Abstract
Although a common occurrence, cystic lesions of the pancreatico-biliary tree (PBT) may pose a diagnostic dilemma because they encompass a large number of neoplastic and benign processes with varied clinical symptoms. Knowledge of lesion classification and characterization are essential in making an accurate prospective diagnosis. This is necessary for identifying clinically significant cystic masses, which at times may require invasive intervention from indolent, nonneoplastic lesions, for which surveillance may suffice. Today, there is an arsenal of modalities for assessing the PBT, however, magnetic resonance imaging (MRI) remains at the forefront for characterizing cystic morphology and fluid content, internal septations, solid component, enhancement patterns, as well as assessing the surrounding normal structures. This pictorial review aims to review the spectrum of MRI features, which will aid in the differential diagnoses of cystic lesions of the PBT and mimickers, enabling the radiologist to reach a more confident diagnosis.
Keywords: Cystic lesions, MRI, pancreatic-biliary tree
Introduction
Cystic lesions of the PBT occur commonly ranging from simple cysts to malignancies, and may be due to developmental, inflammatory, or neoplastic etiologies. Because there is a tremendous variation in the treatment strategies and therapeutic interventions, being able to differentiate noninvasively the types of cystic tumors is of utmost importance. Identifying a benign entity will help prevent unnecessary surgical intervention while pinpointing a sinister process in a timely manner promotes aggressive lifesaving management.
At present, there is an armory of imaging modalities at our disposal for detecting and characterizing cystic lesions of the PBT. Commonly, primary detection is done on ultrasound or computed tomography (CT) scan usually an incidental finding or secondary to vague abdominal symptoms. Well respected modalities, both ultrasound and CT, have undeniable limitations for characterizing cystic tumors of the PBT. Body habitus, size of the lesion, and operator dependency are some of the well-documented boundaries of ultrasound imaging while CT scan has restrictions for actual characterization of the lesion, assessing the fluid content and sometimes even the enhancement pattern.
Magnetic resonance imaging (MRI) remains the leading modality of choice for an accurate assessment of the cystic lesions of the PBT because of its multisequential and multiplanar capabilities and spatial resolution. Morphological features of the cystic lesions on MRI usually correlate with histopathology. MR features that help define the specific entities include signal intensity and internal complexity, such as presence of septations, or enhancing solid components. Furthermore, recently diffusion-weighted imaging (DWI) has proven to be a beneficial tool for differentiating benign from more sinister lesions.[1] The spectrum of MRI features for the various entities will be reviewed. Throughout the manuscript, the educational imaging “pearls” for each tumour are presented in bold italics.
Biliary Tree Cystic Tumors
Congenital and development
Choledochal cysts
A rare developmental anomaly of the intra and extrahepatic biliary tree (IHBT/EHBT), categorized according to the location of cystic dilatation as per the Todani classification.[2] Higher in women of Asian descent the clinical presentation is usually in a younger age group (first or second decade of life), and usually following complications such as cholangitis, pancreatitis, or seldom malignancy. Occasionally, they present as an asymptomatic abdominal mass. Although the etiology is unclear, it is widely accepted that choledochal cysts arise from anomalous pancreatico-biliary ductal junctions. Todani classification remains the mainstay of characterizing these cysts:
Type I: IA – entire EHBT dilatation, IB – focal/segmental EHBT dilatation, IC – dilatation of the CBD segment only
Type II: True diverticulum from EHBD
Type III: EHBD dilatation within duodenal wall (choledochocele)
Type IV: IVA – cystic involvement of IHBT and EHBT, IVB – EHBT cysts only.
MRI can accurately classify cyst types, and calculi (if present) appears as hypointense foci within the T2 bright fluid [Figures 1 and 2].
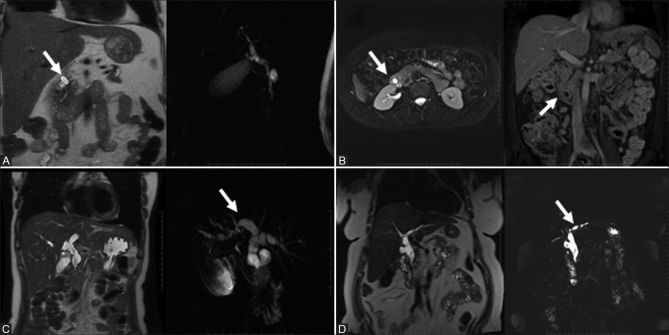
Figure 1(A-C).
Choledochal cysts type 1 (3 subtypes): Type 1A – dilatation of entire EHDT (A, top row); Type 1B – focal or segmental dilatation of the EHBT (B, middle row); Type 1C – dilatation of the CBD segment only (C, bottom row)
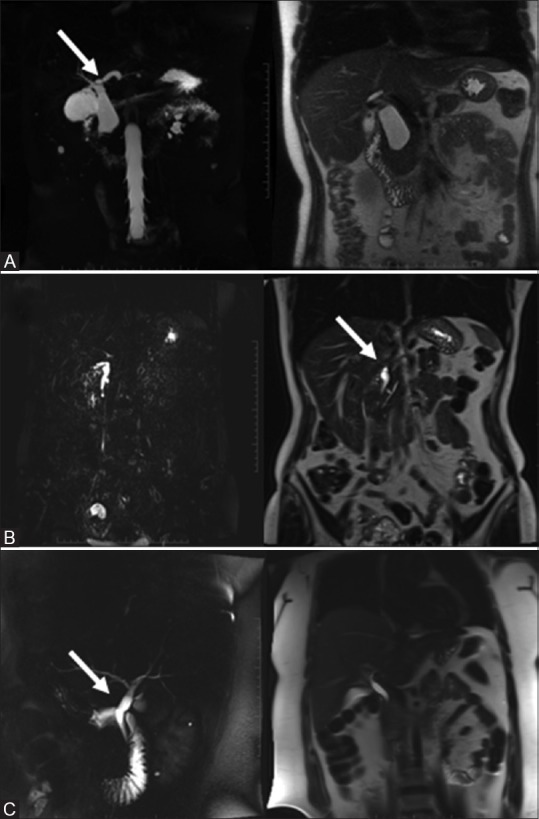
Figure 2(A-D).
Choledochal cysts: Type 2 – true diverticulum from EHBD (A, top row); Type 3 – choledochocele (B, top row); Type 4A – cysts of IHBT and EHDT (C, bottom row) and 4B – only EHBT cysts (D, bottom row)
Bile duct cysts (hamartomas)
Multiple biliary hamartomas are usually discovered incidentally. These Von Meyenburg complexes arise from embryonic bile ducts that fail to involute, and are usually encountered incidentally at imaging, laparotomy, or autopsy. They are well-circumscribed, <1.5 cm, show a hypointense signal to liver parenchyma on T1 weighted images, and usually show no uptake on gadolinium administration; however, recently a thin rim of enhancement has been described, which may represent the surrounding compressed liver tissue. Typically, they are hyperintense on heavily T2-weighted images without evidence of biliary communication [Figure 3A].
Figure 3(A and B).
Bile duct hamartomas: fluid attenuating, T2 hyperintense cyst with thin rim enhancement (A, top row). Caroli's disease: showing T2 bright intrahepatic cysts (arrow) in communication with the biliary tree (B, bottom row)
Caroli disease
Caroli disease (also known as congenital communicating cavernous ectasia of the biliary tree) is classified as a Type V choledochal cyst belonging to the spectrum of fibropolycystic liver disease. This is a developmental defect characterized by saccular dilatation of the intrahepatic bile ducts, and is often associated with ductal plate abnormalities such as hepatic fibrosis. Clinically, patients present with right upper quadrant pain and rarely jaundice. MRI demonstrates only intrahepatic cysts in continuity with the biliary tree [Figure 3B]. These are typically hypointense on T1-weighted images (T1WI) and markedly hyperintense on T2-weighted images (T2WI). The intraluminal portal vein radicals strongly enhance post gadolinium administration. Bridging across dilated ducts (which resemble internal septae) is also revealed on MRI.
Infectious/Inflammatory
Abscess
Biliary abscesses are usually secondary to acute obstructive suppurative cholangitis. The overall appearance of an abscess varies according to the pathological state of the infection. In an acute stage, on MRI, they appear as a cluster of T2 high intensity lesions with irregular margins and varied enhancement in the internal components as well as the surrounding parenchyma. Clinical suspicion helps support diagnosis. In the more common subacute stage, MRI shows a unilocular T1 hypointense, variable T2 hyperintense lesion (comprising central necrosis and liquefaction) with an enhancing, thick, and often increased enhancement in the surrounding parenchyma (”double target” sign) [Figure 4A]. The double target sign is attributed to increased capillary permeability and in the surrounding liver tissue, and this perilesional edema is used to differentiate a hepatic abscess from an indolent cystic lesion.
Figure 4(A and B).
Peribiliary abscess: “double target sign” (A, top row). Bilioma: extrahepatic cystic mass with thick T2 dark enhancing pseudocapsule (B, bottom row)
Bilioma
Biliomas are encapsulated bile collections secondary to rupture and trauma including iatrogenic trauma. They can be intrahepatic or perihepatic and clinical presentation depends on the size and location. Bile extravasation leads to an intense reaction resulting in a T2 hypointense pseudocapsule [Figure 4B]. With hepatobiliary contrast agents, delayed imaging may show contrast within the collection, confirming presence of bile leak. Typically, MRI shows a well-defined T2 hyperintense cystic mass with or without irregular border, calcification, or septations (depending on the chronicity).
Cystic Neoplasms
Benign
Biliary cystadenoma
Biliary cystadenomas (BCA) are rare (<5%), slow growing, multilocular cystic tumors,[3] which are generally intrahepatic, however, extrahepatic variants have been reported. They are seen to occur predominantly in middle-aged women and are considered premalignant. Clinically, patients often present with intermittent pain or symptoms of biliary obstruction. The internal fluid can be clear, proteinaceous, mucinous, and occasionally, gelatinous, purulent, or hemorrhagic.[3] The MR features typically show a T1 hypointense, T2 hyperintense multilocular cystic mass occasionally with thin septations; solid component are rare [Figure 5]. Variable signal intensities depend on internal hemorrhage, protein content, and seldom solid component.
Figure 5.
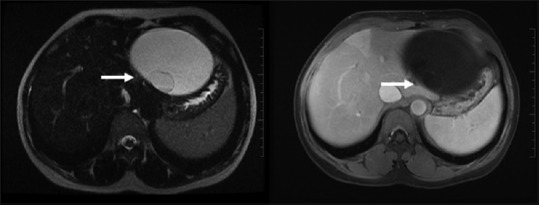
Biliary cystadenoma: Multiloculated cystic lesion showing minimal enhancement of the thin septations/internal locule. No solid components
Gall bladder adenomyomatosis
Also termed adenomatous hyperplasia of the gall bladder, it is a benign hyperplastic cholecystosis. Even though it is usually an incidental diagnosis made in patients in their 50s, age range is wide. This requires no surgical intervention and has a definitive appearance not to be confused with a gall bladder malignancy. It frequently coexists with cholelithiasis and rarely produces abdominal pain. MRI readily shows wall thickening, and the Rokitansky–Aschoff sinuses present as intramural lesions, which are hypointense on T1WI and hyperintense on T2WI without enhancement on gadolinium administration. The “String of pearls/pearl necklace” sign, which alludes to the characteristically curvilinear arrangement of multiple rounded hyperintense intraluminal cavities on T2WI [Figure 6A], are typical for hyperplastic cholesterosis. Intraluminal calculi appear as signal voids, and abnormal enhancement patterns, if seen, should raise the suspicion of a more sinister process at work and warrants a more aggressive approach.
Figure 6(A and B).
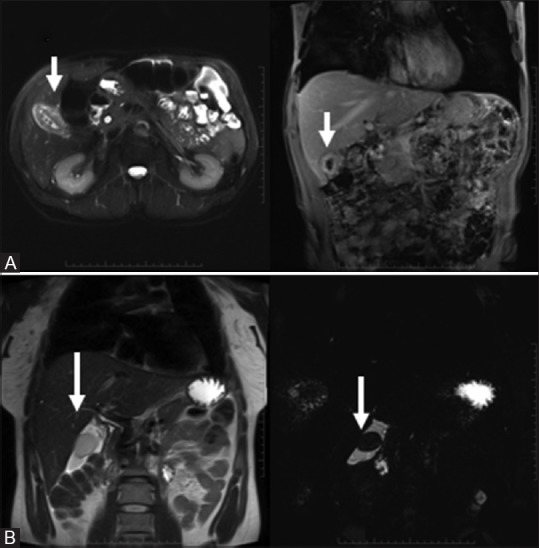
Adenomyomatosis: “String of pearl” on T2WI (A, top row); Lymphangioma: T2 bright structure engulfing the gallbladder without mass effect (B, bottom row)
Lymphangioma
These are benign lesions that show lymphatic differentiation and are of vascular origin. Occurring more commonly in women, the most common presentation is of dull intermittent right upper quadrant pain. Typical MR appearance is that of a cystic process engulfing the gallbladder without mass effect and minimal inhomogeneous enhancement at times [Figure 6B]. Signal drop may be seen on out-of-phase chemical shift sequence. Occasionally, the lymphangioma may compress the gall bladder lumen, however, no communication with the biliary tree is identified.
Malignant
Biliary cystadenocarcinoma
This malignant counterpart of BCA is also a rare, usually slow growing intrahepatic neoplasm arising from hepatobiliary epithelium. Typically seen in middle-aged women who present with abdominal discomfort. MRI and MRCP, because of increased contrast and spatial resolution, are more specific imaging modality in the detection of these neoplasms. MRI demonstrates a T1 dark, T2 bright multilocular appearing cystic mass with thick internal septations and gadolinium administration shows enhancing mural nodules [Figure 7].[4] It remains pertinent to differentiate from a cystadenoma (the benign variant) because, although these are slow growing, they warrant aggressive surgical intervention.
Figure 7.
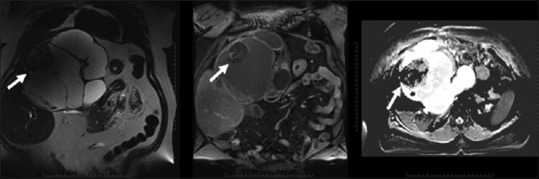
Biliary cystadenocarcinoma: Multilocular cystic mass with enhancing solid component which suppresses on ADC
Mucin hypersecreting carcinoma of the biliary tree
Also known as intraductal papillary mucinous tumors of the bile ducts, these account for approximately 7% of biliary neoplasms.[5] These present clinically as acute cholangitis attributed to intermittent obstruction of the biliary tree. They share a striking similarity to intraductal papillary mucinous tumor of the pancreas in its histopathological features, production of a large amount of mucin, pathophysiologic characteristics, and resultant clinical manifestations.
MR remains the modality of choice for depicting and characterizing the neoplasms. They exhibit distinct dilatation of the EHBT downstream from the cystic lesion secondary to hypersecretion of mucous [Figure 8A]. Depending on tumor size, occasionally an enhancing small flat or fungating mass may be identified within the ducts, however, not necessarily identified.
Figure 8(A and B).
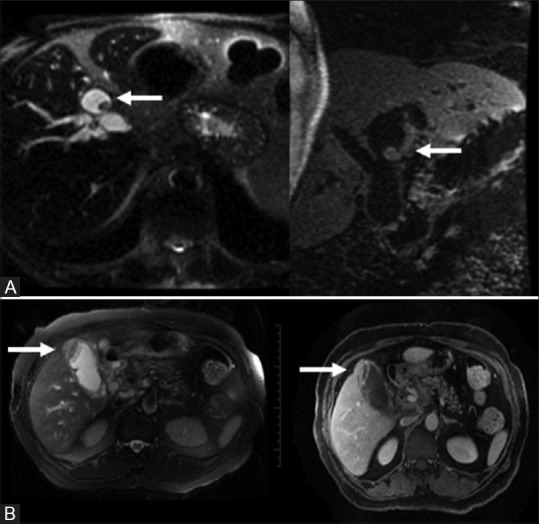
Mucin hypersecreting carcinoma: Mucin filled dilatation of the biliary tree with an enhancing nodule (A, top row). Cystic adenocarcinoma of the gall bladder: complex gall bladder mass with enhancing mural nodules (B, bottom row).
Cystic adenocarcinoma of the gallbladder
While biliary cystadenoma and cystadenocarcinoma of the liver are more common entities, BCA of the gall bladder is an extremely rare lesion often making diagnosis challenging and problematic. The origin of these lesions from the gallbladder can be confirmed by either an endoscopic retrograde cholangiopancreaticography (ERCP) to demonstrate communication with the cystic duct or an angiography to demonstrate the blood supply from a cystic artery; however, MRI is a safe noninvasive modality to illustrate these rare malignancies. MRI imaging shows a complex T1 hypointense, T2 hyperintense intramural mass with enhancing nodules, which are specific for this malignancy [Figure 8B]. Differentiation from lymphangioma and hydatid cyst is warranted but relatively simple on MRI.
Pancreatic Cystic Tumors
Congenital and development
Simple cysts (including mucinous nonneoplastic cysts)
These cysts show mucinous differentiation of the epithelial lining, but lack surrounding ovarian stroma that is characteristic of mucinous cystadenomas. They also have no ductal communication, cellular atypia or internal septae or solid component, and have no malignant potential; hence, reassuring to be followed or even ignored after long-term assessment. Their benign histology correlates excellent on MRI. Typically, these are simple T1 dark, T2 bright unilocular small lesions with thin walls, no internal enhancement or solid components, and interval stability on long term surveillance [Figure 9A].
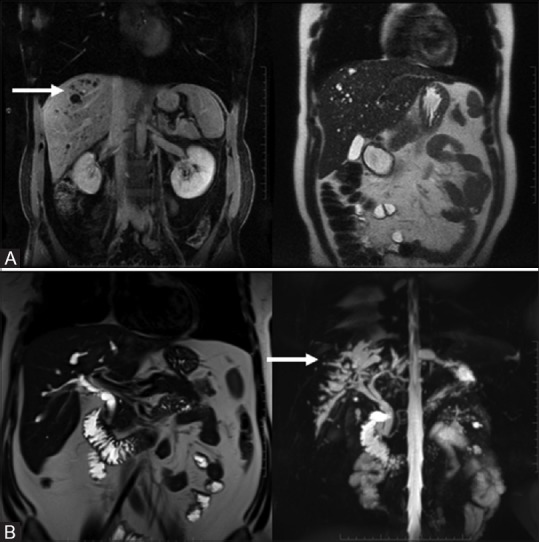
Figure 9(A-C).
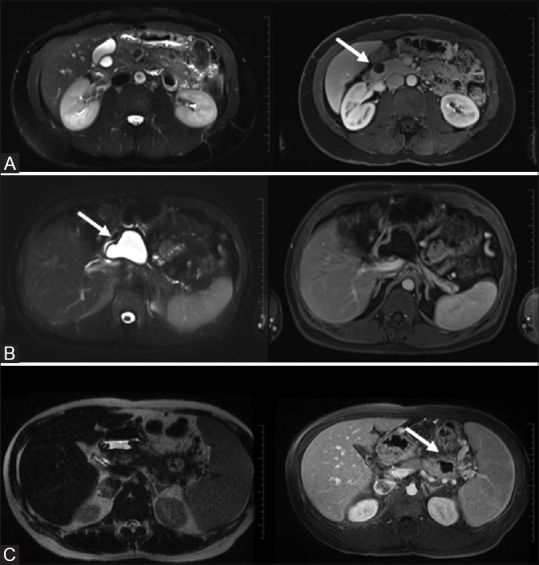
Simple cysts: incidental T2 bright non-enhancing cyst (A, top row). Pseudocyst: well-circumscribed complex cyst with no significant peripancreatic inflammation (B, middle row). Abscess: irregular thick walled cyst with significant peripancreatic inflammation (C, bottom row)
Infectious/Inflammatory
Pseudocyst
Overall, pseudocysts are the most common cystic pancreatic lesions. Usually, occurring as a sequel of pancreatitis, secondary to hemorrhagic fat necrosis and encapsulation of pancreatic secretions by granulation tissue and a fibrous capsule. The MRI appearance of pseudocysts may evolve over time; they are often irregularly marginated early in their formation but become well circumscribed, with a thickened enhancing wall, over a period of several weeks. MRI may be the imaging modality of choice for associated pancreatic parenchymal disease.
MR shows a well-circumscribed cyst with occasional high T1 signal (blood products, necrotic debris), no internal enhancing components, variable wall thickness which is smooth, and at times mild peripancreatic inflammatory changes [Figure 9B].
Abscess
A late complication (>4–6 weeks) of acute necrotizing pancreatitis. Blood products and necrotic or proteinaceous debris are commonly present, and on MRI produce intrinsically increased T1 signal intensity. The thickened and enhancing cyst wall seen on images correspond to granulation tissue and fibrosis that is confirmed on histologic analysis. Inflamed enhancing thick-walled irregular collection with variable signal on MR, secondary to pus, tissue necrosis, and debris [Figure 9C]. Intervention of larger abscesses and psudocysts (>6 cm) is often implemented because there is a concern for superimposed infection increasing morbidity for an already susceptible patient.
Hydatid cyst
A worldwide zoonosis hydatid cyst is produced by the larval stage of the Echinococcus tapeworm (E. granulosus and E. multilocularis). Hydatid disease should be considered when a cystic lesion is identified in a patient who lives in or has come from an area in which the disease is endemic, especially if typical imaging characteristics are seen. Commonly seen in the liver and rarely in the pancreas, appearance of hydatid cyst varies depending on the stage of cyst growth (i.e., whether the cyst is unilocular, contains daughter vesicles, contains daughter cysts, is partially calcified, or is completely calcified [dead]). MRI still remains the more relatable modality for an accurate diagnosis, however, counterpart imaging modalities such ultrasound and CT scan are also very useful. MR usually shows complex multivesicular T2 hyperintense cyst (honeycomb pattern) with low T2 signal fibrous wall (pericyst), incipient membrane detachment (wall irregularities), and daughter cysts [Figure 10]. Internal complexities vary signal changes on the T1WI and gadolinium administration may or may not show enhancement pattern (stage dependent).
Figure 10.
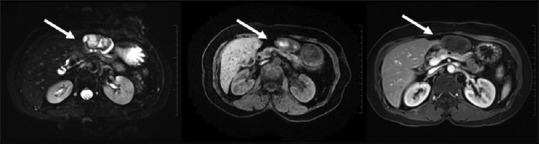
Hydatid cyst: multivesicular cysts with membrane detachment
Cystic Neoplasms
Benign
Serous neoplasms
Also referred to as microcystic cystadenoma, these are commonly found in women over 60 years of age; it is usually an incidental finding however nonspecific complaints of weight loss or even vague abdominal pain have been noted. These tumors are composed of multiple cysts with sizes varying from 0.2 to 2.0 cm. A central stellate scar with calcification has been observed and is known to be pathognomic to this tumor (seen as a signal void on MRI). There are three morphological patterns, namely, polycystic, honeycomb, and oligocystic. On MRI, the common polycystic has multiple cysts measuring <2 cm separated by fibrous septa and an enhancing central scar which may calcify.[6] The honeycomb has well-marginated subcentimeter (micro) cysts with mixed signal intensity and a sharp interface with vessels. The uncommon oligocystic/macrocystic variant is usually seen in the pancreatic head and demonstrates multicystic lobulated morphology with fewer large (>2 cm) cysts [Figure 11A–C]. Asymptomatic serous cystadenomas do not require surgical excision because they are rarely malignant. Tumors smaller than 2 cm have been reported, and are more likely to be serous cystadenomas, although this is not always the norm.
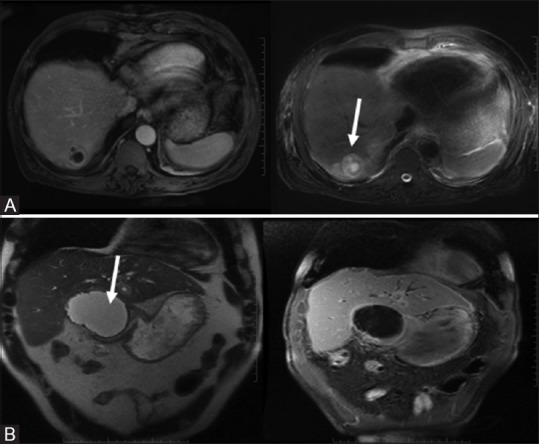
Figure 11(A-C).
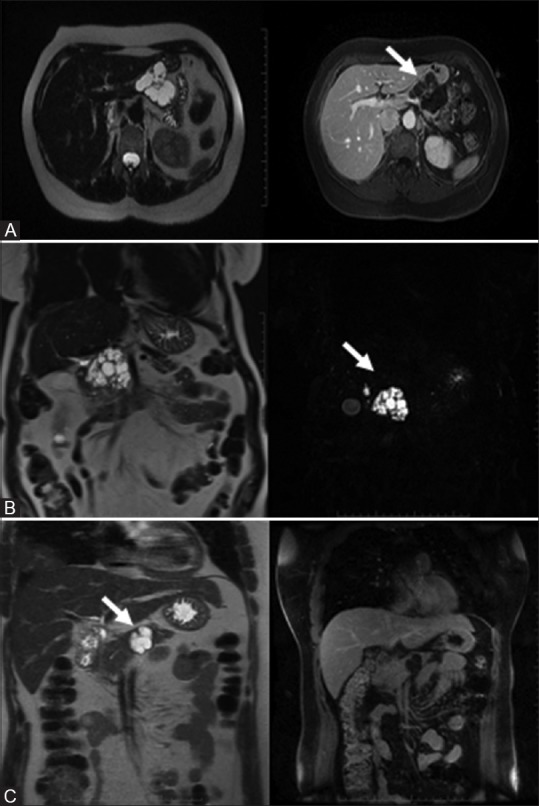
Benign serous neoplasm: Polycystic: multiple (<2 cm) cysts with enhancing central scar (A, top row). Honeycomb: subcentimeter microcysts. No scar (B, middle row). Oligocystic: fewer large cysts ranging from 2 to 5 cm (C, bottom row)
Mucinous cystadenoma
The most common cystic tumors of the pancreas these neoplasms contain large cysts with mucin secreting columnar cells and peripheral calcification (unlike the central calcification seen is serous tumors). These are benign or borderline (dysplastic) unilocular or mildly-septated mucin-producing tumours (variable T1, high T2 signal), with delayed rim enhancement on MRI, and no ductal communication; usually found in pancreatic body/tail [Figure 12]. There is a spectrum of mucinous cystic neoplasms from benign to malignant, however, the confident exclusion of malignancy is rarely possible on the basis of imaging findings alone. Mucinous cystic tumors should always be resected because they are all potentially malignant.
Figure 12.
Mucinous cystadenoma: Benign multiloculated variable T1 and T2 signal without enhancing components, thick septations, or ductal communication
Pancreatic cysts in Von Hippel Lindau disease
Von Hippel Lindau (VHL) is a rare autosomal dominant multisystem disorder with multiple abdominal manifestations, including renal and pancreatic cystic and a variety of benign and malignant neoplasms. Pancreatic involvement in VHL disease includes simple pancreatic cysts, serous microcystic adenomas, and rarely, adenocarcinomas. Pancreatic neuroendocrine tumors can also occur. Combined lesions occur, however, neuroendocrine tumors and cystic lesions only rarely exist together. When symptoms are present, they usually consist of minor abdominal discomfort. Rarely, severe pancreatic cystic disease results in exocrine insufficiency requiring enzyme replacement. Commonly, there is replacement of the pancreas by multiple, T1 dark, T2 bright lobulated cysts (“bunch of grapes” pattern) [Figure 13A].[7] There is no enhancement identified and typically it may be difficult to identify the normal underlying pancreatic parenchyma. It should be noted that pancreatic cysts are extremely rare in the general population; therefore, the presence of even a single cyst in an individual undergoing VHL disease screening because of a family history makes it highly likely that the person has VHL disease.
Figure 13(A and B).
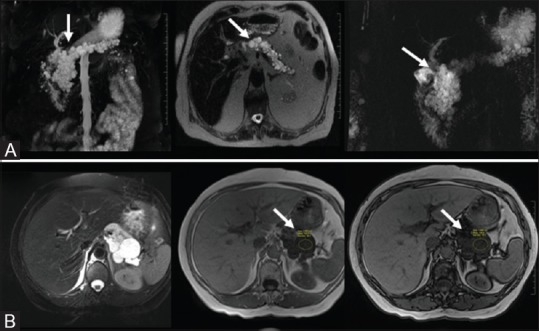
Von Hippel Lindau disease: “Bunch of Grapes” pattern showing innumerable T2 bright cysts (A, top row). Lymphangioma: signal drop on out-of-phase chemical shift imaging (B, bottom row)
Lymphangioma
Lymphangiomas of the abdomen are commonly mesenteric or retroperitoneal. Pancreatic lymphangiomas are extremely rare and warrant differentiation form neoplastic cystic masses of the pancreas. Patients have often presented with epigastric pain and sometimes a palpable mass is felt on clinical examination. MRI characteristics include a T1 dark, T2 bright cystic usually unilocular mass (sometimes polycystic) with no pertinent solid component or enhancement on gadolinium administration [Figure 13B]. A T2 hypointense rim may be seen occasionally, and if large enough, the lymphangioma can be seen compressing adjacent structure in an indolent manner.
Plexiform neurofibroma
Plexiform neurofibroma (PNF) are rare nonepithelial neoplasms similar to neurofibromas found elsewhere in the body, rare mimickers of pancreatic cysts on MR, and are considered a mosaic located form of neurofibromatosis-1. Symptoms are generally related to regional mass effect. Intratumoral edematous and myxoid changes create the characteristic trilobar cystic appearance which has strong T2 hyperintensity with mild enhancement [Figure 14A]. Surgical resection is necessary to exclude malignancy which is more frequently encountered in PNF compared with classical neurofibromas, however, it is still to be considered a benign pancreatic tumor (as malignancy is rare).
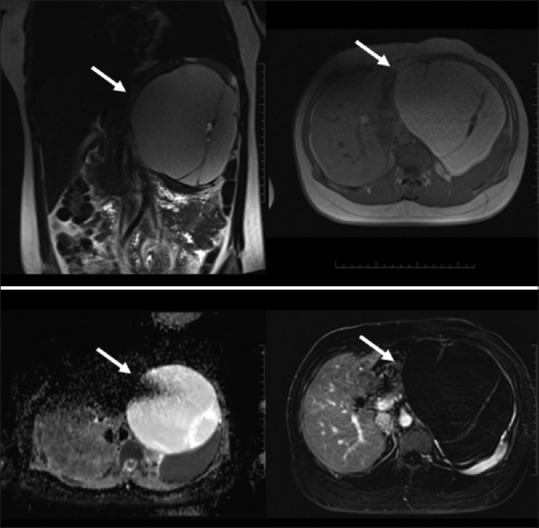
Figure 14(A and B).
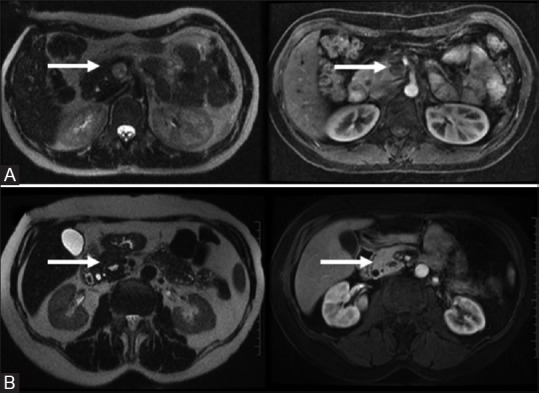
Plexiform Neurofibroma: complex T2 bright “trilobar” appearance (A, top row). Solitary Fibrous Tumors: T1/T2 dark mildly enhancing wall with a central cystic component (B, bottom row)
Solitary fibrous tumor
A solitary fibrous tumor (SFT) is a submesothelial neoplasm that usually arises from the pleura, but also found at other sites such as the lung, mediastinum, pericardium, mesothelium, peritoneum, extraperitoneal space, nose, and paranasal sinus. A pancreatic SFT is exceedingly rare. MRI is more definitive in characterizing these tumors compared to ultrasound and CT scan given its excellent spatial resolution. These are generally asymptomatic and found incidentally with no sex predilection.
SFT is similar in morphology to non-functioning islet cell tumour. On MR imaging SFT may contain a central cystic component surrounded by fibrous rim with low T1/T2 and mild enhancement [Figure 14B]. It is difficult to differentiate an SFT from a nonfunctioning islet cell tumor or solid pseudopapillary tumor (SPT) of the pancreas. SPT, however, appears more multifaceted with internal complexities and irregular margin.
Malignant
Primary pancreatic ductal adenocarcinomas
Solid ductal adenocarcinoma, the most common lethal neoplasm occasionally shows cystic features (cystic degeneration, retention cysts, and attached pseudocysts). Furthermore, primary pancreatic ductal adenocarcinomas (PDAC) with cystic features are sometimes similar to some other cystic pancreatic lesions, including pancreatic serous cystadenoma (SCN), mucinous cystadenoma (MCN), pseudocyst, and so on. On MR, cystic degeneration is seen within/adjacent to the primary malignancy [Figure 15]. The aggressive infiltrative pattern facilitates its diagnosis and differential from true cystic masses warranting immediate intervention. It is hence important to differentiate these entities as indolent cystic masses need no aggressive management versus a ductal malignancy requires urgent attention.
Figure 15.
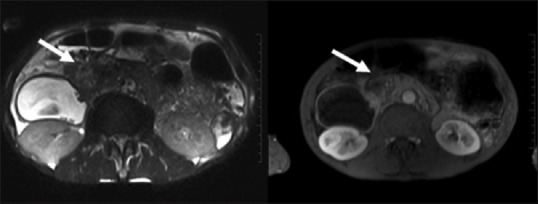
Adenocarcinoma with cystic degeneration: an aggressive neoplasm with central cystic component
Solid pseudopapillary tumors
A solid pesudopapillary tumor (SPT) (also known as papillary and cystic tumors, or solid-cystic tumors) is histologically distinct neoplasms having a low malignant neoplasm, common in young women of Asian or African–American descent. The tumor tends to be a large, well-circumscribed, and slowly growing mass. Capsule and intratumoral hemorrhage are important diagnostic clues rarely found in other neoplasms. MR shows a complex heterogeneous solid-cystic mass with gradual enhancement of the solid component, less than adjacent pancreatic parenchyma [Figure 16].
Figure 16.
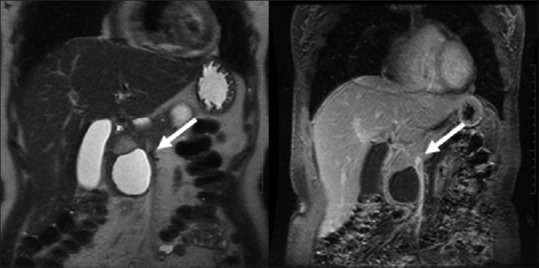
Solid pseudopapillary tumors (SPT): solid-cystic enhancing mass with capsule
Cystic neuroendocrine tumor
Neuroendocrine tumor (NET) of the pancreas are uncommon, most being sporadic, however, there is association with familial syndromes such as MEN1, VHL, and NF1. Cystic pancreatic NET is uncommon and is often considered a component of a larger tumor with cystic degeneration. Thus, they are generally larger than their solid counterparts and usually located within the pancreas or gastrinoma triangle. CT and ultrasound would be limited in evaluating these tumors. MRI imaging shows a T2 hyperintense mass with or without solid component which shows an arterially enhancing solid component or peripheral rim [Figure 17].[8] Most nonfunctioning masses are malignant.
Figure 17.
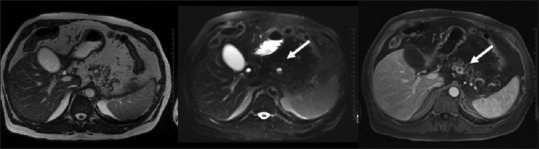
Cystic neuroendocrine tumor: cystic lesion with strong hyper enhancing peripheral rim
Intraductal papillary mucinous neoplasm
Intraductal papillary mucinous neoplasms (IPMNs) are uncommon ductal epithelial tumors. Common in middle-aged men, these may be main duct, branch duct, or mixed type. Branch duct lesions are generally benign whereas main duct tumours are associated with malignancy.[8] Typical location (uncinate process), typical appearance (grapelike locules), and communication with the duct usually separate it from other lesions in the pancreas. A markedly dilated uncinate branch filled with mucus is a typical feature of a side branch IPMN. On MR, a side-branch IPMN appears as T2 bright cluster of small cysts with septated lobulated margins or as a single, unilocular cystic lesion with ductal communication. Complex features include a thick, enhancing wall/septae and nodules. The main duct variant can have either segmental or diffuse ductal involvement. Feature which should raise concern for malignancy include enhancing papillary excrescences/nodules, or wall enhancement [Figure 18].[9] Differentiating feature of segmental main duct IPMN from a mucinous cyst is the presence of MPD dilatation.
Figure 18.
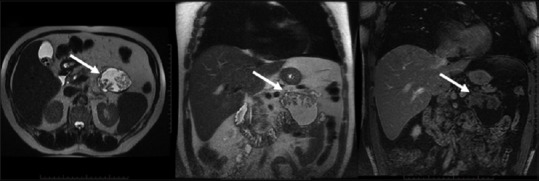
Cystic mass with enhancing papillary excrescences/nodules and ductal dilatation
Mucinous cystadenocarcinoma
The malignant counterpart of the benign cystadenoma is a mucinous cystadenocarcinoma (MCAC) which shows invasive carcinomatous elements within a mucinous cyst with a surrounding ovarian-type stroma. The patients are of an older age group suggesting malignant transformation of an MCA.
MR imaging features show a multilocular macrocystic appearance with thick septations and solid enhancing components. Restricted diffusion on diffusion-weighted imaging may also aid in confirming the diagnosis [Figure 19]. Mucinous cystic tumors should always be resected because they are all potentially malignant.
Figure 19.
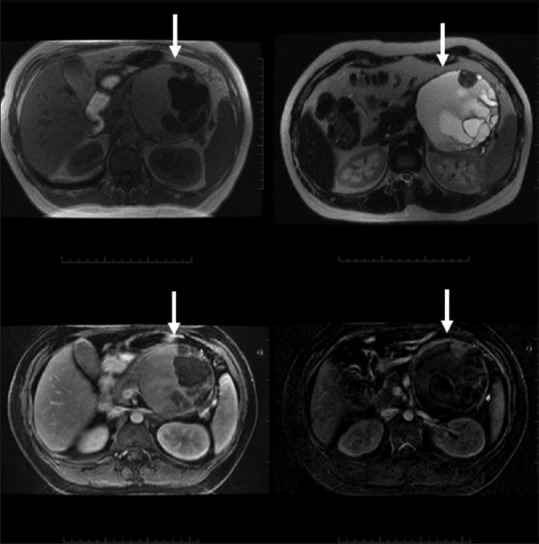
Mucinous cystadenocarcinoma: a multiloculated complex cystic mass containing thick septations and enhancing solid components
Cystic metastases
Cystic pancreatic metastases are unusual, but typically found in a setting of extensive metastatic disease from primary tumours including lung, breast, renal cell carcinoma, and melanoma. MR appearance is of a complex (cystic T1 dark, T2 bright) mass with mural enhancement and/or solid nodules [Figure 20].[10]
Figure 20.
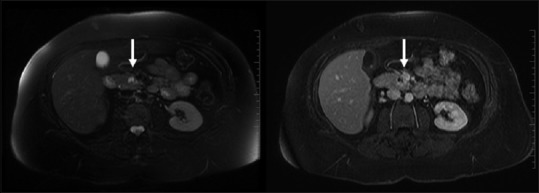
Cystic metastases: Complex T2 bright signal with wall/solid component enhancement and relevant history
Exceedingly rare cystic lesions
These range from infectious lesions such as tuberculosis to aggressive malignancies such as embryonal rhabdomyosarcoma. Although unlikely to be encountered clinically, these should be considered in the differentials diagnosis of cystic pancreatic masses in the appropriate clinical context.
Conclusion
Characterization of cystic lesions of the PBT has always been a challenge for the radiologist and may be a diagnostic quandary. In the last decade or so due to refined and improved imaging techniques, especially high-resolution MRI, there has been significant advancement in the characterization and accurate diagnoses of PBT cystic masses. In most cases, a correct presumptive diagnosis can be made on the basis of MRI criteria alone. Specific MRI findings that are important to recognize are the size of the lesion; the presence and thickness of a wall; the presence of septa, calcifications, or internal nodules; the enhancement pattern and the signal intensity spectrum. In addition, access to critical clinical information remains useful. MR features described in this pictorial review may aid radiologists in narrowing the diffentials and distinguishing indolent masses from more aggressive lesions requiring timely intervention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr Korosh Khalili for his contribution to this article.
References
- 1.Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL, et al. Diffusion-Weighted MR Imaging of Solid and cystic Lesions of the Pancreas. RadioGraphics. 2011;31:E47–64. doi: 10.1148/rg.313105174. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Turner MA, Fulcher AS, Halvorsen RA. Congenital anomalies and normal variants of the pancreaticobiliary tract and the pancreas in adults: Part 1, Biliary tract. Am J Roentgenol. 2006;187:1536–43. doi: 10.2214/AJR.05.0772. [DOI] [PubMed] [Google Scholar]
- 3.Koenraad J, Mortele, Pablo RR. Cystic Focal Liver lesions in the Adult; Differential CT and MR Imaging Features. Radiographics. 2001;21:895–910. doi: 10.1148/radiographics.21.4.g01jl16895. [DOI] [PubMed] [Google Scholar]
- 4.Korobkin M, Stephens DH, Lee JK, Stanley RJ, Fishman EK, Francis IR, et al. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. Am J Roentgenol. 1989;153:507–11. doi: 10.2214/ajr.153.3.507. [DOI] [PubMed] [Google Scholar]
- 5.Barton JG, Barrett DA, Maricevich MA, Schnelldorfer T, Wood CM, Smyrk TC, et al. Intraductal papillary mucinous neoplasm of the biliary tract: A real disease? HPB. 2009;11:684–91. doi: 10.1111/j.1477-2574.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JY, Kim MJ, Lee JY, Lim JS, Chung JJ, Kim KW, et al. Typical and Atypical Manifestations of Serous Cystadenoma of the Pancreas: Imaging Findings with Pathologic Correlation. Am J Roentgenol. 2009;193:136–42. doi: 10.2214/AJR.08.1309. [DOI] [PubMed] [Google Scholar]
- 7.Graziani R, Mautone S, Vigo M, Manfredi R, Opocher G, Falconi M. Spectrum of magnetic resonance imaging findings in pancreatic and other abdominal manifestations of Von Hippel-Lindau disease in a series of 23 patients: A pictorial review. JOP. 2014;15:1–18. doi: 10.6092/1590-8577/1757. [DOI] [PubMed] [Google Scholar]
- 8.Herwick S, Miller FH, Keppke AL. MRI of Islet Cell Tumors of the Pancreas. Am J Roentgenol. 2006;187:W472–80. doi: 10.2214/AJR.05.0809. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto S, Horton KM, Lawler LP, Hruban RH, Fishman EK. Intraductal papillary mucinous Neoplasms of the Pancreas: Can Benign Lesions Be Differentiated from Malignant lesions with multidetector CT? Radiographics. 2005;25:1451–68. doi: 10.1148/rg.256055036. [DOI] [PubMed] [Google Scholar]
- 10.Zagoria RJ, Wolfman NT, Karstaedt N, Hinn GC, Dyer RB, Chen TM. CT features of renal cell carcinoma with emphasis on relation to tumor size. Invest Radiol. 1990;25:261–6. doi: 10.1097/00004424-199003000-00010. [DOI] [PubMed] [Google Scholar]