Abstract
Purpose:
To evaluate the technical and clinical success of radiofrequency ablation of osteoid osteoma and analyze the factors responsible for clinical success. We also tried to investigate the role of follow-up computed tomography (CT) imaging.
Materials and Methods:
This is a prospective study approved by the institute's ethics committee involving 87 patients with appendicular osteoid osteoma. CT-guided radio frequency ablation was performed using a bipolar ablation system. Patients were followed up over 15.4 (4–24) months for pain, and clinical success/failure was determined using established criteria. Patients with clinical failure were taken for repeat ablation. Follow-up CT was obtained at 6 months and correlated with clinical success. Procedural scans were later reviewed for technical success in a blinded manner and correlated with clinical success along with other imaging and patient characteristics.
Results:
Mean pre-procedure visual analog scale (VAS) score was 7.0 ± 0.8. Primary success rate after single session was 86.2%(75/87 patients), and overall success rate after one/two sessions was 96.6%(84/87). No major complications were noted. Technical success rate was 89.7%(78/87). All 9 patients who had a suboptimal needle positioning had recurrence where as three patients had recurrence despite technical success. None of the imaging characteristics or history of prior intervention was significantly associated with clinical success. Follow-up CT showed advanced bone healing in 48 lesions, and was confined to the treatment success group. Alternately, minimal/absent bone healing was seen in all (12) patients of primary treatment failure and 27 patients with treatment success.
Conclusions:
CT-guided percutaneous radio frequency ablation is a safe and highly effective treatment for osteoid osteomas even in recurrent and residual cases. Technical success is the most important parameter affecting the outcome. Post radio frequency ablation CT findings have a good positive but a poor negative predictive value in prognostication.
Keywords: Interventional radiology, osteoid osteoma, therapy
Introduction
Osteoid osteoma is a benign bone tumor composed of a nidus of woven bone and osteoid surrounded by osteoblasts and peripheral reactive zone of thickened cortical or trabecular bone and loose fibrovascular tissue.[1] It commonly occurs in children and young adults, with long bones of the lower limbs being the sites of predilection.[2] Pain is the most common presenting symptom, which is characteristically worse at night and promptly relieved by nonsteroidal anti-inflammatory drugs (NSAIDs). In neglected cases, it may also present as growth disturbance, scoliosis, osteoarthritis, and if located within the capsule of a joint, swelling, synovitis, restricted movement, and contracture. The classic radiographic appearance is of a small central radiolucent nidus surrounded by a zone of bony sclerosis and cortical thickening caused by endosteal and subperiosteal new bone formation.[3] Computed tomography (CT) is the best imaging modality for the diagnosis of osteoid osteomas[4] as it allows easy identification and precise localization of the nidus [Figure 1]. Magnetic resonance imaging (MRI) is considered less useful than CT in the detection of osteoid osteomas. However, MRI better demonstrates osteoid osteomas located in cancellous bones and the associated soft tissue and intramedullary changes. In inconclusive lesions, Tc 99 HDP bone scintigraphy is performed, which shows intense radiotracer uptake in the region of nidus and surrounding bone with a typical double density sign.[5]
Figure 1(A-D).
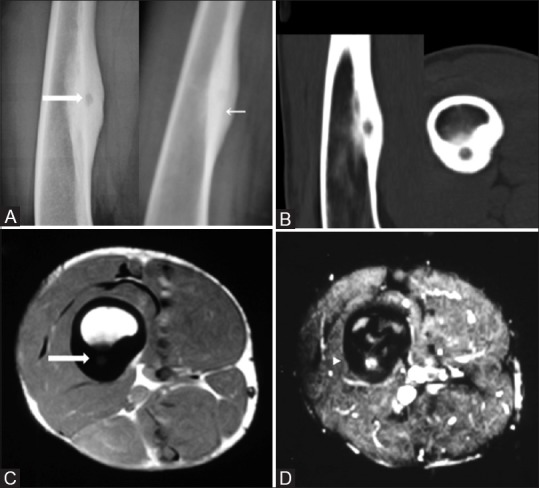
Anteroposterior and lateral radiographs (A) show a radiolucent nidus (thick arrow) amid an area of fusiform cortical thickening in the diaphysis of right femur (thin arrow) better seen on sagittal and axial CT images (B). Axial MRI shows a localized T1 dark periosteal bone formation (C) with central nidal enhancement on post contrast images (D)
Treatment options classically include long-term analgesia and surgical excision. Aspirin or other NSAIDs frequently provide effective pain control, however, long-term therapy may be unacceptable because of refractory pain, recurrent nocturnal pain with resultant sleep deprivation, or gastrointestinal complications. Articular or periarticular osteoid osteomas are particularly resistant to conservative therapy, and more aggressive intervention is often necessary.[3] Ever since Rosenthal et al.[6] reported the efficacy of CT-guided thermal ablation in osteoid osteoma, there has been a steady paradigm shift towards minimally invasive percutaneous treatment options such as interstitial laser ablation,[7] cryotherapy,[7,8,9] and especially radio frequency ablation (RFA).[10,11,12]
Because intraoperative localization of these small lesions can be very difficult, open surgical removal often necessitates considerable resection of bone, and consequently, internal fixation and/or bone grafting may be required.[13] Thermocoagulation of osteoid osteoma using RFA requires only small osseous access to allow insertion of the electrode. Therefore, loss of bone substance is minimal and does not cause significant structural weakening.[3] Furthermore, lesions located in anatomic areas that are technically difficult to access, such as the acetabulum and femoral head and neck, have lower surgical success rates.[14] Percutaneous RFA has now become the modality of choice for the treatment of osteoid osteoma because of the high success rate and minimal morbidity associated with this form of treatment.[12] Follow-up CT is not recommended but may demonstrate partial or complete replacement of the nidus with sclerotic bone within 2 months to 2 years after ablation. After 2 years, the lesion may be completely indistinguishable from parent bone. Follow-up MR imaging should show resolution of bone marrow edema. A CT finding of persistent radiolucency of the ablated site, or MR imaging findings of arterial enhancement of the nidus and residual marrow edema in patients with negative findings at CT, are suggestive of residual tumor.[3] If residual symptoms are present, a second application of RFA is safe and is often successful,[11] with reported response rates of 80–90%.[12]
Majority of the studies to date have used the monopolar system to evaluate the role of RFA in osteoid osteomas. Bipolar RFA of osteoid osteomas has been used with a high success rate without any major complications in many small series.[15] We tried to evaluate the technical and clinical success rates and any associated factors with a bipolar RFA device in a large subset of patients (n = 87). We also evaluated the role of postprocedural imaging for documenting healing and to prognosticate outcome.
Patients and Methods
This was prospective study conducted from June 2009 to June 2015 involving 87 patients. The study was started after approval by the institutional ethics committee. Written informed consent was obtained from all the patients.
Patients
Patients with radiologic diagnosis of osteoid osteoma in painful bone lesions were included in the study. We excluded lesions located in the hand or in the posterior arch of vertebrae and tumor <1 cm away from a major nerve due to probable neural damage.[12] Coagulation disorders/local site infection also precluded the procedure.
Preprocedure
Detailed history was obtained from the patients regarding the duration of illness, treatment underwent, etc. Severity of pain was assessed subjectively using the visual analog scale (VAS) score. Preprocedural workup included a coagulation profile including prothrombin time/International normalized ratio (INR) and pre-anesthetic workup including blood investigations, chest radiograph, and clinical exam by the anesthetist. Plain radiographs and non-contrast (NCCT) of the bone lesion were performed in all cases to establish the diagnosis. In inconclusive cases, bone scintigraphy or MRI was performed additionally.
Procedure
All the procedures were performed by an interventional radiologist (SG) with 10 years of experience in image-guided orthopedic interventions under either general or spinal anesthesia. The procedures were performed under CT guidance (Somatom Sensation 40 slice CT scanner, Siemens, Erlangen, Germany).
Thin axial sections of 1.2 mm thickness were acquired to localize the lesion precisely and planning the entry point and approach. We tried to puncture in the scan plane. Keeping the entry point perpendicular to the proximal cortex helped in avoiding needle skidding. In general, the shortest distance through the bone was selected for access [Figure 2A], however, if such an approach was unsafe because of neurovascular (or other anatomic) structures [Figure 2B], or technically difficult because of a steeply oblique approach to the bone surface with a risk of needle skidding off the cortex [Figure 2C], the lesion was approached via opposite cortex. A prone position was generally avoided because of the difficulty in monitoring during anesthesia [Figure 2D]. Liberal use of limb rotation was done to make the approach technically easier. While approaching intraarticular osteoid osteomas, a transarticular approach was usually avoided to reduce the risk of synovitis/septic arthritis and articular cartilage damage.
Figure 2(A-D).
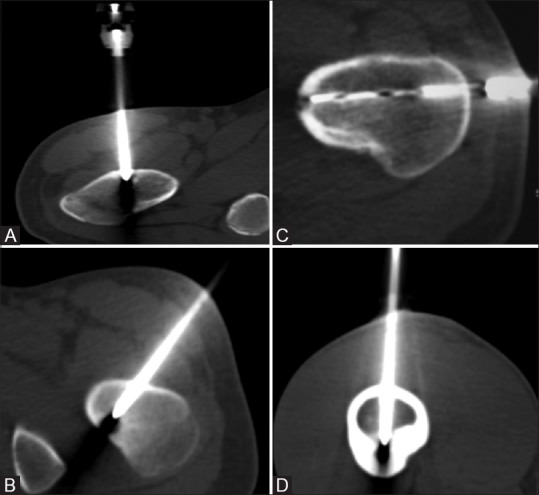
Procedural axial CT images (A-D) show that a direct vertical approach (A), an oblique approach (B) to avoid the neurovascular bundle, lateral approach through opposite cortex (C) to avoid needle skidding and an anterior approach (D) for lesions in posterior cortex to obviate prone position could be used
Because we used bipolar RF system, there was no requirement for the placement of grounding pads. An 11G bone biopsy needle set (Osteo Site, COOK Medical, Bloomington, USA), and a hammer or a drill was used to gain access to the nidus. Once the needle was placed in the centre of the nidus, the inner stylet was removed and the RF probe was introduced through the cannula into the nidus [Figure 3A]. Biopsy was not obtained in any of the cases.
Figure 3(A and B).
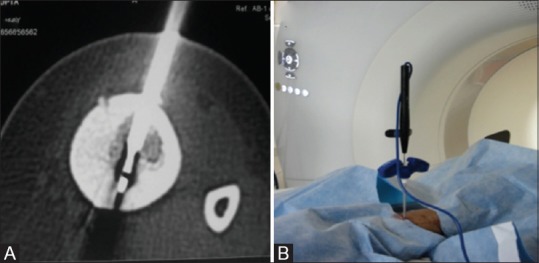
Axial CT image (A) depicting RFA probe (block arrow) across the nidus with the outer cannula of access needle withdrawn approximately 1 cm from the tip. Configuration of the RF probe and outer access needle (B) in the CT gantry
The outer cannula was subsequently withdrawn by approximately 1 cm from the active tip to avoid contact between the electrode and the cannula to prevent burning of the access track [Figure 3A and B]. We used CelonLab POWER bipolar RF generator (Celon AG Medical Instruments, Rheinstrasse, Teltow, Germany) and bipolar RF applicators–CelonProSurge micro- T09 or CelonProSurge micro-T15 (Celon AG Medical Instruments, Rheinstrasse, Teltow, Germany). The probes had a shaft length of 15 cm and size of 18G, and electrode lengths of 9 mm and 15 mm. We used non-cooled electrodes of 9 mm active tip in lesions less than 1cm and 15mm in lesions more than 1cm in maximum diameter. The electrode was connected to the RF generator and the power was set at 3W. Completion of the ablation procedure was defined by reaching the target energy of 0.5kJ. Often the ablation would reach the so-called “roll-off” (i.e., significant increase of impedance resulting in the loss of AC flow) indicating a complete local coagulation necrosis/charring. In such cases, a manual repositioning of the electrode tip and restarting of the RFA was done till the deposition of desired energy. A “dry tip” would also cause an early “roll-off,” and hence, it is essential to wet the probe tip with a saline soaked gauze and test conductivity before application. The application time ranged between 4 and 5.5 minutes. In larger lesions, the probe was repositioned and the procedure was repeated such that the overlapping zones of ablation were produced and the entire lesion was covered. However, this maneuver could be done in a few cases because making a new tract juxtaposed with previous bony tract was difficult due to repeated needle slippage into the old tract. Often a fresh entry site in the proximal bony cortex had to be made to be able to achieve this. Local compression and bandage was done post procedure. The total time required from the time of entry into the CT scanner to recovery from anesthesia ranged 60–100 minutes.
Mechanism of radiofrequency ablation
RFA utilizes a generator which is a source of alternating current and an electrode with an insulated shaft and a noninsulated tip, which is placed into the lesion under image guidance. High frequency alternating current from the generator is passed through the needle into the surrounding tissues and the alternating polarity of the electrode produces oscillation of ions in the vicinity. This results in frictional heating of surrounding tissues and coagulation necrosis.[16] Maximum amount of heat is produced in tissues with lower resistance than that in tissues with higher resistance, and the tissue resistance in marrow bone is significantly lower compared to cortical bone. Hence, during ablation, an intact cortical bone provides an insulating effect protecting the surrounding soft tissues and cartilage from thermal damage.[17] Organ[18] demonstrated, during ablation through radiofrequency, the long axis of treatment zone = 2 × length of bare tip and transverse axis = 2/3 long axis.
Postprocedure care
The patients were observed in the procedure room until there was complete recovery from anesthesia and establishment of spontaneous breathing. Oral or intramuscular analgesics were administered because patients often had increased need of analgesia in the immediate perioperative period. The patients were discharged 6 hours post procedure. Patients with lesions in the weight bearing bones were instructed to restrain from strenuous activities for a minimum of 1 month. Otherwise, patients were not restricted from normal daily activities.
Follow up
As per the protocol, patients were followed up in the outpatient department (OPD) 1 month and 6 months after the procedure. The VAS pain score was obtained, and the change in analgesic intake following the procedure was assessed. A follow-up CT scan was performed at 6 months after the procedure. Patients with recurrent/residual symptoms were identified and considered for repeat RFA. Any periprocedural (less than 30 days following treatment) or delayed complications (more than 30 days following treatment), such as infections, neurovascular damage, growth plate disturbance, or articular bone damage, were assessed.
Definitions
A procedure was considered technically successful if the electrode was placed so that no portion of the lesion was more than 5–7 mm (depending on the coagulation diameter of the electrode) away from the exposed tip and if the target energy was deposited.[12] Residual symptoms were defined as pain or impaired function or both identical to the presenting complaints that persisted for more than 2 weeks after radiofrequency thermal ablation. Recurrent symptoms were defined as the reappearance of symptoms that followed a symptom-free period after radiofrequency thermal ablation.[17]
Clinical outcome
The outcome was assessed clinically by the presence or absence of pain and the requirement for analgesic intake. The patients were categorized into three groups. As described by Rosenthal et al.[12]
Clinical success –pain free and did not require medications/additional procedures
Indeterminate–pain was neither severe enough nor frequent enough to necessitate additional investigations or procedures
Clinical failure–recurrent/persistent pain requiring analgesic intake and additional procedures.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software (version 11.5.1; SPSS, Chicago, Ill). Descriptive statistics such as mean and standard deviation were calculated where appropriate. Differences with a p value of less than 0.05 were considered to be statistically significant. Interval estimate (95% confidence interval) was used to analyze the success rates of the procedures. Parametric data such as patient age and lesion size were tested with the t-test. Categorical data namely lesion location, patient sex, nidus calcification, prior intervention, technical success, and CT healing pattern were evaluated with the Fisher exact test.
Results
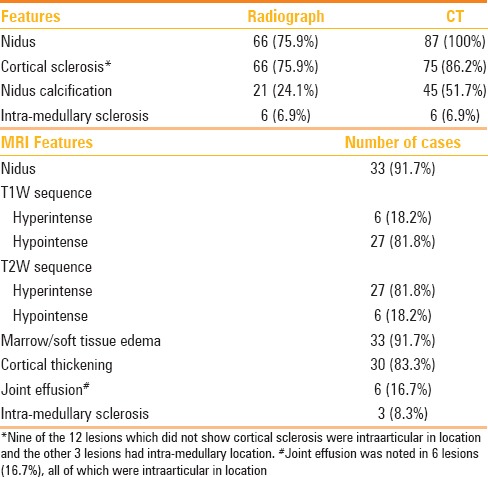
Patient demographics, duration, and nature of complaints along with lesion characteristics have been tabulated in Table 1. Preprocedural diagnostic workup included radiography, CT, and MRI. Imaging features have been tabulated in Table 2.
Table 1.
Patient characteristics and lesion location
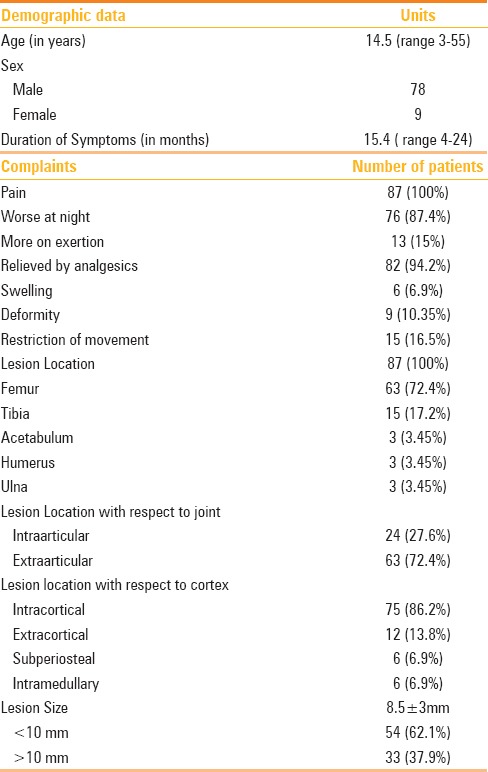
Table 2.
Imaging appearance of osteoid osteoma
Outcome
Clinical success
Of the 87 patients, 75 patients (86.2%) had complete pain relief after RFA and required no further analgesics (treatment success group). Nine of the remaining 12 patients (10.3%) experienced persistent/recurrent pain and repeat RFA was performed (treatment failure group), following which they were asymptomatic (secondary success rate: 96.5%). The remaining 3 patients (3.4%) had residual pain even after a repeat ablation and underwent surgical excision of the lesions (clinical failure). The success rate after one session of RFA was 86.2% (75 of 87 patients). The overall success rate after 1–2 ablation procedures was 96.5%(84 of 87 patients). The mean pre-procedure VAS score of 87 patients was 7.0 ± 0.8. In most of the patients treated successfully, the VAS score dropped to 0 by the end of first month. A few patients who were treated successfully (n = 3) experienced residual pain (VAS = 2–6) for 6–8 weeks before there was complete resolution of pain. The average period of follow-up in our study was 15.4 months (range = 4–24 months).
Complications
Two patients who were treated for a tibial osteoid osteoma developed puncture site infection with pus discharge approximately 2 weeks after the procedure. The infection was confined to the skin and subcutaneous tissues without involvement of bone, which resolved on oral antibiotics. In addition, a drill bit got broken in situ during one of the procedures. However, it could be retrieved surgically and no serious effects were noted.
Technical success
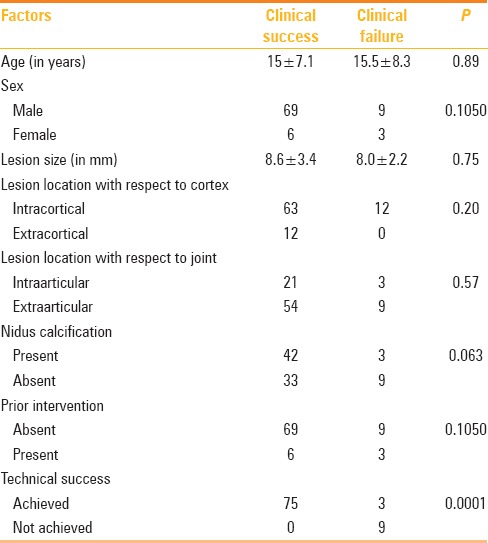
Out of the 87 procedures, 78 were technically successful (89.7%). Optimal needle positioning could not be achieved in the remaining 9 patients (10.3%). All 9 patients who had a suboptimal needle positioning in the nidus had recurrence, and 3 patients who had an optimal needle positioning in the nidus had recurrence. Difference between the groups was statistically significant (P = 0.0001). It was the only factor found to have significant statistical association with clinical success. Other factors assessed for possible correlation with clinical success are tabulated in Table 3.
Table 3.
Factors influencing outcome after RF ablation
Healing patterns of osteoid osteoma on computed tomography
Follow-up CT scan was performed in all the patients at 6 months after the procedure. The follow-up scans were compared with the preprocedure scans and the changes were categorized into five groups, as stated by Vanderschueren et al.[19 Their classification was (1) complete ossification of the nidus, (2) presence of a minimal nidus rest, (3) decrease in the size of the nidus, (4) unchanged size of the nidus, and (5) changed configuration of the nidus. Distribution of CT healing pattern of lesions is shown in Table 4.
Table 4.
CT healing pattern of osteoid osteoma

For the purpose of statistical analysis, the five groups were grouped in two main categories, namely,(A) advanced bone healing (complete ossification, minimal residual nidus and altered configuration of nidus) [Figure 4] and (B) minimal or absent bone healing (decreased size, and unchanged size of the nidus) [Figure 5]. Relationship between CT healing patterns and the clinical outcome were analyzed.
Figure 4(A-D).
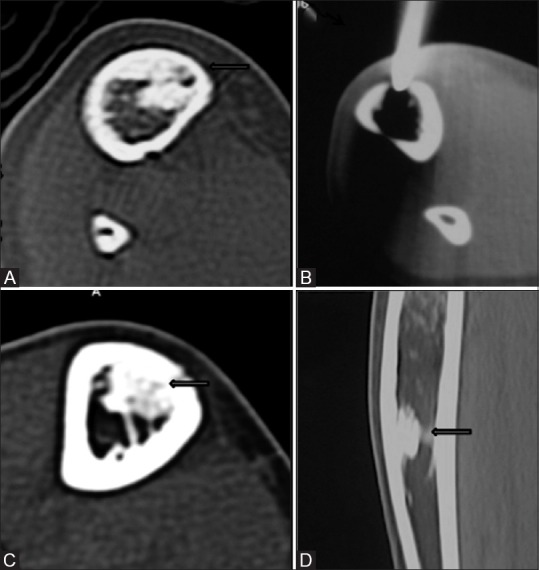
Unenhanced axial CT image (A) shows a hypodense nidus (thick arrow) in the anteromedial cortex of right tibia with significant intramedullary sclerosis. Procedural CT image (B) shows the tip of the biopsy needle in the nidus (thin arrow). Axial (C) and sagittal (D) CT images obtained at 6 months follow-up show complete ossification of the nidus (thick arrow)
Figure 5(A-D).
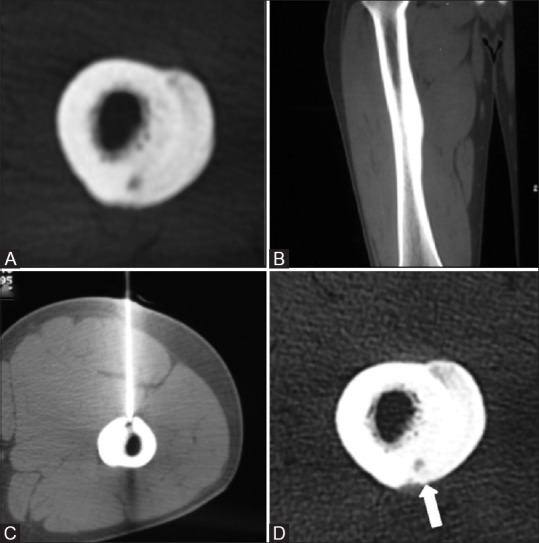
Unenhanced axial (A) and sagittal (B) CT images show a small nidus in the posterior cortex of femoral diaphyses with extensive periosteal new bone formation. Procedural CT (C) shows the tip of the biopsy needle in the nidus with patient in prone position (exception). Follow up axial (D) CT images show no change in nidus size (thick arrow) or periosteal reaction
All the 12 patients who had treatment failure showed minimal or absent bone healing in the follow-up scans and 27 of the 75 patients who had treatment success showed minimal bone healing in the follow-up imaging. Advanced bone healing was observed in 48 lesions and was confined to the treatment success group. None of the lesions with treatment failure showed advanced bone healing. Difference between the groups was statistically significant (P = 0.0001).
Discussion
In the current study, a typical patient was a young male with a dull deep ache in the thigh or leg with nocturnal exacerbation. The most common bone involved was femur with a predisposition for femoral neck, as seen in one-third (21/87) of the cases. Majority of the lesions were subcentimetric in size and cortical in location producing a significant cortical perinidal sclerosis except in the intraarticular cases. Intraarticular osteoid osteomas show minimal/absent cortical thickening [Figure 6] because of the lack of inner periosteal layer of cambium.[20] In addition, intramedullary lesions more often show perinidal intramedullary sclerosis than cortical thickening, as seen in our cases.[20]
Figure 6(A-D).
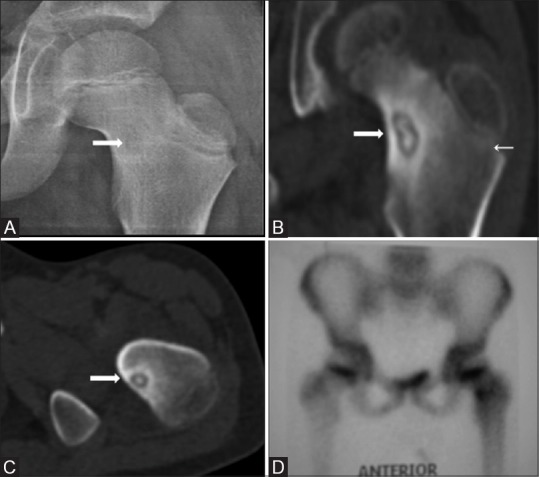
AP radiograph of left hip joint (A) shows a faint radiolucent focus (thick arrow) in the neck of the left femur with no significant cortical thickening. CT coronal MPR (B) and axial (C) images show central nidal calcification with minimal reactive periosteal bone formation. Bone scan shows intense radiotracer uptake in the corresponding location (D)
We employed general/spinal anesthesia in all our patients. Local anesthesia or intravenous sedation usually results in suboptimal pain control and immobility, especially during needle entry into the nidus. General anesthesia was preferred in pediatric patients because of increased patient anxiety and slower recovery with spinal anesthesia.
We did not performa preprocedure biopsy for the osteoid osteoma in any of our patients.
Hoffman[21] concluded that a biopsy prior to treatment is not mandatory due to a remarkable amount of false negative findings in clinically and morphologically unambiguous cases of osteoid osteoma. Biopsy was able to prove diagnosis in 14 of 29 (48%) cases in their study.
We utilized a non-cooled tip RF probe to have an entirely predictable treatment zone and no ablation of surrounding normal tissue.[17] Though cooled tip RF probes with its larger ablation zone would be desirable in a larger lesion, we preferred to have a limited and controlled ablation because majority of our lesions were subcentimetric.
Bipolar RF applicators with variable lengths of the exposed electrode were used for the RF procedures. In bipolar probes, the active and return electrodes are mounted on the active tip of the same probe, and hence, there is no requirement for the placement of grounding pads, which serve as the return electrodes in the monopolar system, and thus, no risk of a ground pad site burn.[15] Moreover, metallic materials, such ascardiac pacemakers, may get included in the electrical circuit leading to unwanted effects, and hence, are a contraindication to the use of monopolar system. In addition, unpredictable electrical current paths between the grounding pads and the RF probe may lead to inhomogeneous energy deposition and consequently irregular ablation zones, which is especially of concern in osteoid osteomas considering their small size. Whereas, in the bipolar system, there is restriction of the RF current to a precise zone around the probe tip which enhances the ablative effect.[15] Majority of the studies to date have used the monopolar system to evaluate the role of RFA in osteoid osteomas. Mahnken et al.[15] were the first to report on bipolar RFA of osteoid osteomas. Though they observed a high success rate without any major complications, the sample size was limited to 12 cases, which is insufficient to draw any major conclusions regarding the use or safety of bipolar ablation devices.
In our study, the average duration of a single ablation procedure was between 4 and 5.5 minutes. This is similar to the time required with the monopolar system wherein the energy is applied and maintained at 90°C for 4–6 minutes.[12,17]
In our study, primary success rate was 86.2% (75 of 87 cases) and the overall secondary success rate after one or two RF procedures was 96.5% (84 of 87 cases). Mahnken et al.[15] also reported a high success rate of 92% (11 of 12 cases) after one or two procedures using bipolar RFA. In the study by Rosenthal et al.,[12] which is the largest study on RFA of osteoid osteoma to date using monopolar RF ablation, a primary success rate of 91% was reported.
Factors affecting clinical outcome
Technical success
It was positively correlated with clinical success in our study (p = 0.001). The problems leading to a suboptimal needle positioning were categorized into two groups: Visualization and access problems, as stated by Vanderscheuren et al.[22] In one patient, who had a small, approximately 4mm lesion in the medial cortex of the femoral neck, drilling from the lateral cortex was employed becausethere was a risk of needle skidding off the cortex if a vertical approach was used. Retrospective analysis of the images showed that tip of the electrode was not placed in the nidus but a little inferior to it, which may be attributed to the long distance that had to be drilled from the lateral cortex to reach the nidus [Figure 7]. In another patient, who had a lesion in the medial femoral cortex, a vertical approach was employed. Because of the obliquity of the cortex, there was repeated skidding of the needle off the cortex, and hence, there was a suboptimal needle positioning in the nidus. Repeat RF treatment was performed in both the patients, following which they were asymptomatic. Vanderscheuren et al.[22] also reported that inaccurate procedures, either from access or visualization problems significantly affected the probability of a clinically successful outcome. It is also stated that lesions which are technically difficult to approach such as in pelvis, spine andclose to joint carry a relatively higher recurrence rate compared to lesions at approachable locations.[17,22]
Figure 7(A-C).
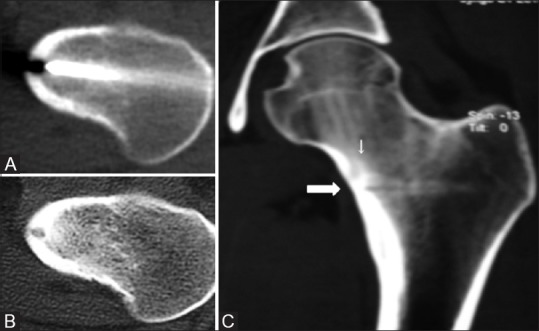
Procedural CT (A) shows the tip of the biopsy needle in the nidus. Follow-up axial (B) CT images show no significant change in lesion. Because patient continued to have pain, retrospective analysis of 3D MPR coronal images (C) revealed that the biopsy track (thick arrow) is running a little inferior to the nidus (thin arrow)
Imaging characteristics
Our evaluation revealed that none of the imaging characteristics, includinglocation of the lesion with respect to cortex or joint or calcification of nidus, were significantly associated with a favorable outcome, which is concordant with previous studies.[22]
Prior intervention
History of a previously failed treatment did not appear to affect the probability of a clinically successful outcome in our study (P = 0.105). Our results are consistent with Vanderscheuren et al.,[22] however, in contradiction to Rosenthal et al.,[12] who reported a statistically significant difference in the success rates between RF treatment in fresh cases and RF treatment in recurrent cases and concluded that a prior failed intervention reduces the probability of success after RF treatment. In addition, we had a primary success rate of 86.2% (75/87) and a secondary success rate of 75% (9/12), which do not have a statistically significant difference between them (p = 0.31).
A retrospective analysis of possible reasons of secondary clinical failure in 3 patients revealed that a very small nidus (4 mm) which was difficult to target in one and a larger nidus (15 mm) with possibly incomplete ablation was noted in another patient. We used a single RF probe with 15 mm active tip in lesions larger than 10 mm. Multiple authors recommend that two or more probes with overlapping ablation zones, which may include multiple needle positions, may be required for larger lesions (>10 mm), especially in repeat ablations.[17,22] Third patient had a densely calcified nidus along with extensive perinidal cortical sclerosis, which caused difficulty in gaining access to the centre of the nidus. Two of these patients never had reduction in pain whereasone patient (with larger lesion) had immediate reduction in the pain score after each ablation session but had recurrence of pain within 6–8 weeks postprocedure each time.
Computed tomographhealing pattern versus clinical outcome
In our study, an advanced pattern of healing of nidus is seen only in successfully treated lesions; absent/minimal pattern of healing is seen both in lesions with treatment success and treatment failure. Very similar results were reported in the study by Vanderscheuren et al.,[19] with a follow up imaging done as late as 24 months. Thus, CT imaging solely may not help to differentiate treatment success and treatment failure. Moreover, postprocedural imaging has a good positive predictive value but has a poor negative predictive value.
Limitations of the study
Spinal osteoid osteomas were not treated in our study; hence, efficacy of the bipolar system at such critical locations could not be studied. We did not seek histological confirmation of the lesion by performing a pre-ablation biopsy, however, we share the opinion that it is not necessary in presence of typical clinical history and imaging findings.
Our treatment protocol of thermal ablation with only RFis limited in scope. Because success rates were compared with reports on other procedures, a comparison of RFA and promising techniques such as laser photocoagulation or microwave ablation would be desirable.
Conclusions
RFA is a safe and highly effective form of treatment for osteoid osteomas with high success rates. It is a cost effective and minimally invasive treatment, and the post procedure recovery is brief. Technical success is the most important parameter, which determines the outcome. Prior intervention either surgical or percutaneous does not affect the outcome. Post RFA CT findings have a good positive but a poor negative predictive value. In case of residual/recurrent disease, a second thermocoagulation is usually successful in eliminating symptoms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Mr. Bhushan Sharma and Mr. Bhanu Pratap, our chief CT technologists for helping us perform these procedures without a glitch and the CT nursing staff headed by sister Sushma for assisting us during the procedures.
References
- 1.O'Connell JX, Nanthakumar SS, Nielsen GP, Rosenberg AE. Osteoid osteoma: The uniquely innervated bone tumor. Mod Pathol. 1998;11:175–80. [PubMed] [Google Scholar]
- 2.Sans N, Galy-Fourcade D, Assoun J, Jarlaud T, Chiavassa H, Bonnevialle P, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212:687–92. doi: 10.1148/radiology.212.3.r99se06687. [DOI] [PubMed] [Google Scholar]
- 3.Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, et al. Thermal Ablation of Osteoid Osteoma: Overview and Step-by-Step Guide1. RadioGraphics. 2009;29:2127–41. doi: 10.1148/rg.297095081. [DOI] [PubMed] [Google Scholar]
- 4.Assoun J, Richardi G, Railhac JJ, Baunin C, Fajadet P, Giron J, et al. Osteoid osteoma: MR imaging versus CT. Radiology. 1994;191:217–23. doi: 10.1148/radiology.191.1.8134575. [DOI] [PubMed] [Google Scholar]
- 5.Helms CA. Osteoid osteoma. The double density sign. Clin Orthop. 1987;222:167–73. [PubMed] [Google Scholar]
- 6.Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: A new procedure. Radiology. 1992;183:29–33. doi: 10.1148/radiology.183.1.1549690. [DOI] [PubMed] [Google Scholar]
- 7.Gangi A, Dietemann JL, Gasser B, Mortazavi R, Brunner P, Mourou MY, et al. Interstitial laser photocoagulation of osteoid osteomas with use of CT guidance. Radiology. 1997;203:843–8. doi: 10.1148/radiology.203.3.9169714. [DOI] [PubMed] [Google Scholar]
- 8.Adam G, Keulers P, Vorwerk D, Heller KD, Füzesi L, Günther RW. The percutaneous CT-guided treatment of osteoid osteomas: Acombined procedure with a biopsy drill and subsequent ethanol injection. RöFo. 1995;162:232–5. doi: 10.1055/s-2007-1015871. [DOI] [PubMed] [Google Scholar]
- 9.Skjeldal S, Lilleås F, Follerås G, Stenwig AE, Samset E, Tillung T, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii-a case report. Acta Orthop Scand. 2000;71:637–8. doi: 10.1080/000164700317362307. [DOI] [PubMed] [Google Scholar]
- 10.Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol. 2001;12:717–22. doi: 10.1016/s1051-0443(07)61443-2. [DOI] [PubMed] [Google Scholar]
- 11.Vanderschueren GM, Taminiau AHM, Obermann WR, Bloem JL. Osteoid osteoma: Clinical results with thermocoagulation. Radiology. 2002;224:82–6. doi: 10.1148/radiol.2241011135. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: Percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171–5. doi: 10.1148/radiol.2291021053. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;361:186–91. doi: 10.1097/00003086-199904000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wörtler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83:391–6. doi: 10.1302/0301-620x.83b3.11679. [DOI] [PubMed] [Google Scholar]
- 15.Mahnken AH, Tacke JA, Wildberger JE, Günther RW. Radiofrequency ablation of osteoid osteoma: Initial results with a bipolar ablation device. J Vasc Interv Radiol. 2006;17:1465–70. doi: 10.1097/01.RVI.0000235737.22496.6A. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SN, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: Proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–45. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 17.Pinto CH, Taminiau AH, Vanderschueren GM, Hogendoorn PC, Bloem JL, Obermann WR. Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: Tricks of the trade. Am J Roentgenol. 2002;179:1633–42. doi: 10.2214/ajr.179.6.1791633. [DOI] [PubMed] [Google Scholar]
- 18.Organ LW. Electrophysiologic principles of radiofrequency lesion making. Appl Neurophysiol 1976. 1977;39:69–76. doi: 10.1159/000102478. [DOI] [PubMed] [Google Scholar]
- 19.Vanderschueren GM, Taminiau AHM, Obermann WR, van den Berg-Huysmans AA, Bloem JL, van Erkel AR. The healing pattern of osteoid osteomas on computed tomography and magnetic resonance imaging after thermocoagulation. Skeletal Radiol. 2007;36:813–21. doi: 10.1007/s00256-007-0319-1. [DOI] [PubMed] [Google Scholar]
- 20.Chai JW, Hong SH, Choi J-Y, Koh YH, Lee JW, Choi J-A, et al. Radiologic diagnosis of osteoid osteoma: From simple to challenging findings. Radiographics. 2010;30:737–49. doi: 10.1148/rg.303095120. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann RT, Jakobs TF, Kubisch CH, Trumm CG, Weber C, Duerr HR, et al. Radiofrequency ablation in the treatment of osteoid osteoma—5-year experience. Eur J Radiol. 2010;73:374–9. doi: 10.1016/j.ejrad.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Vanderschueren GM, Taminiau AHM, Obermann WR, van den Berg-Huysmans AA, Bloem JL. Osteoid osteoma: Factors for increased risk of unsuccessful thermal coagulation. Radiology. 2004;233:757–62. doi: 10.1148/radiol.2333031603. [DOI] [PubMed] [Google Scholar]


