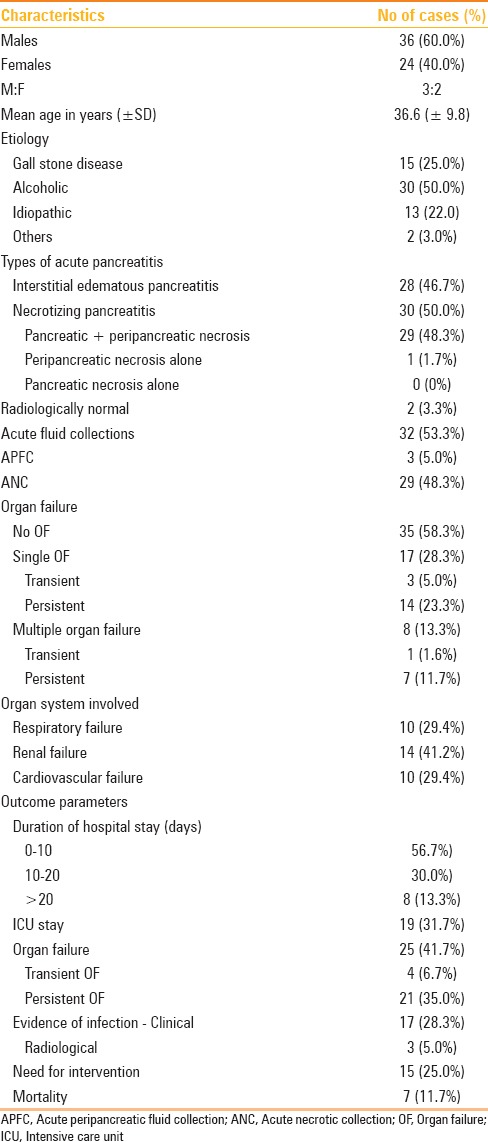
The median duration of hospital stay was 8 days (range, 3–71 days), and 43.3% cases had a hospital stay of more than 10 days. Seventeen (40.0%) patients had clinical evidence of infection, where as only 3 out of these 17 showed radiological evidence of infection. Intervention was required in 15 (25%) cases. Out of 60 patients, 53 recovered while 7 died. All the patients who died had evidence of infection. Moreover, of all the patients with evidence of infection, 7/17 (41%) cases died. There was a significant association (P < 0.001) between evidence of infection and mortality. All the patients who died had persistent OF, and there was a significant association between presence of persistent OF and mortality.
Extrapancreatic findings
Extrapancreatic complications occurred in 33/60 (55%) patients in our study. The most common was pleural effusion, seen in 30/60 (50%) cases, followed by ascites. Venous thrombosis (involving splenoportal axis) was the most common vascular complication, seen in 16/60 (27%) patients.
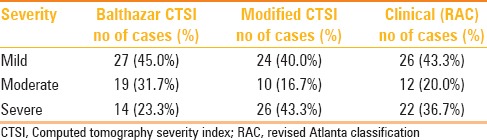
Severity grading of pancreatitis
As per the RAC, 26 (43.3%) patients had mild AP, 12 (20%) had moderately severe disease (transient OF in n = 3, presence of local complications in n = 9), and 22 (36.7%) had severe AP. Table 2 describes the distribution of severity (mild, moderately severe, or severe AP) –according to the revised Atlanta classification, CTSI, and MCTSI scoring systems.
Table 2.
Comparison of pancreatitis severity according to the Balthazar CTSI, modified CTSI scoring system, and revised Atlanta classification
Interobserver agreement
The calculated kappa statistic for interobserver agreement between the two radiologists was 0.89 for CTSI and 0.92 for MCTSI score, indicating excellent interobserver agreement for both scores.
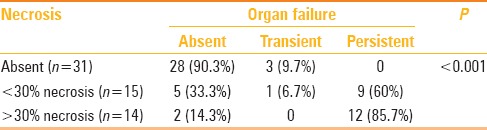
Presence of necrosis and organ failure
Table 3 outlines the correlation of pancreatic necrosis and OF. The amount of necrosis was directly related to the incidence of OF. Among patients with >30% necrosis, 12/14 (85.7%) had persistent OF. We found a significant association between necrosis on CECT and occurrence of persistent OF (P value < 0.001).
Table 3.
Correlation of pancreatic necrosis and organ failure
Correlation of CT scoring indices with outcome parameters
We found that CT severity assessment using both CTSI and MCTSI showed significant correlation with outcome parameters including mean duration of hospital stay, presence of persistent OF, evidence of infection, need for intervention, and mortality. Duration of ICU stay was the only outcome parameter, which did not show a significant correlation with CTSI/MCTSI scores. Table 4 outlines the correlation of adverse clinical outcomes and days of hospitalization with severity grading according to CTSI and modified CTSI. Table 5 shows the AUROC for CTSI and MCTSI scores in the prediction of severity parameters. Both CTSI and MCTSI were excellent in the prediction of these severity parameters. Pearson's correlation coefficient for CTSI and MCTSI for calculating duration of hospital stay was 0.634 and 0.594, respectively. For all severity parameters studied, no statistically significant difference was observed between CTSI and MCTSI scoring systems.
Table 4.
Comparison of patients with clinical severity parameters and days of hospitalization with severity grading according to CTSI and modified CTSI
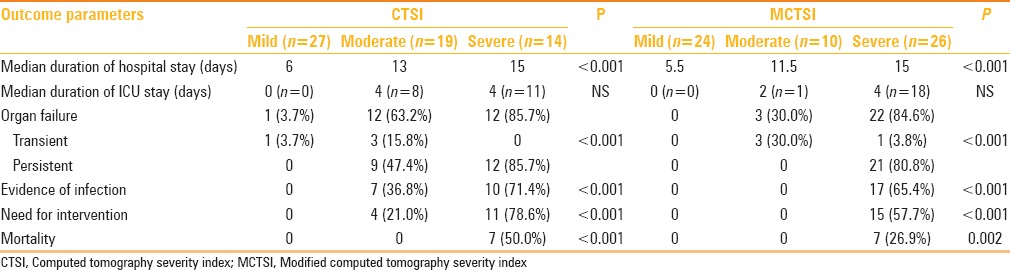
Table 5.
Area under ROC curves (AUROC) for CTSI and MCTSI for prediction of severity parameters
In our study, all patients who had evidence of infection (17/17 i.e., 100%), as well as all patients who required intervention (15/15 i.e., 100%) were categorized on MCTSI as “severe” pancreatitis. In contrast, on CTSI scoring, 7/17 (41.2%) cases who had evidence of infection were categorized as “moderate” and not severe pancreatitis. Similarly, 4/15 (26.7%) cases who required intervention were categorized as “moderate” pancreatitis on CTSI. Thus, MCTSI (and not CTSI) correctly categorized all cases that had evidence of infection or needed intervention. Similarly, all patients who developed persistent OF (21/21 i.e., 100%) were correctly categorized as “severe” pancreatitis by MCTSI scoring. On the other hand, while using CTSI scoring system, only 12/21 (57.1%) of these cases were labelled as “severe,” while as many as 9/21 (42.9%) were categorized as “moderate” pancreatitis. Thus, MCTSI (and not CTSI) correctly categorized all cases that developed persistent OF [Figure 1].
Figure 1(A-D).
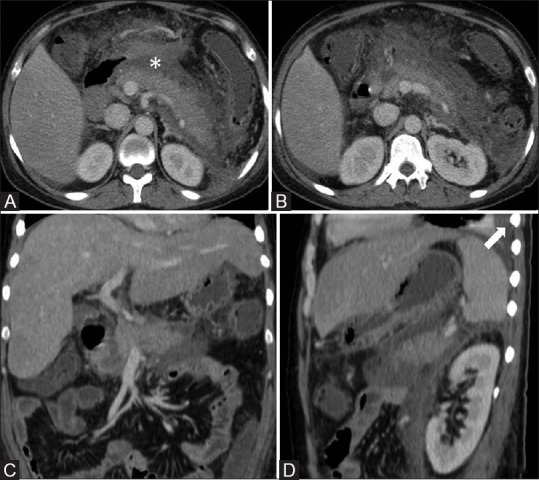
A 34-year-alcoholic-male with acute pancreatitis and persistent organ failure. Contrast-enhanced CT (Day 7) [axial (A and B), coronal (C), sagittal (D)] images show pancreatic necrosis <30%, acute necrotic collection (asterisk), and left pleural effusion (arrow). CTSI score was 6(discordant) and MCTSI score was 8 (concordant with RAC grading)
Concordance of CT scoring systems with revised Atlanta grading of acute pancreatitis
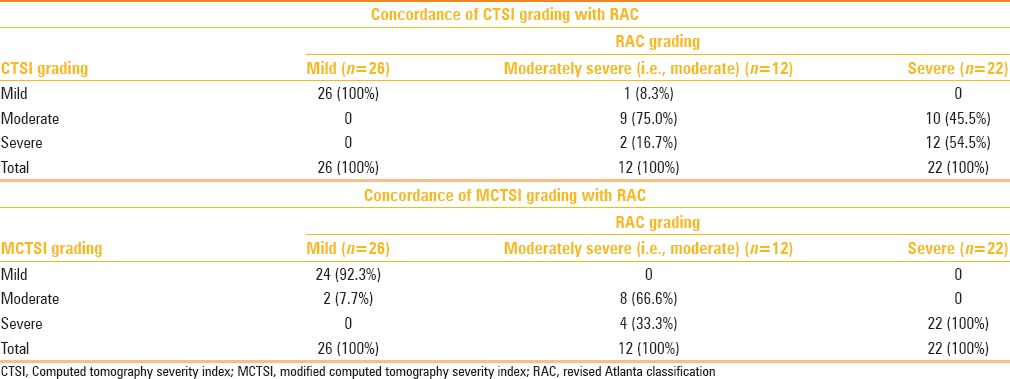
Table 6 outlines the concordance of CT scoring indices with the RAC grading of AP. With assessment of severity as per the RAC, MCTSI was concordant overall in 54 (90.0%) cases; CTSI was concordant overall in 47 (78.3%) cases, and both of them were concordant in 43 (71.7%) cases.
Table 6.
Concordance of CT scoring indices with the revised Atlanta grading of acute pancreatitis
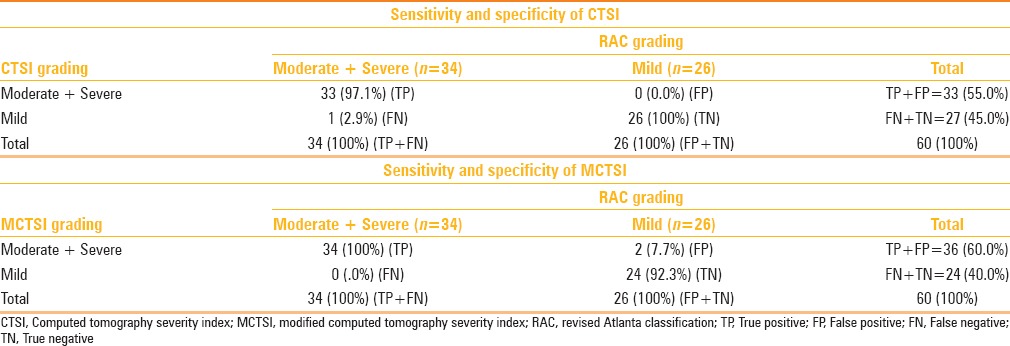
Sensitivity and specificity of CT scoring systems
For clinical decision-making, it is helpful to classify cases of AP as “mild” or “not mild”(i.e., moderately severe or severe). Hence, we assessed the sensitivity and specificity of CTSI and MCTSI for the diagnosis of moderate and severe disease, as shown in Table 7. The sensitivity, specificity, PPV, and accuracy of CTSI for detecting moderate and severe disease were 97.1%, 100%, 100%, and, 98.3% respectively. On the other hand, the sensitivity, specificity, PPV, and, accuracy of MCTSI for detection of moderate and severe disease were 100%, 92.3%, 94.4%, and, 96.7% respectively. Overall, MCTSI classification tended to assign a higher severity grade than CTSI to the same patient. In other words, MCTSI correctly categorized all severe cases, and CTSI correctly categorized all mild cases. Thus, CTSI had 100% specificity for categorization of moderate and severe cases; while its sensitivity was 97.1%. On the other hand, MCTSI had a sensitivity of 100%, whereas specificity was 92.3%.
Table 7.
Sensitivity and specificity of CTSI and MCTSI for detection of moderate/severe disease
Out of the 26 cases who were categorized as “severe” pancreatitis on MCTSI, 4/26 (15.4%) had no OF and no evidence of infection. These patients had a mild clinical course. On the basis of presence of local complication (i.e., collection, ANC), these cases were finally assigned a “moderately severe” category according to the RAC. All these 4 patients were young males (mean age of 35 years) who had a history of chronic alcohol abuse.
Discussion
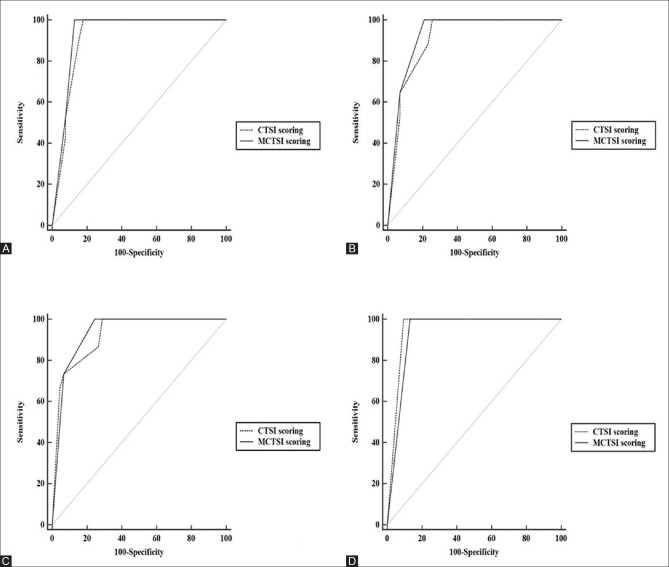
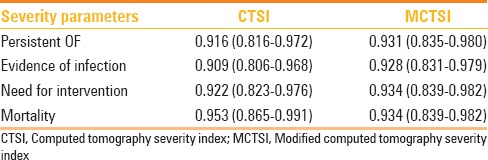
This study was performed to assess the severity of AP by two CT scoring systems i.e., CTSI and MCTSI, and correlate these scorings with clinical outcomes and assessment of severity according to the RAC, 2012. CT severity assessment using both CTSI and MCTSI showed significant correlation with outcome parameters including mean duration of hospital stay, presence of persistent OF, evidence of infection, need for intervention, and mortality [Figure 2]. Both CTSI and MCTSI showed good concordance with severity grading as per the RAC and excellent interobserver agreement between the two observers. MCTSI showed 100% sensitivity in categorizing moderate or severe disease, where as CTSI was less sensitive, though 100% specific, in doing so. However, between them overall, CTSI and MCTSI did not show a statistically significant difference in severity assessment.
Figure 2(A-D).
Area under ROC curves for CTSI (dashed line) and MCTSI (solid line) in predicting severity parameters [A, Persistent OF, B, Evidence of infection, C, Need for intervention, D, Mortality]
Modified CTSI differs from CTSI in two important aspects.[9] It includes points for extrapancreatic complications such as pleural effusion, ascites, vascular, or GI complications. Such complications were seen in 55% of our cases in this study who accordingly received an extra 2 points on MCTSI scoring as compared to CTSI. Moreover, MCTSI has a simpler quantification of amount of necrosis (as <30% or >30% only), which is easier to apply. In MCTSI scoring, points are given in increments of 2 – i.e., even scores only.
Previous studies[1,23] showed that both CTSI and MCTSI were significantly associated with all clinical outcome parameters, including length of hospital stay, admission to and length of ICU stay, persistent OF, pancreatic infection, need for intervention, mortality, and clinical severity of AP. The present study also produced similar results, except that we did not find significant association of these scores with the length of ICU stay. This may be partly due to the relatively small number of our patients (19/60) who had a stay in the ICU (the rest of the patients were managed in the ward). Furthermore, in the present study, both CTSI and MCTSI showed good concordance with severity grading as per the RAC, with MCTSI performing a little better (although this difference was not statistically significant). This is similar to the results of a recent study by Raghuwanshi et al.[24] Compared with the study by Bollen et al., a greater proportion of our cases had OF. This is likely because a higher percentage of our cases belonged to the severe and moderately severe categories of AP.
The present study showed a significant association between necrosis and persistent OF, which is in accordance with the study done by Wig et al.,[25] which showed a direct and significant relationship between the amount of necrosis and incidence of OF. We also found a significant association between persistent OF and evidence of infection with mortality. All of our patients who died had persistent OF as well as evidence of infection; while no mortality occurred in patients with no OF or transient OF; or those without evidence of infection. This is in accordance with previous studies, such asthose by Buchler et al.[26] and Johnson et al.[27]
We assessed the sensitivity and specificity of CTSI and MCTSI in the categorization of cases as “mild” or “not mild”(i.e., moderately severe or severe). MCTSI had a sensitivity of 100%, where as a specificity was 92.3%. CTSI had 100% specificity, where as its sensitivity was 97.1%. In other words, MCTSI correctly categorized all severe cases, though it slightly overestimated some mild and moderate cases. CTSI correctly categorized all mild cases. However, in moderate cases, it both underestimated and overestimated a few cases; among severe cases, it underestimated almost half the cases as moderate grade. MCTSI (and not CTSI) correctly categorized all cases that had evidence of infection or needed intervention and also all cases that developed persistent OF. In the clinical setting, this increased sensitivity of MCTSI gives it an edge over CTSI assessment – it is imperative to identify all patients of AP who may have a potentially complicated clinical course to institute appropriate management strategies in a timely manner.
We encountered a subset of patients (4/26, 15.4%) with CT findings suggestive of severe AP–all these cases had necrosis of more than 30%, acute necrotic collections, as well as extrapancreatic complications. However, the clinical course of these patients did not match the CT assessment of severity–they had no OF, no evidence of infection and had a mild clinical course. These patients were all young males with AP related to chronic alcohol intake. These findings do suggest that such patients may do better clinically than their CT findings suggest. Further studies with larger sample sizes are needed to confirm these results.
The present study had certain limitations. Our study population was biased towards cases of more severe AP. This is because there was a significant proportion of patients of AP who had mild symptoms and were diagnosed clinically (on the basis of typical clinical presentation and elevated serum amylase and/or lipase activity) and these patients did not undergo CECT scanning. This is in accordance with the current recommendations for cross-sectional imaging in cases of AP.[1,18,28] Patients with mild AP usually do not require pancreatic imaging.
We compared the CT assessment of severity with clinical grading, as per the RAC, which is currently the recommended classification for AP. However, as the RAC is being increasingly applied in clinical practice, few limitations are getting recognized.[29] Some of these include underestimation of the effect of infected necrosis and/or extrapancreatic infections on the outcome of AP. In the present study, we did separately assess, and found significant correlation between, the grading according to CTSI and MCTSI scoring systems, and various outcome parameters, including evidence of infection. In addition, the “moderately severe” category of AP (according to RAC) includes patients with transient OF, which is a clinical parameter, as well as patients with local complications, which are detected on imaging, usually on CECT. Hence, the final severity category assigned to our patients, according to the RAC, required information regarding local complications that was derived from the CT study itself. Patients who did not have OF, but CECT showed local complications, were graded as “moderately severe” AP. This, however, is what is followed as the standard clinical practice. Another limitation of this study was that we did not compare CECT scoring systems (CTSI/MCTSI) with clinical scoring systems such as bedside index for severity in acute pancreatitis (BISAP) score, which have also been shown to be accurate in severity assessment of AP.[22] Moreover, both CTSI and MCTSI scores were recorded in the same interpretation session. This implies that the radiologist assigning the MCTSI score was aware of the CTSI score and vice-versa, thus introducing a potential bias.
Conclusion
In conclusion, we found that both CTSI and MCTSI showed significant correlation with clinical outcome parameters, as well as good concordance with grading of severity as per the revised Atlanta classification. MCTSI showed a higher sensitivity whereas CTSI showed a higher specificity in differentiating between mild AP and moderate or severe disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, et al. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. Am J Roentgenol. 2011;197:386–92. doi: 10.2214/AJR.09.4025. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan S, Forsmark CE. The difficulty in predicting outcome in acute pancreatitis. Am J Gastroenterol. 2010;105:443–5. doi: 10.1038/ajg.2009.623. [DOI] [PubMed] [Google Scholar]
- 3.Ranson JHC, Ritkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in Acute Pancreatitis. SurgGynecolObstet. 1974;139:69–81. [PubMed] [Google Scholar]
- 4.Knaus WA, Zimmermann JE, Wagner DP, Draper EA, Lawrence DE. APACHE- acute physiology and chronic health evaluation: A physiologically based classification system. Crit Care Med. 1981;9:591–7. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: Alarge population- based study. Gut. 2008;57:1698–703. doi: 10.1136/gut.2008.152702. [DOI] [PubMed] [Google Scholar]
- 6.Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340–6. doi: 10.1136/gut.25.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 8.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JHC. Acute pancreatitis: Value of CT in establishing prognosis. Radiology. 1990;174:331–6. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 9.Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN. A modified CT severity index for evaluating acute pancreatitis: Improved correlation with patient outcome. Am J Roentgenol. 2004;183:1261–5. doi: 10.2214/ajr.183.5.1831261. [DOI] [PubMed] [Google Scholar]
- 10.De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: Evaluation of a new scoring system. Pancreas. 2007;34:185–90. doi: 10.1097/mpa.0b013e31802d4136. [DOI] [PubMed] [Google Scholar]
- 11.Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612–9. doi: 10.1038/ajg.2011.438. [DOI] [PubMed] [Google Scholar]
- 12.Leung T-K, Lee C-M, Lin S-Y, Chen H-C, Wang H-J, Shen L-K, et al. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World J Gastroenterol. 2005;14(11):6049–52. doi: 10.3748/wjg.v11.i38.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21:2387–94. doi: 10.3748/wjg.v21.i8.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koziel D, Gluszek S, Matykiewicz J, Lewitowicz P, Drozdzak Z. Comparative analysis of selected scales to assess prognosis in acute pancreatitis. Can J GastroenterolHepatol. 2015;29:299–303. doi: 10.1155/2015/392643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley EL., III A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga. Arch Surg. 1993;128:586–90. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 16.Bollen TL, van Santvoort HC, Besselink MG, van Leeuwen MS, Horvath KD, Freeny PC, et al. Dutch Acute Pancreatitis Study Group. The Atlanta Classification of acute pancreatitis revisited. Br J Surg. 2008;95:6–21. doi: 10.1002/bjs.6010. [DOI] [PubMed] [Google Scholar]
- 17.Thoeni RF. The revised Atlanta classification of acute pancreatitis: Its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751–64. doi: 10.1148/radiol.11110947. [DOI] [PubMed] [Google Scholar]
- 18.Banks PA, Bollen TL, Dervenis C, Goosven HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definations by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 19.Zaheer A, Singh VK, Qureshi RO, Fishman EK. The revised Atlanta classificationfor acute pancreatitis: Updates in imaging terminology and guidelines. Abdom Imaging. 2013;38:125–36. doi: 10.1007/s00261-012-9908-0. [DOI] [PubMed] [Google Scholar]
- 20.Sureka B, Bansal K, Patidar Y, Arora A. Imaging lexicon for acute pancreatitis: 2012 Atlanta Classification revisited. Gastroenterol Rep. 2016;4:16–23. doi: 10.1093/gastro/gov036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarr MG, Banks PA, Bollen TL. Revision of the Atlanta classification of acute pancreatitis. Acute Pancreatitis Classification Workgroup [Internet] 2008. [cited 2011 Apr 8]. Available from http://www.pancreasclub.com/resources/AtlantaClassification .
- 22.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Sharma V, Rana SS, Sharma RK, Kang M, Gupta R, Bhasin DK. A study of radiological scoring system evaluating extrapancreatic inflammation with conventional radiological and clinical scores in predicting outcomes in acute pancreatitis. Ann Gastroenterol. 2015;28:399–404. [PMC free article] [PubMed] [Google Scholar]
- 24.Raghuwanshi S, Gupta R, Vyas MM, Sharma R. CT Evaluation of Acute Pancreatitis and its Prognostic Correlation with CT Severity Index. J ClinDiagn Res. 2016;10:6–11. doi: 10.7860/JCDR/2016/19849.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wig JD, Bharathy KG, Kochhar R, Yadav TD, Kudari AK, Doley RP, et al. Correlates of organ failure in severe acute pancreatitis. JOP. 2009;10:271–5. [PubMed] [Google Scholar]
- 26.Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: Treatment strategy according to the status of infection. Ann Surg. 2000;232:619–26. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–4. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillip V, Steiner JM, Algül H. Early phase of acute pancreatitis: Assessment and management. World J Gastrointest Pathophysiol. 2014;5:158–68. doi: 10.4291/wjgp.v5.i3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manrai M, Kochhar R, Thandassery RB, Alfadda AA, Sinha SK. The Revised Atlanta Classification of Acute Pancreatitis: A Work Still in Progress? JOP. 2015;16:356–64. [Google Scholar]