Abstract
Introduction
Frailty used as predictive tool is still not carried out in daily practice, although many studies confirm the great clinical importance of the frailty syndrome in surgical outcomes. There is no standardized method of measuring the physiological reserves of older surgical patients.
The aim of this study was to analyze a cohort of older urological patients according to various frailty indices, in order to evaluate whether they are predictors of post-operative complications after urological procedures.
Material and methods
This is a prospective observational study on 78 consecutive older (≥70 years) patients, subjected to major urological (both endoscopic and ’open surgical’) procedures. Frailty was defined according to the Edmonton Frail Scale. Several risk models and biochemical parameters were evaluated.
Post-operative outcomes were surgical and medical complications, mortality and rehospitalisation within 3 months.
Results
An overall prevalence of frailty of 21.8% was found. Patients with complications were frailer than those without complications (univariate analysis), considering both total patients (p = 0.002) and endoscopic (p = 0.04) and ’open surgical’ patients (p = 0.013). However, in multivariate analysis, a significant correlation was not found between all frailty indices tested and the risk of major complications. Limitation of the study: the small sample size (lack of statistical power), although this is a prospective study focused on older urological patients.
Conclusions
New urology-tailored pre-operative assessment tools may prove beneficial when calculating the risks/benefits of urological procedures, so that objective data can guide surgical decision- making and patient counselling. Further large clinical studies specifically focusing on elderly in urology will be needed.
Keywords: frailty, complications, comorbidity
INTRODUCTION
The world population is gradually becoming older, but age alone cannot describe the health status of individuals, nor can it be the decisive factor in choosing, for example, which level of treatment a patient should undergo. It has been suggested that ‘health status’ may be represented by the number of health deficits accumulated by individuals during their lives and thus lead to the introduction of the concept of frailty. Frailty (Latin: fragilitas; brittleness) is generally defined as a state of reduced physiological reserves [1]. Following the Cardiovascular Health Study, Fried et al. [2] formulated specific criteria defining the frailty syndrome, which offer an empirically derived and validated definition based on at least three or more characteristics: unexplained weight loss, muscle weakness (low grip strength), self-reported exhaustion, poor endurance (as shown by slow walking speed) and low activity level.
In our opinion, from the surgical viewpoint, the best definition of frailty is that proposed by Clegg et al., as “the reduced capacity to cope with stressors which increase the risk of adverse outcomes in older patients” [3].
Frailty is a multi-dimensional geriatric syndrome, linked not only to physical, but also to social and psychological factors [4]. For example, geriatricians have associated cognitive impairment with poor outcomes in the elderly [5].
Several studies have reported the effect of frailty on falls, hospitalization and mortality, but few have focused on surgical patients and frailty is not one of the traditional surgical risk indices [6]. Studies have associated loneliness [7], functional limitations [8], cognitive impairment [9, 10, 11], poor nutritional status [12] and depression [13] with various post-operative complications, but there is no consensus on how frailty should be measured. For this reason, some frailty indices have been proposed to identify patients at risk of complications before operation, quantifying the balance between the risks and benefits of a treatment, such as a surgical procedure. In response to this need, a comprehensive assessment tool of characteristics of elderly cancer patients before elective surgery (PACE) was developed and validated in a multispecialty study, including only a few cases of genito-urinary cancers [14]. However, not all surgical patients are identical, and nor are all surgical specialities!
The aims of the present study were to analyze a cohort of older urological patients according to various frailty indices, to verify whether they are predictive of post-operative complications after urological procedures.
MATERIAL AND METHODS
In this prospective observational study, data was collected from 106 consecutive patients aged ≥70 years, subjected to major urological (both endoscopic and ’open surgical’) procedures. In the ’open surgical’ group we included only radical cystectomy, or radical nephrectomy, or radical prostatectomy; in the endoscopic group trans-urethral resection of the prostate (TURP), or transurethral resection of large (>4 cm) bladder neoplasms. Patients also underwent a standardized pre-operative interview and frailty assessment.
Frailty was defined according to the Edmonton Frail Scale (EFS) [15], a validated multifactorial scale which screens for cognitive impairment, dependence in instrumental activities of daily living, recent burden of illness, self-perceived health, depression, weight loss, medication issues, incontinence, inadequate social support, and mobility problems. Scores range from 0 (not frail) to 17 (very frail). A patient is considered as frail with a score >7, non-frail with <4, and intermediate with 5–7 .
Comprehensive medical and urological history was acquired for all patients. Data was collected on age, gender, BMI, and preoperative haemoglobin, albumin and creatinine. All the components of the PACE tool were used (see Table 1A): Satarian Index of Comorbidities (SIC, scores 0–7); Mini-Mental State Examination to assess cognitive ability (MMSE, 0-30, with ≤24 defining cognitive impairment); Activities of Daily Living (ADL, 0–6); Instrumental Activities of Daily Living (IADL, 0–8); Geriatric Depression Scale (GDS, 0–15); Eastern Cooperative Oncology Group Performance Status (ECOG, from 0 for health, to 5 for patient dead); Brief Fatigue Inventory (BFI, fatigue level increasing from 0 to 10); and ASA risk (1–5), calculated by an anesthesiologist.
Table 1.
(A) Components of PACE and (B) Dichotomisation Thresholds for each parameter
| Table 1A | |||
| Components of PACE | |||
| a. American Society of Anesthesiologists Physical Status – ASA | |||
| b. Mini-Mental State Examination – MMSE c. Activities of Daily Living – ADL | |||
| d. Instrumental Activities of Daily Living – IADL e. Geriatric Depression Scale – GDS g. Eastern Cooperative Oncology Group Performance Status – ECOG | |||
| h. Satarian Index of Comorbidities – SIC | |||
| Table 1.B | |||
| Parameter | RANGE | Dichotomisation threshold | Groups |
| Demographic parameters | |||
| Age, years | 70–94 | 80 | <80 Adults ≥80 Elderly |
| BMI (kg/m2) | 19–37.9 | 25 | <25 Normal weight ≥25 Overweight |
| Biochemical parameters | |||
| Albumin (g/l) | 32–47 | 37 | ≤37 Low >37 Normal |
| Hemoglobin (g/l) | 92–168 | 120 | ≤120 Low >120 Normal |
| Creatinine (g/l) | 46–290 | 130 | ≤130 Normal >130 High |
| Other parameters | |||
| CCI | 0–37 | 3 | ≤3 Low risk >3 High risk |
| ACCI | 0–42 | 7 | ≤7 Low risk >7 High risk |
| Operative time (min) | 15–590 | 210 | <210 Low ≥210 High |
| Blood loss (ml) | 0–1800 | 100 | ≤100 Low >100 High |
| Frailty indices | |||
| EFS | 0–17 | 7 | ≤7 Not frail >7 Frail |
| Components of PACE | |||
| a. ASA | 1–5 | 2 | ≤2 Low risk >2 High risk |
| b. MMSE | 0–30 | 24 | ≤24 Cognitive impairment |
| c. ADL | 0–6 | 5 | ≤5 Functional dependency 6 Independency |
| d. IADL | 0–8 | 7 | ≤7 Physical dependency 8 Independency |
| e. GDS | 0–15 | 4 | ≤4 Non depression >4 Depression |
| f. BFI | 0–10 | 2 | ≤ 2 No fatigue/Mild fatigue >2 Fatigue |
| g. ECOG | 0–5 | 1 | 1 Healthy >1 Sick |
| h. SIC | 0–7 | 1 | 1 No comorbidity >1 Comorbidities |
In addition, comorbidities were evaluated with the Charlson Comorbidity Index (CCI, score 0–37) and Age- corrected Charlson Score (ACCI, 0–42). Operation variables were also recorded: type of intervention (endoscopy vs. ’open surgery’), operative time, blood loss, transfusion rate, and need for transfer to intensive care unit (ICU). Post-operative outcomes were surgical and medical complications, mortality, and rehospitalisation within 3 months. Complications within 3 months, classified according to the Clavien- Dindo system, were considered both as categorical variables and categorized as absent/minor (Clavien 0–I) or major (Clavien II–V).
Several risk models were evaluated: EFS, various components of PACE, CCI and ACCI, and biochemical parameters.
Statistical analysis
Statistical analysis was performed with SPSS 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Data were tested for normality (Kolmogorov-Smirnov test). Categorical data are expressed as numbers (%) and continuous data as means (SD) or medians (interquartile range), if normally or non-normally distributed, respectively. Means, SDs, medians and frequencies were used as descriptive statistics. Results were analyzed with Pearson’s chi-square test and Fisher’s exact test, as appropriate, and for proportions in the case of categorical data. For means with continuous numeric data, ANOVA/Student’s t-test and the Mann-Whitney U-test were used, for normally and non-normally distributed data, respectively.
Because of the small sample size, some variables were dichotomised, according to the thresholds reported in the relative literature, and considered as categorical (see Table 1B): age was dichotomised by subdividing patients into <80 (adults) and ≥80 years (elderly), BMI <25 (normal weight) and ≥25 (overweight), ASA ≤2 (low risk) and >2 (high risk), CCI ≤3 (low risk) and >3 (high risk), ACCI ≤7 (low risk) and >7 (high risk), SIC in ≤1 and >1 comorbidity, MMSE ≤24 (cognitive impairment) and >24 (normal cognitive functions), GDS ≤4 (non depression) and >4 (depression), ADL ≤5 (functional dependency) and 6 (independency) and IADL ≤7 (physical dependency) and 8 (independency). In neoplastic patients ECOG was dichotomised into ≤1 (healthy) and >1 (sick/dead). For biochemical parameters, albumin was dichotomised into ≤37 g/L and >37g/L, hemoglobin ≤120 g/L and >120 g/L and creatinine ≤130 g/L and >130 g/L. For the EFS, ≤7 was used for non-frail patients and >7 for frail ones. For multivariate analysis, logistic regression was performed.
A two-sided p value < 0.05 was considered statistically significant.
RESULTS
A total of 106 eligible patients were identified: 28 declined participation in the study. The final sample size was 78.
Overall patient characteristics and subdivision according to frailty indices are listed in Table 2: data are reported for total patients and sub-groups according to type of surgical procedure (endoscopy vs. surgery). The complete list of procedures is given in Table 3.
Table 2.
Overall patient characteristics according to frailty indices (sub-groups according to type of surgical procedure)
| Parameters | Total | Endoscopy | Surgery | p |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age, mean (SD) | 78.51 (3.88) | 81.61 (4.76) | 75.91 (4.1) | <0.001 |
| Gender | 0.195 | |||
| Female | 11 (14.1) | 4 (9.1) | 7 (20.6) | |
| Male | 67 (85.9) | 40 (90.9) | 27 (79.4) | |
| BMI (kg/m2), median (IQR) | 24.71 (4.04) | 24.1 (2.75) | 25,65 (4.34) | 0.432 |
| Biochemical parameters | ||||
| Albumin (g/l), median (IQR) | 42.05 (5.52) | 40.9 (6.3) | 42.2 (4.1) | 0.586 |
| Hemoglobin (g/l), mean (SD) | 136.33 (15.39) | 136.61 (16.40) | 135.97 (14.22) | 0.569 |
| Creatinine (g/l), median (IQR) | 95 (35) | 99 (35) | 91.5 (28.0) | 0.295 |
| Other parameters | ||||
| CCI, n°(%) | 0.671 | |||
| 0–3 | 43 (55.1) | 23 (52.3) | 20 (58.8) | |
| 4–5 | 28 (35.9) | 16 (36.4) | 12 (35.3) | |
| >5 | 7 (9.0) | 5 (11.4) | 2 (5.9) | |
| ACCI, n°(%) | 0.282 | |||
| 0–7 | 49 (62.8) | 25 (56.8) | 24 (70.6) | |
| 8–9 | 23 (29.5) | 14 (31.8) | 9 (26.5) | |
| >9 | 6 (7.7) | 5 (11.4) | 1 (2.9) | |
| Operative time (min), median (IQR) | 70 (185) | 30 (44) | 210 (110) | <0.001 |
| Blood loss (ml), median (IQR) | 0 (200) | <100 | 350 (600) | <0.001 |
| IUC (days), median (IQR) | 0 (0) | / | 0 (1) | <0.001 |
| Frailty indices | ||||
| 1. EFS | 0.418 | |||
| ≤4 | 37 (47.4) | 18 (40.9) | 19 (55.9) | |
| 5–7 | 24 (30.8) | 15 (34.1) | 9 (26.5) | |
| >7 | 17 (21.8) | 11 (25.0) | 6 (17.6) | |
| 2. Components of PACE | ||||
| 2.a ASA, n°(%) | 1 | |||
| 1 | 0 (0) | 0 (0) | 0 (0) | |
| 2 | 39 (50%) | 22 (50) | 17 (50) | |
| 3 | 39 (50%) | 22 (50) | 17 (50) | |
| 4 | 0 (0) | 0 (0) | 0 (0) | |
| 2.b MMSE, n°(%) | 0.131 | |||
| >27 | 26 (33.3) | 11 (25.0) | 15 (44.1) | |
| 25–27 | 31 (39.7) | 18 (40.9) | 13 (38.2) | |
| ≤24 | 21 (26.9) | 15 (34.1) | 6 (17.6) | |
| 2.c ADL, n°(%) | 0.392 | |||
| 6 | 44 (56.4) | 22 (50.0) | 22 (64.7) | |
| 4–5 | 31 (41.0) | 21 (47.7) | 11 (32.4) | |
| <4 | 2 (2.6) | 1 (2.3) | 1 (2.9) | |
| 2.d IADL, n°(%) | 0.391 | |||
| 8 | 51 (65.4) | 26 (59.1) | 25 (73.5) | |
| 6–7 | 22 (28.2) | 15 (34.1) | 7 (20.6) | |
| <6 | 5 (6.4) | 3 (6.8) | 2 (5.9) | |
| 2.e GDS, n°(%) | ||||
| ≤4 | 50 (64.1) | 23 (52.3) | 27 (79.4) | 0.042 |
| 4–8 | 22 (28.2) | 17 (38.6) | 5 (14.7) | |
| >8 | 6 (7.7) | 4 (9.1) | 2 (5.9) | |
| 2.f BFI, n°(%) | 0.491 | |||
| ≤3 | 33 (42.3) | 19 (43.2) | 14 (41.2) | |
| 4–6 | 39 (50.0) | 23 (52.3) | 16 (47.1) | |
| >6 | 6 (7.7) | 2 (4.5) | 4 (11.8) | |
| 2.g ECOG, n°(%) | 0.334 | |||
| 0 | 12 (15.4) | 3 (10.7) | 9 (30.6) | |
| 1 | 30 (38.5) | 16 (57.1) | 14 (46.7) | |
| 2 | 12 (15.4) | 7 (25.0) | 5 (16.7) | |
| 3 | 4 (5.1) | 2 (7.1) | 2 (6.7) | |
| 2.h SIC, n°(%) | 0.005 | |||
| 0 | 24 (30.8) | 7 (15.9) | 17 (50.0) | |
| 1–2 | 36 (46.2) | 25 (56.8) | 11 (32.4) | |
| >2 | 18 (23.1) | 12 (27.3) | 6 (17.6) | |
BMI – body mass index; CCI – charlson comorbidity index; ACCI – age-corrected charlson score; IUC – intensive unit care; EFS – edmonton frail scale; ASA – american society of anesthesiologists physical status; MMSE – mini-mental state examination; ADL – activities of daily living; IADL – instrumental activities of daily living; GDS – geriatric depression scale; BFI – brief fatigue inventory; ECOG – eastern cooperative oncology group performance status; SIC – satarian index of comorbidities
Table 3.
Distribution of endoscopic and surgical procedures
| Procedures | n. (%) |
|---|---|
| Endoscopic Procedures | 44 (56.4%) |
| TURP | 10 (12.8%) |
| Endoscopic Resection of Large Bladder Neoplasm (>3 cm) | 34 (43.6%) |
| Surgical Procedures | 34 (43.6%) |
| Hysterosacropexy | 1 (1.3%) |
| Open partial nephrectomy | 4 (5.1%) |
| Open radical cystectomy | 5 (6.4%) |
| Open radical nephrectomy | 10 (12.8%) |
| Open radical prostatectomy | 12 (15.4%) |
| Open surgical repair of rectal-bladder fistula | 1 (1.3%) |
| Urethrectomy | 1 (1.3%) |
| Total | 78 (100%) |
TURP – trans-urethral resection of the prostate
Male patients appeared to be frailer than female ones (p = 0.003), but this was due to the difference in gender distribution (85.9% men, 14.1% women).
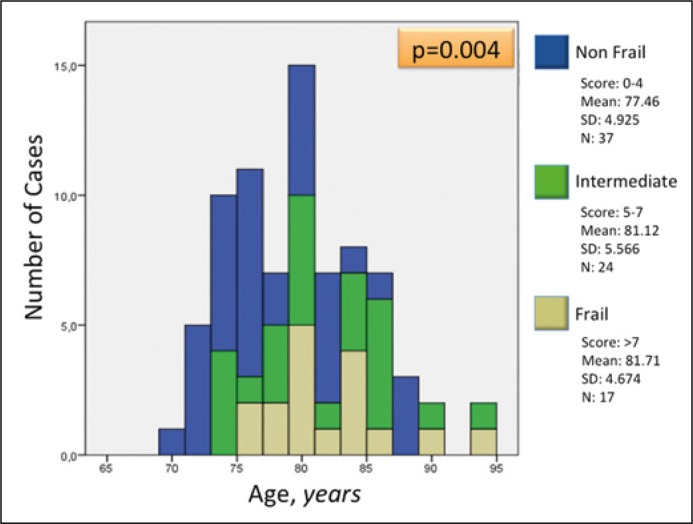
An overall prevalence of frailty of 21.8% was found. Patients with high and intermediate frailty generally fell into the older age groups (p = 0.004), as shown in Figure 1.
Figure 1.
Distribution of patients according to the EFS (edmonton frail scale) score and age.
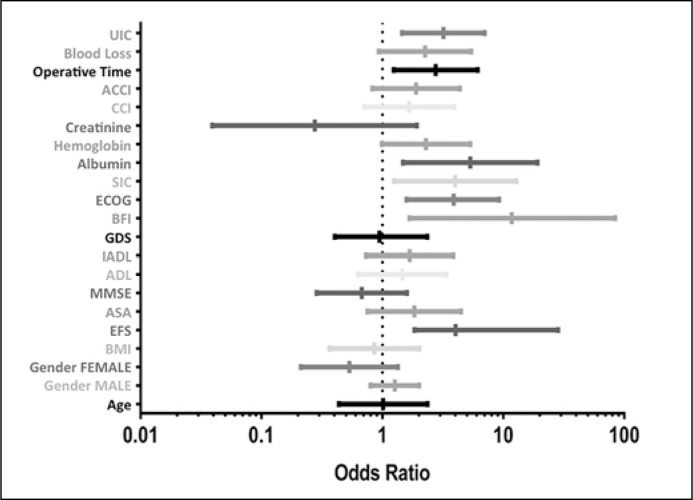
Correlations between variables and presence/absence of complications (Grade ≥2) are listed in Table 4. In univariate analysis, patients with complications (Grade ≥2) were frailer than those without complications, considering both total patients (p = 0.002) and endoscopic (p = 0.04) and surgical patients (p = 0.013). The complete list of odds ratios for each parameter is given in Table 5 and Figure 2.
Table 4.
Correlations between variables and presence/absence of complications (Grade ≥2) (univariate analysis)
| Total | Endoscopy | Open surgery | |||
|---|---|---|---|---|---|
| Parameter | Complications | p | p | p | |
| No | Yes | ||||
| Demographic parameters | |||||
| Age, mean (SD) | 78.52 (5.28) | 78.29 (5.59) | 0.437 | 0.118 | 0.144 |
| Gender | 0.242 | 0.456 | 0.656 | ||
| Female | 7 (9) | 4 (5.1) | |||
| Male | 54 (69.2) | 13 (16.7) | |||
| BMI (kg/m2), median (IQR) | 24.8 (3.63) | 24.4 (5.9) | 0.24 | 0.644 | 0.122 |
| Biochemical parameters | |||||
| Albumin (g/l), median (IQR)*** | 42.2 (3.4) | 36.0 (11.9) | 0.254 | 0.148 | 0.05 |
| Hemoglobin (g/l), mean (SD) | 138.91 (13.51) | 144.0 (21.60) | 0.343 | 0.608 | 0.407 |
| Creatinine (g/l), median (IQR) | 94 (24) | 100 (46) | 0.44 | 0.925 | 0.699 |
| Other parameters | |||||
| CCI, n°(%) | 0.736 | 0.082 | 0.599 | ||
| 0–3 | 35 (44.9) | 8 (10.3) | |||
| 4–5 | 21 (26.9) | 7 (9.0) | |||
| >5 | 5 (6.4) | 1 (1.3) | |||
| ACCI, n°(%) | 0.311 | 0.059 | 0.551 | ||
| 0–7 | 41 (52.6) | 8 (10.3) | |||
| 8–9 | 16 (20.5) | 7 (9.0) | |||
| >9 | 4 (5.1) | 1 (1.3) | |||
| Operative time (min), median (IQR) | 52.5 (169) | 225 (320) | 0.009 | 0.609 | 0.109 |
| Blood loss (ml), median (IQR) | 0 (275) | 500 (1700) | 0.066 | / | 0.392 |
| IUC (days), median (IQR) | 0 (0) | 1 (4) | 0.003 | / | 0.038 |
| Frailty indices | |||||
| 1. EFS | 0.002 | 0.04 | 0.013 | ||
| ≤4 | 32 (41) | 5 (6.4) | |||
| 5–7 | 21 (26.9) | 3 (3.8) | |||
| >7 | 8 (10.3) | 9 (11.5) | |||
| 2. Components of PACE | 0.17 | 0.664 | 0.465 | ||
| 2.a ASA, n°(%) | |||||
| 1 | 0 (0) | 0 (0) | |||
| 2 | 33 (42.3) | 6 (7.7) | |||
| 3 | 28 (35.9) | 11 (14.1) | |||
| 4 | 0 (0) | 0 (0) | |||
| 2.b MMSE, n°(%) | 0.679 | 0.668 | 0.58 | ||
| >27 | 21 (26.9) | 5 (6.4) | |||
| 25–27 | 25 (32.1) | 6 (7.7) | |||
| ≤24 | 15 (19.4) | 6 (7.7) | |||
| 2.c ADL, n°(%) | 0.613 | 0.587 | 0.329 | ||
| 6 | 35 (44.9) | 9 (11.5) | |||
| 4–5 | 25 (32.1) | 7 (9.0) | |||
| <4 | 1 (1.3) | 2 (2.6) | |||
| 2.d IADL, n°(%) | 0.392 | 0.319 | 0.652 | ||
| 8 | 42 (53.8) | 9 (11.5) | |||
| 6–7 | 16 (20.5) | 6 (7.7) | |||
| <6 | 3 (3.8) | 1 (1.3) | |||
| 2.e GDS, n°(%) | 0.1 | 0.081 | 0.037 | ||
| ≤4 | 39 (50.0) | 11 (14.1) | |||
| 4–8 | 20 (25.6) | 2 (2.6) | |||
| >8 | 2 (2.6) | 4 (5.1) | |||
| 2.f BFI, n°(%) | <0.001 | 0.038 | 0.015 | ||
| ≤3 | 32 (41.0) | 1 (1.3) | |||
| 4–6 | 27 (36.4) | 12 (15.4) | |||
| >6 | 2 (2.6) | 4 (5.1) | |||
| 2.g ECOG, n°(%) | <0.001 | 0.04 | 0.09 | ||
| 0 | 10 (17.2) | 2 (3.4) | |||
| 1 | 27 (46.6) | 12 (15.4) | |||
| 2 | 7 (12.1) | 5 (8.6) | |||
| 3 | 0 (0) | 4 (6.9) | |||
| 2.h SIC, n°(%) | 0.158 | 0.013 | 0.016 | ||
| 0 | 22 (28.2) | 1 (1.3) | |||
| 1–2 | 26 (33.3) | 10 (12.8) | |||
| >2 | 13 (16.7) | 5 (6.4) | |||
BMI – body mass index; CCI – charlson comorbidity index; ACCI – age-corrected charlson score; IUC – intensive unit care; EFS – edmonton frail scale; ASA – american society of anesthesiologists physical status; MMSE – mini-mental state examination; ADL – activities of daily living; IADL – instrumental activities of daily living; GDS – geriatric depression scale; BFI – brief fatigue inventory; ECOG – eastern cooperative oncology group performance status; SIC – satarian index of comorbidities
Table 5.
Complete list of odds ratios for each parameter
| TOTAL | ||
|---|---|---|
| Parameters | OR (IC95%) | p |
| Demographic parameters | ||
| Age≥80 | 1.02 (0.35–2.99) | 0.972 |
| Gender | ||
| Female | 0.53 (0.21–1.34) | 0.242 |
| Male | 1.27 (0.8–2.01) | 0.242 |
| BMI (kg/m2) ≥25 | 0.83 (0.29–2.45) | 0.788 |
| Biochemical parameters | ||
| Albumin (g/l) ≤37 | 9.67 (1.56–60.01) | 0.02 |
| Hemoglobin (g/l) ≤120 | 3.21 (0.87–11.88) | 0.121 |
| Creatinine (g/l) >130 | 0.22 (0.27–1.85) | 0.173 |
| Other parameters | ||
| CCI >3 | 1.92 (0.65–5.73) | 0.279 |
| ACCI >7 | 2.30 (0.77–6.87) | 0.16 |
| Operative time (min) ≥210 | 4.04 (1.27–12.81) | 0.023 |
| Blood loss (ml) >100 | 2.86 (0.87–9.33) | 0.106 |
| IUC: Yes | 5.14 (1.57–16.82) | 0.008 |
| Frailty indices | ||
| EFS >7 | 7.45 (2.23–24.95) | 0.001 |
| Components of PACE | ||
| ASA >2 | 2.16 (0.71–6.59) | 0.272 |
| MMSE ≤24 | 0.60 (0.19–1.89) | 0.374 |
| ADL <6 | 1.62 (0.5–4.77) | 0.418 |
| IADL <8 | 1.97 (0.66–5.88) | 0.257 |
| GDS >4 | 0.97 (0.31–2.97) | 0.953 |
| BFI >3 | 17.66 (2.02–141.58) | 0.001 |
| ECOG >1 | 7.05 (1.86–26.89) | 0.004 |
| SIC >1 | 5.50 (1.43–21.11) | 0.012 |
BMI – body mass index; CCI – charlson comorbidity index; ACCI – age-corrected charlson score; IUC – intensive unit care; EFS – edmonton frail scale; ASA –American Society of Anesthesiologists Physical Status; MMSE – mini- mental state examination; ADL – activities of daily living; IADL – instrumental activities of daily living; GDS – geriatric depression scale; BFI – brief fatigue inventory; ECOG: Eastern Cooperative Oncology Group Performance Status; SIC – satarian index of comorbidities
Figure 2.
Complete list of odds ratios for each parameter analyzed.
BMI – body mass index; CCI – charlson comorbidity index; ACCI – age-corrected charlson score; IUC – intensive unit care; EFS – edmonton frail scale; ASA –American Society of Anesthesiologists Physical Status; MMSE – mini- mental state examination; ADL – activities of daily living; IADL – instrumental activities of daily living; GDS – geriatric depression scale; BFI – brief fatigue inventory; ECOG: Eastern Cooperative Oncology Group Performance Status; SIC – satarian index of comorbidities
However, multivariate analysis (even using dichotomised variables) revealed no significant correlations (probably due to the low number of cases).
DISCUSSION
Although average life expectancy is increasing in the western world, resulting in a growing number of frail individuals, ‘biological age’ is often taken as a crude index of frailty, whereas chronological age is a poor correlate. Up to 75% of patients over the age of 85 are not frail, although, as confirmed in our analysis, frailty does tend to increase with age. The reported prevalence of frailty in the community varies enormously – from 4.0% to 59.1% – because the differing definitions of frailty result in widely differing prevalences between studies [16].
Frailty has been used to identify community-dwelling older adults at risk of poor clinical outcomes, and the European Male Ageing Study Group identified it as a predictor of all-cause mortality [17].
Few reports specifically related to surgery are available, indicating that frailty assessment may predict outcomes in older neoplastic patients – with the result that its influence on survival is comparable with that of the TNM stage [18].
Most notably, Makary et al. [19] demonstrated that any degree of pre-operative frailty was predictive of post-operative complications, but currently available evidence is too inconsistent to guide clinical decision- making [20].
In addition, in our opinion, examining large heterogeneous cohorts of patients undergoing operations in different fields of surgery is not exactly the most correct method to follow, because of the enormous differences among various specialties. Some reported studies do describe analyses of cohorts of urological patients, but they are not ordered in a clear-cut manner according to one particular system or another [17, 21].
We decided to test the above-mentioned frailty indices only in a cohort of urological patients, in order to evaluate them in particular. We included in our analysis of patients undergoing major surgical interventions and endoscopic procedures, to assess the capability of the elderly to cope with stressors.
Univariate analysis confirms the predictive value of the EFS score, which indicated a significant association between frailty and the risk of major complications, after both urological surgery and endoscopy. We decided not to include in the outcomes parameters such as length of hospital stay, because of the influence of various confounding factors often associated with organizational difficulties at home, problems or delays in providing cots, materials and equipment for preventing pressure sores, etc. Results for the multivariate analysis were not significant, probably due both to the small sample size and the low overall incidence of complications.
In the literature, pure frailty is measured according to several indices [22]: in our experience, EFS is a simple, easy and quick-to-administer test which can exhaustively assess patients’ physical and psychosocial characteristics, and no prior geriatric assessment is required. Conversely, PACE is complex and lengthy to administer.
We found many similarities and dissimilarities when we compared EFS with various PACE components:
EFS uses simple questions, whereas PACE requires a specific test for each component. Some components, such as cognitive status, functional independence and mood, may be evaluated with both indices, but they are not significant when individually analyzed in the PACE test. We therefore speculate that these domains are not the most important when assessing surgical risk; they may have more influence from the medical viewpoint in the case of chronic diseases.
Other domains, such as social support, nutritional status and physical performance, are taken into account in EFS but not in PACE. Are these precisely the parameters which are also essential in evaluating frail surgical patients?
As in regards to the assessment of general health status, PACE contains the component ECOG performance status (specifically for oncological patients) and this domain may be compared with the evaluation of general health status in EFS. Because we found that ECOG was significantly correlated with complications, we consider it to be important in evaluating both oncological and non-oncological patients.
Some domains not included in EFS, such as Fatigue (measured with BFI) turned out to be significant predictors of complications.
Physical performance items (included in EFS) are not comparable to BFI and both should therefore be included in new tests, if their specific values are to be maintained.
In our study, the ASA risk did not predict complications, mainly because all patients were distributed in Groups 2 and 3. For these intermediate degrees of risk, the ASA risk has been demonstrated as limited to patients suitable for elective surgery [23]. Reported analyses are often limited to physical parameters and psychosocial aspects. Only a few reports contain analyses of other biochemical parameters, such as albumin, haemoglobin and creatinine values [19]. In our opinion, these aspects are essential in multifactorial analysis. Some studies, focusing on general surgery, have found a correlation between low albumin levels and post-operative complications [24–27]. The reasons for this are not only related to nutritional status, but also because of the fact that low albumin seems to be related to general inflammation or stress and may be an indicator of a patient’s vulnerability. A contemporary evaluation of comorbidities should also be considered, for a more complete picture of a patient’s health status.
This study is the first analysis of frailty focusing exclusively on urological patients. Other reports have evaluated cohorts of surgical patients, including urological patients, but not limited only to them.
We realize that this study also has several limitations. First, we did not evaluate the influence of frailty on long-term functional outcomes and quality of life. Second, the small sample size implies a lack of statistical power, although this is a prospective study of selected old patients recommended for major urological procedures.
Our analysis suggests the need for new, highly specific urological indices, comprising simple items about cognitive status, health status, functional and physical independence, social support, comorbidities, nutritional status, mood, functional performance, and fatigue.
CONCLUSIONS
Further well-designed large clinical studies specifically focusing on urology will be needed to develop targeted risk-reduction strategies for frail older urological patients, testing various proposed indices in order to refine specific items.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Mitnitski A, Rockwood K. Aging as a process of deficit accumulation: its utility and origin. Interdiscip Top Gerontol. 2015;40:85–98. doi: 10.1159/000364933. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent E, O Hoogendijk E. Psychosocial factors modify the association of frailty with adverse outcomes: a prospective study of hospitalised older people. BMC Geriatrics. 2014;4:108–116. doi: 10.1186/1471-2318-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 7.Herlitz J, Wiklund I, Caidahl K, et al. The feeling of loneliness prior to coronary artery bypass grafting might be a predictor of short- and long-term postoperative mortality. Eur J Vasc Endovasc Surg. 1998;16:120–125. doi: 10.1016/s1078-5884(98)80152-4. [DOI] [PubMed] [Google Scholar]
- 8.Arozullah AM, Khuri SF, Henderson WG, Daley J. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–857. doi: 10.7326/0003-4819-135-10-200111200-00005. [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–139. [PubMed] [Google Scholar]
- 10.Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127:886–891. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 11.Schoufour JD, Evenhuis HM, Echteld MA. The impact of frailty on care intensity in older people with intellectual disabilities. Res Dev Disabil. 2014;35:3455–3461. doi: 10.1016/j.ridd.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Maurer MS, Luchsinger JA, Wellner R, Kukuy E, Edwards NM. The effect of body mass index on complications from cardiac surgery in the oldest old. J Am Geriatr Soc. 2002;50:988–994. doi: 10.1046/j.1532-5415.2002.50251.x. [DOI] [PubMed] [Google Scholar]
- 13.Galanakis P, Bickel H, Gradinger R, Von Gumppenberg S, Forstl H. Acute confusional state in the elderly following hip surgery: incidence, risk factors and complications. Int J Geriatr Psychiatry. 2001;16:349–355. doi: 10.1002/gps.327. [DOI] [PubMed] [Google Scholar]
- 14.Pope D, Ramesh H, Gennari R, et al. Pre-operative assessment of cancer in the elderly (PACE): a comprehensive assessment of underlying characteristicsof elderly cancer patients prior to elective surgery. Surg Oncol. 2006;15:189–197. doi: 10.1016/j.suronc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community - dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 17.Ravindrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: results from the European Male Ageing Study (EMAS) Arch Gerontol Geriatr. 2013;57:360–368. doi: 10.1016/j.archger.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Ommundsen N, Wyller TB, Nesbakken A, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014 doi: 10.1634/theoncologist.2014-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Amer Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist. 2012;17:1439–1449. doi: 10.1634/theoncologist.2012-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revenig LM, Canter DJ, Taylor MD. Too frail for surgery? Initial results of a large multidisciplinary prospective study exmining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg. 2013;217:665–670. doi: 10.1016/j.jamcollsurg.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Malmstrom TK, Miller DK, Morley JE. A Comparison of Four Frailty Model. J Am Geriatr Soc. 2014;62:721–726. doi: 10.1111/jgs.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung JM, Dzankic S. Relative importance of preoperative health status versus intraoperative factors in predicting postoperative adverse outcomes in geriatric surgical patients. J Am Geriatr Soc. 2001;49:1080–1085. doi: 10.1046/j.1532-5415.2001.49212.x. [DOI] [PubMed] [Google Scholar]
- 24.Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 25.Detsky AS, Baker JP, O’Rourke K, et al. Pre-dicting nutrition-associated complications for patients undergoing gastrointestinal surgery. JPEN. 1987;11:440–446. doi: 10.1177/0148607187011005440. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: Results from the national VA surgical risk study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 27.Rady MY, Ryan T, Starr NJ. Clinical characteristics of preoperative hypoalbuminemia predict outcome of cardiovascular surgery. JPEN. 1997;21:81–90. doi: 10.1177/014860719702100281. [DOI] [PubMed] [Google Scholar]