Sir,
A 35-year-old man came to our observation with dyspnea, which started 5 years after he began working as turner. He worked for a company of hydraulic systems using disposable tools, mainly composed of hard metals and aluminum oxides.
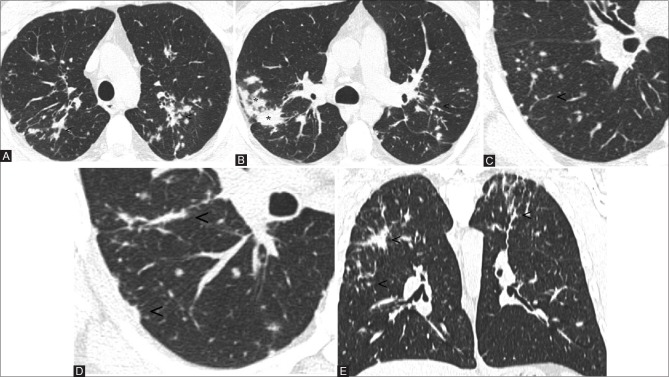
He was a mild former smoker (6 pack-years) who quitted smoking 10 years before the onset of his symptoms. Pulmonary function tests were within normal levels for vital capacity (4.1 L; percentage predicted vital capacity 99%), forced expiratory volume in 1s (3.2 L; percentage predicted forced expiratory volume in 1s 96%), total lung capacity (5.7 L; percent predicted total lung capacity 100%), forced expiratory volume in 1s/forced vital capacity of 80%, and diffusing capacity of the lung for carbon monoxide equal to 127%. Bronchoalveolar lavage (BAL) fluid showed lymphocytosis with a CD4/CD8 ratio 7:1, multinucleated giant cells were not observed. On diagnosis, high-resolution computed tomography (HRCT) of the chest showed multiple small nodules with a perilymphatic distribution, occasionally with the tendency toward coalescence or to give confluent consolidations with irregular contours [Figure 1A–D]. These lesions involved the upper, middle, and lower zones of both the lungs, with a predominance of the upper and middle zones [Figure 1E]. Some mediastinal lymph nodes were found, with short axis up to approximately 10mm. No pleural effusion was present. A thoracoscopic lung biopsy (video-assisted thoracoscopic surgery) of the left upper lobe was performed, and histopathological examination revealed non-necrotizing, nonconfluent, epithelioid granulomas with multinucleated cells. Although a few multinucleated cells were observed in the alveolar spaces, no features diagnostic of giant cell interstitial pneumonitis (GIP) were evident [Figure 2A–D]. A metallic analysis was performed on the surgical specimen of the patient, specifically, it was performed a multielement analysis with inductively coupled plasma mass spectrometry (ICP-MS, Elan DRC II, Perkin Elmer, Waltham, MA). Pathologic specimens contained a concentration of tungsten of 0.29 μg/g (7.3 times greater than those of median values found in autoptic lung samples from non-professionally exposed subjects), and 2.4 μg/g for titanium (3.4 times than normal).
Figure 1(A-E).
Axial images at diagnosis show bilateral lung nodules with a perilymphatic pattern. Note the peribronchovascular (A and B; see arrowheads), interlobular (C; see arrowheads), and subpleural involvement (D; see arrowheads). The confluence of many small nodules forms consolidations with irregular margins (B; see asterisk). The lesions mainly involve the upper and middle zones (E, coronal view; see arrowheads)
Figure 2(A-D).
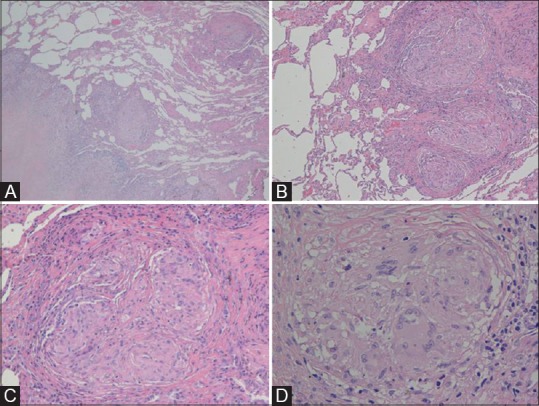
The thoracoscopic biopsy shows an interstitial fibrotic lesion with a reticular and nodular pattern (A, H and E stain, magnification 40×) and a subpleural and septal distribution. The lesion is characterized by multiple non-confluent granulomas (B, 100×) accompanied by collagen deposition devoid of significant inflammation and necrosis (C, 200×). The granulomas is made of epitheliod hystiocytes with few multinuclear giant cells (D, 400×)
Based on these results and the history of exposure, we recommended the patient to quit his job or transfer his task to avoid exposure. Eight months later, he was transferred to a different work unit devoid of hard metal dust, and his clinical symptoms improved and completely disappeared within 2 months. The patient was followed over time, and his clinical state and pulmonary function tests were satisfactory. In particular, a follow-up HRCT obtained at 2 years after ending hard metal dust exposure showed an almost complete resolution of parenchymal abnormalities [Figure 3A and B]. Notably, the patient did not take any corticosteroids or other immunosuppressive drugs neither at diagnosis nor during the follow-up period.
Figure 3(A and B).
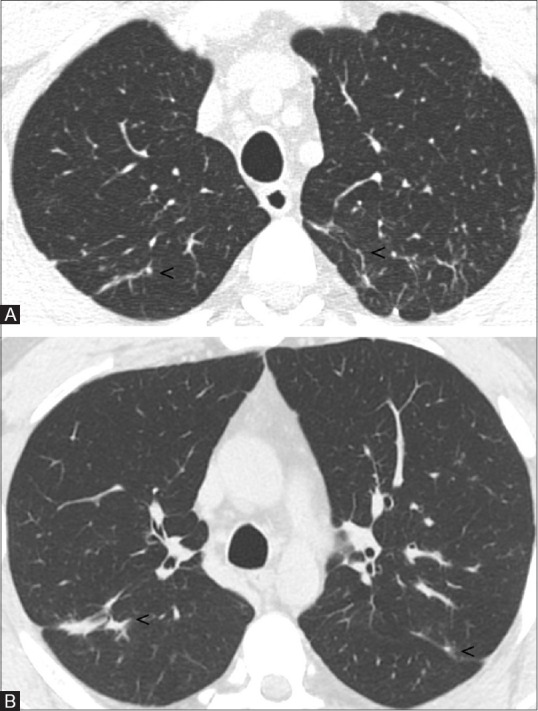
Two years later follow-up HRCT images. Bilaterally, some small linear opacities persist (A and B; see arrowheads), no further parenchymal abnormalities are appreciable
Discussion
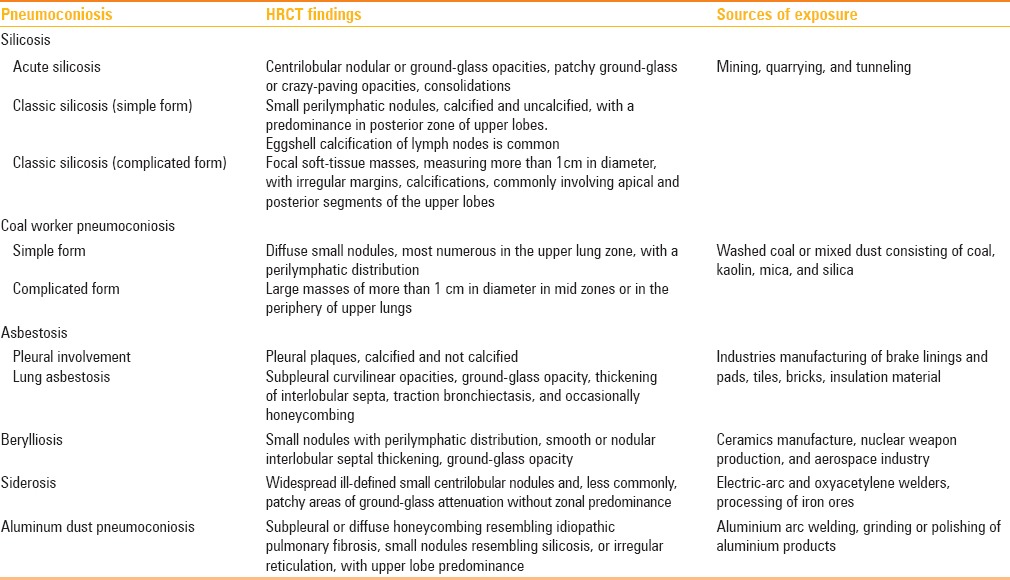
Hard metal lung disease is a rare form of occupational lung disease [Table 1] that can occur in workers employed in the manufacture, utilization, or maintenance of tools composed of hard metal, a material composed mainly of tungsten carbide, cobalt or diamond-cobalt, and occasionally of other metals such as titanium, tantalum, chromium, nickel.[1,2] Clinically, the condition resembles hypersensitivity pneumonitis, with subacute presentations and possible evolution to pulmonary fibrosis.[3]. This interstitial lung disease is generally characterized by the presence of bizarre cannibalistic multinucleated giant cells in the alveoli and the bronchoalveolar lavage.[4] It is noted that cobalt and tungsten carbide interact with oxygen, resulting in the augmented production of toxic activated oxygen species. In addition, cobalt ions are potent inducers of hypoxia-inducible factor (HIF)-1, an important intracellular regulator. Probably, these processes stimulate macrophages to become the giant multinucleated cells, which is characteristic of GIP.[4]
Table 1.
The most common occupational lung diseases, with their HRCT patterns and more frequent sources of exposure
A pathological diagnosis of giant cell interstitial pneumonitis is, therefore, specific for hard metal lung disease, even though not all affected patients exhibit this pathognomonic feature.[5] One important aspect of hard-metal lung disease is that the disease may occur after a short duration of exposure, implying individual susceptibility rather than cumulative exposure.[4,6] The diagnostic criteria for hard metal lung disease include the following: (a) a history of exposure to metal dust; (b) characteristic clinical features, including shortness of breath, cough, and dyspnea on exertion over a prolonged period; (c) radiologic findings of interstitial lung disease; (d) histologic findings of interstitial lung disease or a giant cell interstitial pneumonia pattern (presence of a large number of giant cells filling the airspace), with thickening of the interstitium and alveolar walls by mononuclear cells; and (e) a histopathologic finding of metallic content in lung tissue.[7] Interestingly, cobalt, although critical factor in hard metal lung disease, is detected in approximately 10% of lung samples from patients with hard metal lung disease, presumably for its high solubility in body fluids.[4] In our case, the HRCT and histopathological findings raised a differential diagnosis between sarcoidosis and occupational interstitial lung disease. A diagnosis of hard metal lung disease was made on the basis of the positive occupational history, the resolution of the symptoms, and the improvement of HRCT findings following the removal of dust exposure, and in absence of specific treatments, the radiological and histopathological findings consistent with an interstitial lung disease, and finally, the concentrations of tungsten and titanium in lung tissue. It is interesting to note that the HRCT findings of GIP have been described in few case reports and articles giant cell interstitial pneumonia shows HRCT images of mixed ground-glass opacities and reticulation.[5] Moreover, although the finding of giant cell interstitial pneumonia is almost pathognomonic for hard metal pneumoconiosis, the histopathologic manifestations of hard-metal disease range from bronchitis to subacute fibrosing alveolitis to interstitial fibrosis.[3,5,8] The corresponding HRCT patterns are ground-glass, irregular linear opacities, consolidations, centrilobular nodules, and, in advanced disease, parenchymal distortion, traction bronchiectasis and honeycombing.[2,3,7,9] In the present case, HRCT findings show a pattern of perilymphatic nodules and peribronchovascular consolidation, data not previously reported in English literature. Moreover, the histopathologic manifestation of nonnecrotizing granulomatous inflammation with giant cells, reported in our case, has not been previously reported. Sarcoid-like granulomatous inflammation has been reported in occupational exposure to beryllium and rare earths dust.
In conclusion, the present case should raise awareness that hard metal lung disease may present unconventional radiologic patterns, in particular, a perilymphatic pattern underlying non-GIP histopathological interstitial lung disease.
Teaching point
Hard metal lung disease is a rare occupational lung disease. GIP is known as its typical pathological finding, and the reported HRCT abnormalities, for this occupational disease, include patchy lobular ground-glass opacities, centrilobular nodularity, and occasionally, honeycombing. The present case suggests that hard metal lung disease may show a granulomatous sarcoid-like interstitial lung disease with a perilymphatic HRCT pattern.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflict of interest.
References
- 1.Hiroya T, Satoshi K, Kichizo K. Two cases of hard metal lung disease showing gradual improvement in pulmonary function after avoiding dust exposure. J Occup Med Toxicol. 2015;4:10–29. doi: 10.1186/s12995-015-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KI, Kim CW, Lee MK, Lee KS, Park CK, Choi SJ, et al. Imaging of Occupational Lung Disease. RadioGraphics. 2001;21:1371–91. doi: 10.1148/radiographics.21.6.g01nv011371. [DOI] [PubMed] [Google Scholar]
- 3.Junichi T, Hiroshi M, Masaki T. An observational study of giant cell interstitial pneumonia and lung fibrosis in hard metal lung disease. BMJ Open. 2014;27:4. doi: 10.1136/bmjopen-2013-004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemery B, Abraham JL. Hard Metal Lung Disease Still Hard to Understand. Am J Respir Crit Care Med. 2007;176:2–3. doi: 10.1164/rccm.200704-527ED. [DOI] [PubMed] [Google Scholar]
- 5.Choi JW, Lee KS, Chung MP, Han J, Chung MJ, Park JS. Giant Cell Interstial Pneumonia: High Resolution CT and Pathologic Findings in Four Adult Patient. AJR Am J Roentgenol. 2005;184:268–72. doi: 10.2214/ajr.184.1.01840268. [DOI] [PubMed] [Google Scholar]
- 6.Naqvi AH, Hunt A, Burnett BR, Abraham JL. Pathologic spectrum and lung dust burden in giant cell interstitial pneumonia (hard metal disease/cobalt pneumonitis): Review of 100 cases. Arch Environ Occup Health. 2008;63:51–70. doi: 10.3200/AEOH.63.2.51-70. [DOI] [PubMed] [Google Scholar]
- 7.Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Pneumoconiosis: Comparison of Imaging and Pathologic Findings. RadioGraphics. 2006;26:59–77. doi: 10.1148/rg.261055070. [DOI] [PubMed] [Google Scholar]
- 8.Moriyama H, Kobayashi M, Takada T, Shimizu T. Two-dimensional analysis of elements and mononuclear cells in hard metal lung disease. Am J Respir Crit Care Med. 2007;176:70–7. doi: 10.1164/rccm.200601-134OC. [DOI] [PubMed] [Google Scholar]
- 9.Gotway MB, Golden JA, Warnock M. Hard metal interstitial lung disease: High-resolution computed tomography appearance. J Thorac Imaging. 2002;17:314–8. doi: 10.1097/00005382-200210000-00009. [DOI] [PubMed] [Google Scholar]