Abstract
Introduction
Currently alpha1-adrenoceptor blockers (AB) are widely used as first-line therapy to improve lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). We compared the efficacy and safety profile of tamsulosin, alfuzosin and silodosin in LUTS due to BPH.
Material and methods
Consecutive consenting male patients (N = 269) undergoing medical management of BPH with AB from February 2012 to October 2015 were enrolled. Patients were randomized to a 0.4 mg tamsulosin (group T), 10 mg alfuzosin (group A) or a 8 mg silodosin (group S) by double-blind randomization. All patients were assessed for improvements and post-void residual urine (PVR) and for adverse drug events (ADE).
Results
IPSS showed significant improvement in Group S at the first week (11.7 ±4.18, p = 0.027) and at 3 months (7.97 ±3.84, p = 0.020). QOL showed significant improvement at 1 (2.2 ±0.76, p = 0.020), 4 (1.47 ±0.63, p <0.001) and 12 (1.2 ±0.66, p <0.001) weeks in Group S. The mean Qmax improvement was the maximum (13.76 ±2.44, p = 0.028) in Group S at 1 week. Reduction in PVR was the maximum in Group S, but it was not statistically significant. Adverse drug events (ADE) were observed in 20.07% (54/269) patients and distribution was similar in the three groups with decreasing incidence with progression of time.
Conclusions
Silodosin is the most efficacious AB with rapid onset of action. Silodosin also improves the quality of life in patients with LUTS due to BPH and objectively improves maximum flow rate. However, silodosin has more adverse events when compared to tamsulosin and alfuzosin.
Keywords: benign prostatic hyperplasia, alpha 1-adrenoceptor blocker, tamsulosin, alfuzosin, silodosin
INTRODUCTION
Benign prostatic hyperplasia (BPH) results in lower urinary tract symptoms (LUTS) and is common in 50% of men past the age of 60 years. BPH causes resistance to urinary flow due to the increase in prostate capsule tone in prostates <30 cc [1]. LUTS is caused by increased prostatic smooth muscle contraction due to the sympathetic hyperactivity of alpha 1-adrenoceptors. LUTS are bothersome and interfere with quality of life (QoL) of aging males [2]. Autopsy studies in Asian and Caucasian men showed an overall prevalence of BPH in 74.8% of men. Alpha 1-adrenoceptor blockers (AB) are currently the recommended first-line therapies for benign prostatic hyperplasia (BPH) [2]. They have high efficacy, are less expensive and have fewer adverse events (ADE) in the treatment of LUTS [1, 3, 4]. They act principally by blocking α1A-adrenoreceptors, which is most prevalent in the prostatic smooth muscle and produce relaxation in the smooth muscle component of the prostate [4]. Multiple drugs are available and each drug has its own advantages and disadvantages. Tamsulosin is the most widely used AB, alfuzosin is used in sexually active males and silodosin is a newer agent with greater α-1A receptor selectivity. We compared the safety and efficacy of the three drugs namely tamsulosin, alfuzosin and silodosin.
MATERIAL AND METHODS
This was a hospital-based, double-blind, randomized trial, performed in 269 patients undergoing medical management of BPH in the Department of Urology, in a tertiary care institute, in South India. After obtaining approval from the Institute Research Council and Ethics committee, the study was conducted from February 2012 to October 2015.
Study population
All consecutive consenting male patients with BPH aged more than 50 years, LUTS with mild to moderate International Prostate Symptom Score (IPSS), Maximum flow rate (Qmax) <15 ml/sec on uroflowmetry (UFR), prostate size measuring 25–50 cm3 and post void residue (PVR) <100 ml on trans abdominal ultrasound (US) were eligible for the study after obtaining a written informed consent. The exclusion criteria were the following: use of AB within 2 weeks/ 5-alpha reductase inhibitors (5ARI) within 6 months or phytotherapy, active urinary tract infection (UTI), bladder outlet obstruction due to any other cause like urethral stricture, bladder neck stenosis or diagnosed to have vesical calculus, urethral or bladder diverticulum, neurogenic bladder, prostatic or bladder cancer, significant orthostatic hypotension (OH), post-prostatectomy and renal impairment (serum creatinine >2 mg/dl).
Randomization, allocation concealment and blinding
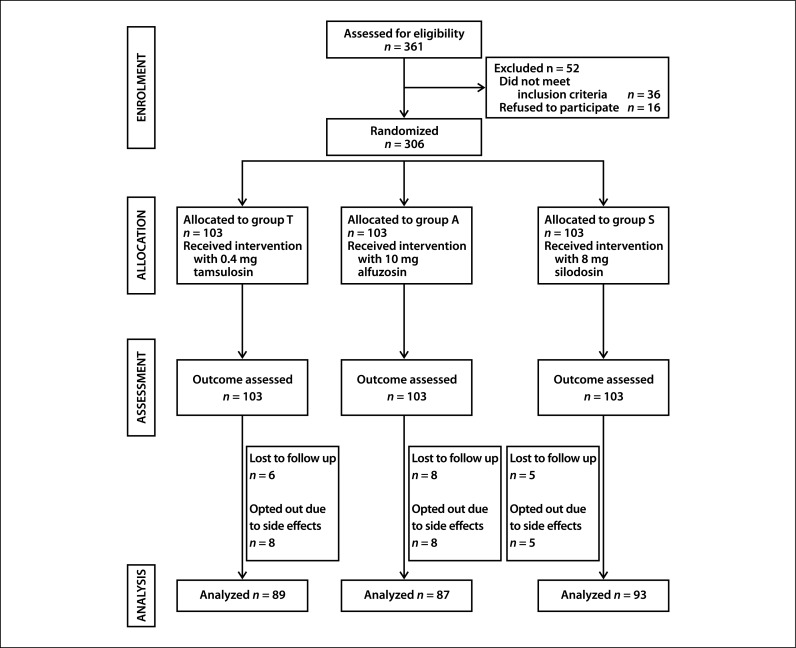
Patients were randomized to receive oral tamsulosin 0.4 mg or alfuzosin 10 mg or silodosin 8 mg by block randomization (block size of 10) performed using Microsoft Excel 2010. Allocation concealment was performed using opaque sealed envelopes. Randomization and sealed envelopes were done by a person independent of the investigators. Envelopes were opened in the outpatient department by a nursing staff not involved in the research. The drug was placed in a sealed envelope with the code. The patient and the investigators who assessed the outcomes were also blinded. Hence, the study was double-blind. At the end of the study, the groups were decoded and analyzed (Figure 1).
Figure 1.
Flow of study participants through the study.
Intervention
A standard protocol was used for all our patients. Patients were evaluated with detailed history regarding LUTS. Clinical and digital rectal examination was done. LUTS was assessed using the IPSS questionnaire (English and native language Kannada) including QoL. UFR, ultrasound of the kidney, ureters, bladder (KUB) and prostate, with PVR, serum prostate specific antigen (PSA), blood urea, serum creatinine, blood pressure (BP) in supine and standing position and electrocardiogram were done in all patients at the beginning of the study. Follow-up was done at 1,4 and 12 weeks with LUTS assessment using IPSS, QoL, Qmax using UFR and US for PVR and side effects of each drug and BP in supine and standing positions. Eight patients each in groups T and A and five patients in group S opted out due to side effects.
Study endpoints
The primary endpoints were improvement in IPSS LUTS scores, QoL, Qmax and PVR from baseline at 1,4 and 12 weeks. The secondary endpoints were ADE to the drugs.
Statistical analysis
Statistical analysis was performed using SPSS version 19 for Windows (IBM Corporation, Armonk, New York). The normality of the data was initially assessed using a Box and Whisker plot. The variables were summarized using mean, standard error, median, interquartile range, and percentages based on the characteristics of the variable. One-way ANOVA and the Kruskal-Wallis test were used as appropriate for the analysis of continuous variables based on the normality of the distribution. Chi-Square test was used for categorical variables. The P value of <0.05 was considered statistically significant.
RESULTS
For the purpose of tabulation and analysis, the groups were denoted as: Group T receiving 0.4 mg of oral tamsulosin, group A receiving 10 mg of alfuzosin and Group S receiving 8 mg of silodosin. The baseline patient characteristics like age, duration of symptoms, prostate weight, Qmax, and PVR were comparable (Table 1).
Table 1.
Baseline characteristics
| Parameter | Group T N = 89 | Group A N = 87 | Group S N = 93 | P value |
|---|---|---|---|---|
| Age, years Mean ±SD | 58.47 ±6.16 | 56.90 ±10.26 | 59.10 ±8.79 | 0.451 |
| IPSS Mean ±SD | 16.27 ±6.07 | 16.23 ±6.48 | 14.27 ±5.33 | 0.337 |
| Mild: Moderate IPSS, N | 18:71 | 14:73 | 21:72 | 0.544 |
| QoL Mean ±SD | 2.40 ±0.86 | 2.37 ±0.89 | 2.47 ±0.82 | 0.465 |
| Prostate weight, cc Mean ±SD | 33.82 ±11.38 | 37.76 ±12.10 | 35.18 ±11.20 | 0.342 |
| Voided volume, cc Mean ±SD | 162.36 ±45.46 | 165.67 ±39.51 | 159.78 ±43.41 | 0.654 |
| Qmax, ml/sec Mean ±SD | 11.75 ±2.64 | 12.69 ±2.58 | 12.13 ±2.62 | 0.381 |
| PVR, cc Mean ±SD | 44.74 ±27.14 | 41.88 ±19.33 | 53.61 ±29.00 | 0.218 |
| Serum creatinine, mg/dl Mean ±SD | 1.45 ±0.89 | 1.56 ±0.24 | 1.39 ±0.78 | 0.260 |
| PSA ng/ml Mean ±SD | 2.8 ±1.54 | 3.1 ±1.72 | 2.7 ±0.61 | 0.129 |
IPSS and QoL
The mean IPSS scores at baseline were comparable between the three groups (Table 1; p = 0.337). At follow-up at first week (11.7 ±4.18, p = 0.027 and at 3 months (7.97 ± 3.84, p = 0.020) the maximum improvement was observed in Group S and this was statistically significant. At end of the first month, the maximum improvement was seen in Group S; however, it did not reach statistical significance (9.43 ±3.89, p = 0.077). The mean QoL scores at baseline were comparable between the three groups (Table 1; p = 0.465). At follow-up at 1 (2.2 ±0.76, p = 0.020), 4 (1.47 ±0.64, p <0.001) and 12 (1.2 ±0.66, p <0.001) weeks after starting AB, improvement in QoL was the maximum in Group S and this was statistically significant (Table 2).
Table 2.
IPSS and QoL in tamsulosin, alfuzosin and silodosin at 1, 4 and 12 weeks
| Time | Parameter | Group T Mean ±SD | Group A Mean ±SD | Group S Mean ±SD | P value |
|---|---|---|---|---|---|
| 1 week | IPSS | 15.23 ± 6.67 | 15.4 ±6.52 | 11.7 ±4.18 | 0.027 |
| QoL | 2.77 ±0.9 | 2.3 ±0.79 | 2.2 ±0.76 | 0.020 | |
| 4 weeks | IPSS | 11.83 ±5.31 | 12.33 ±6.22 | 9.43 ±3.89 | 0.077 |
| QoL | 2.17 ±0.7 | 1.67 ±0.61 | 1.47 ±0.63 | <0.001 | |
| 12 weeks | IPSS | 11.03 ±5.07 | 11.43 ±6.19 | 7.97 ±3.84 | 0.020 |
| 2.03 ±0.61 | 1.53 ±0.63 | 1.2 ±0.66 | <0.001 |
Qmax and PVR
The mean Qmax at baseline was comparable between the three groups (Table 1; p = 0.381). At follow-up, the improvement was the maximum in Group S and it was statistically significant at 1 week (13.76 ±2.44, p = 0.028). At 4 and 12 weeks after starting the drug, the improvement was the maximum in Group S; however, it was not statistically significant. The PVR was similar in the three groups at baseline. Though the PVR was reduced in Group S at all three intervals, it was not significant (Table 3).
Table 3.
Qmax and PVR in tamsulosin, alfuzosin and silodosin at 1, 4 and 12 weeks
| Time | Parameter | Group T Mean ±SD | Group A Mean ±SD | Group S Mean ±SD | P value |
|---|---|---|---|---|---|
| 1 week | Vvol | 156.12 ±39.12 | 155.17 ±16.28 | 151.29 ±37.11 | 0.572 |
| Qmax | 11.90 ±2.95 | 13.11 ±2.63 | 13.76 ±2.44 | 0.028 | |
| PVR | 38.64 ±24.78 | 35.27 ±19.33 | 43.31 ±22.93 | 0.435 | |
| 4 weeks | Vvol | 153.21 ±31.11 | 159.19 ±43.12 | 154.13 ±23.18 | 0.440 |
| Qmax | 13.87 ±2.33 | 15.00 ±2.18 | 15.77 ±4.91 | 0.097 | |
| PVR | 27.96 ±17.36 | 30.18 ±17.24 | 29.25 ±17.86 | 0.910 | |
| 12 weeks | Vvol | 161.23 ±27.34 | 157.32 ±28.98 | 159.14 ±32.45 | 0.683 |
| Qmax | 14.33 ±2.15 | 15.76 ±2.08 | 16.15 ±4.81 | 0.083 | |
| PVR | 24.42 ±14.73 | 25.80 ±17.99 | 25.74 ±15.9 | 0.949 |
Vvol – voided volume (ml); Qmax – maximum flow rate (ml/second); PVR – post void residual urine (ml)
Adverse drug events (ADE)
ADE were observed in 54 out of 269 patients (20.07%). At 1,4 and 12 weeks, the proportion of patients developing side effects was least prominent in group S. Dizziness was the most common side effects in all of the 3 groups. Abnormal ejaculation (AE) was most common in group S (6.5% at 4 weeks and 9.7% at 12 weeks) and insomnia (3/93; 3.2% at 1 and 12 weeks) and syncope (6/93; 6.45% at 1 week) were observed only in group S. Fatigue was observed in groups T and A. Headache was observed only in 6 (6.9) patients in group A at 1 and 12 weeks. The incidence of ADE reduced with progression of time. However, there was no statistically significant difference at any point of time between the three groups (Table 4).
Table 4.
Adverse effects to tamsulosin, alfuzosin and silodosin at 1, 4 and 12 weeks
| Side effect | Time | Group T N = 89 N (%) |
Group A N = 87 N (%) |
Group S N = 93 N (%) |
P value |
|---|---|---|---|---|---|
| Nil | 1 week | 81 (91.1) | 72 (82.8) | 64 (68.9) | 0.199 |
| 4 weeks | 75 (84.3) | 84 (94.4) | 73 (78.5) | ||
| 12 weeks | 73 (82.1) | 76 (87.4) | 68 (73.1) | ||
| Abnormal ejaculation | 1 week | 0 | 0 | 0 | 0.223 |
| 4 weeks | 0 | 0 | 6 (6.5) | ||
| 12 weeks | 3 (3.4) | 0 | 9 (9.7) | ||
| Dizziness | 1 week | 3 (3.4) | 3 (3.5) | 11 (11.8) | 0.189 |
| 4 weeks | 8 (9) | 0 | 13 (14) | ||
| 12 weeks | 3 (10) | 3 (3.4) | 4 (13.3) | ||
| Fatigue | 1 week | 4 (4.6) | 9 (10.3) | 0 | 0.254 |
| 4 weeks | 3 (3.4) | 5 (5.7) | 0 | ||
| 12 weeks | 3 (3.4) | 6 (6.9) | 0 | ||
| Orthostatic hypotension | 1 week | 3 (3.4) | 0 | 7 (7.5) | 0.278 |
| 4 weeks | 2 (2.3) | 0 | 0 | ||
| 12 weeks | 3 (3.4) | 1 (1.2) | 0 |
DISCUSSION
There are no studies in literature comparing the following three drugs: tamsulosin, alfuzosin and silodosin in the medical management of LUTS due to BPH. We administered these three drugs as monotherapy in symptomatic LUTS due to BPH in 269 patients and observed for improvements in IPSS, QoL, Qmax, PVR and also for ADE. Robert et al. recommended newer drugs like PDE5I and combination therapies like AB with 5-alpha- reductase inhibitors (5ARI). They however suggested that selection of therapy is to be individualized [5]. Wang et al. assessed the effect of alpha adrenoceptor antagonists, 5-alpha reductase inhibitors, PDE-5 inhibitors and muscarinic receptor antagonists in a meta-analysis on 29,384 patients [6]. Yuan et al. assessed the effect of AB, 5ARI, muscarinic receptor antagonists (MRA) and PDE5I in 58548 patients. They found that AB, 5ARI and PDE5I were the most effective agents. Among alpha1-adrenoceptor blockers, doxazosin and terazosin were most effective. They also concluded that medical therapy in BPH is safe and drugs have a comparable ADE profile [3]. Novara et al. analyzed the effect of silodosin over placebo in a pooled analysis [7].
IPSS and QoL
Wang et al. showed that IPSS score reduction with all drug groups when compared with the placebo. They also observed that there was no significant difference between AB and ARI or PDE5I. AB with PDE5I had the best symptom score improvement [2, 6]. However, they did not find which of the three alpha1-adrenoceptor blockers is better [6]. Improvement in IPSS was comparable among tamsulosin, alfuzosin, silodosin, naftopidil, dutasteride, vardenafil, sildenafil, and tadalafil [3]. We observed that all three drugs had improvement in IPSS scores and it was the maximum with silodosin. Pande et al. observed that tamsulosin and silodosin were comparable for efficacy [8]. Zhang et al. observed alfuzosin 10 mg to be effective and well tolerated in LUTS due to BPH with or without antihypertensive medications [9]. Oelke et al. observed that the overall satisfaction and satisfaction with efficacy was greater with tadalafil when compared with the placebo, than tamsulosin when compared with the placebo [10]. In our study, the maximum improvement in QoL was with silodosin. Novara et al. observed that silodosin significantly improved IPSS and QoL compared to placebo [7]. In a recent study, Takeshita et al. proved that 4 mg of silodosin has a similar efficacy to 0.4 mg of tamsulosin in improving IPSS. They also observed that nocturia was exclusively improved by silodosin [11].
Maximum flow rate
Wang et al. observed Qmax improvement with AB which was similar to the placebo. However, AB with 5ARI combination had the maximum improvement. PDE5I showed improvement in IPSS scores, but not in Qmax [6]. Novara et al. identified that doxazosin and dutasteride showed the maximum improvement in Qmax. With placebo, doxazosin, dutasteride, terazosin, alfuzosin, tamsulosin, naftopidil, and silodosin all had significantly higher Qmax post-treatment [3, 7]. Silodosin objectively improved Qmax, while tamsulosin did not improve Qmax in a recent study [11]. In our study, Qmax improvement was the maximum at first week with silodosin. In the following weeks, similar Qmax improvement was observed with all three drugs. We observed that although the baseline IPSS scores were comparable among the three groups, it was lower among patients of the silodosin arm. However, the distribution of mild and moderate IPSS categories was similar across the three arms. Also, baseline Qmax, a more objective measurement was comparable among the three arms (Table 1).
Adverse drug events (ADE)
When compared to placebo, doxazosin, terazosin, silodosin and tadalafil had a significantly higher incidence of adverse drug events [3]. The primary specific ADE reported for AB was dizziness, headache and asthenia. Pande et al. observed abnormal ejaculation only with silodosin and orthostatic hypotension only with tamsulosin. They reported silodosin to be useful for elderly patients and alfuzosin for younger sexually active men. However, we observed that ADE was the maximum with silodosin and least with tamsulosin and alfuzosin. Zhang et al. observed that alfuzosin with antihypertensive medication decreases systolic and diastolic blood pressure. Orthostatic hypotension was observed only with tamsulosin in 3% patients, but not with alfuzosin and silodosin [9]. In a study on silodosin, the most common ADE was abnormal ejaculation in 22% patients. The incidence of dizziness, orthostatic hypotension and cardiovascular ADE was similar to placebo [7]. Takeshita et al. identified that patients did not have preference to either tamsulosin or silodosin in their crossover open label randomized trial [11]. Lower dose of silodosin can be more cost efficacious and reduce the incidence of side effects like abnormal ejaculation and orthostatic hypotension [11]. We observed that silodosin was the efficacious alpha1-adrenoceptor blocker and also had the highest incidence of abnormal ejaculation.
CONCLUSIONS
Silodosin is the most efficacious alpha-1 adrenoceptor blocker with a rapid onset of action and had consistent improvement in LUTS at 3 months in Indian men. Silodosin also improves the quality of life of patient with LUTS due to BPH and objectively improves maximum flow rate. However, silodosin has more adverse events in the form of abnormal ejaculation and dizziness when compared to tamsulosin and alfuzosin.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Van Asseldonk B, Barkin J, Elterman DS. Medical therapy for benign prostatic hyperplasia: a review. Can J Urol. 2015;22(Suppl 1):7–17. [PubMed] [Google Scholar]
- 2.Wang XH, Wang X, Shi MJ, Li S, Liu T, Zhang XH. Systematic review and meta-analysis on phosphodiesterase 5 inhibitors and α-adrenoceptor antagonists used alone or combined for treatment of LUTS due to BPH. Asian J Androl. 2015;17:1022–1032. doi: 10.4103/1008-682X.154990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan JQ, Mao C, Wong SY, et al. Comparative Effectiveness and Safety of Monodrug Therapies for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Network Meta-analysis. Medicine (Baltimore) 2015;94:e974. doi: 10.1097/MD.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osman NI, Mangera A, Chapple CR. Non-Hormonal treatment of BPH/BOO. Indian J Urol. 2014;30:194–201. doi: 10.4103/0970-1591.126906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert G, Descazeaud A, Delongchamps NB, et al. Benign prostatic hyperplasia medical treatment: systematic review of the literature by the CTMH/AFU. Prog Urol. 2012;22:7–12. doi: 10.1016/j.purol.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Wang X, Li S, Meng Z, Liu T, Zhang X. Comparative effectiveness of oral drug therapies for lower urinary tract symptoms due to benign prostatic hyperplasia: a systematic review and network meta-analysis. PloS One. 2014;9:e107593. doi: 10.1371/journal.pone.0107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novara G, Chapple CR, Montorsi F. A pooled analysis of individual patient data from registrational trials of silodosin in the treatment of non-neurogenic male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) BJU Int. 2014;114:427–433. doi: 10.1111/bju.12712. [DOI] [PubMed] [Google Scholar]
- 8.Pande S, Hazra A, Kundu AK. Evaluation of silodosin in comparison to tamsulosin in benign prostatic hyperplasia: a randomized controlled trial. Indian J Pharmacol. 2014;46:601–607. doi: 10.4103/0253-7613.144912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang LT, Lee SW, Park K, et al. Multicenter, prospective, comparative cohort study evaluating the efficacy and safety of alfuzosin 10 mg with regard to blood pressure in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia with or without antihypertensive medications. Clin Interv Aging. 2015;10:277–286. doi: 10.2147/CIA.S74102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oelke M, Giuliano F, Baygani SK, Melby T, Sontag A. Treatment satisfaction with tadalafil or tamsulosin vs placebo in men with lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH): results from a randomised, placebo-controlled study. BJU Int. 2014;114:568–575. doi: 10.1111/bju.12733. [DOI] [PubMed] [Google Scholar]
- 11.Takeshita H, Moriyama S, Arai Y, et al. Randomized crossover comparison of the short-term efficacy and safety of single half-dose silodosin and tamsulosin hydrochoride in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. Low Urin Tract Symptoms. 2016;8:38–43. doi: 10.1111/luts.12106. [DOI] [PubMed] [Google Scholar]