Abstract
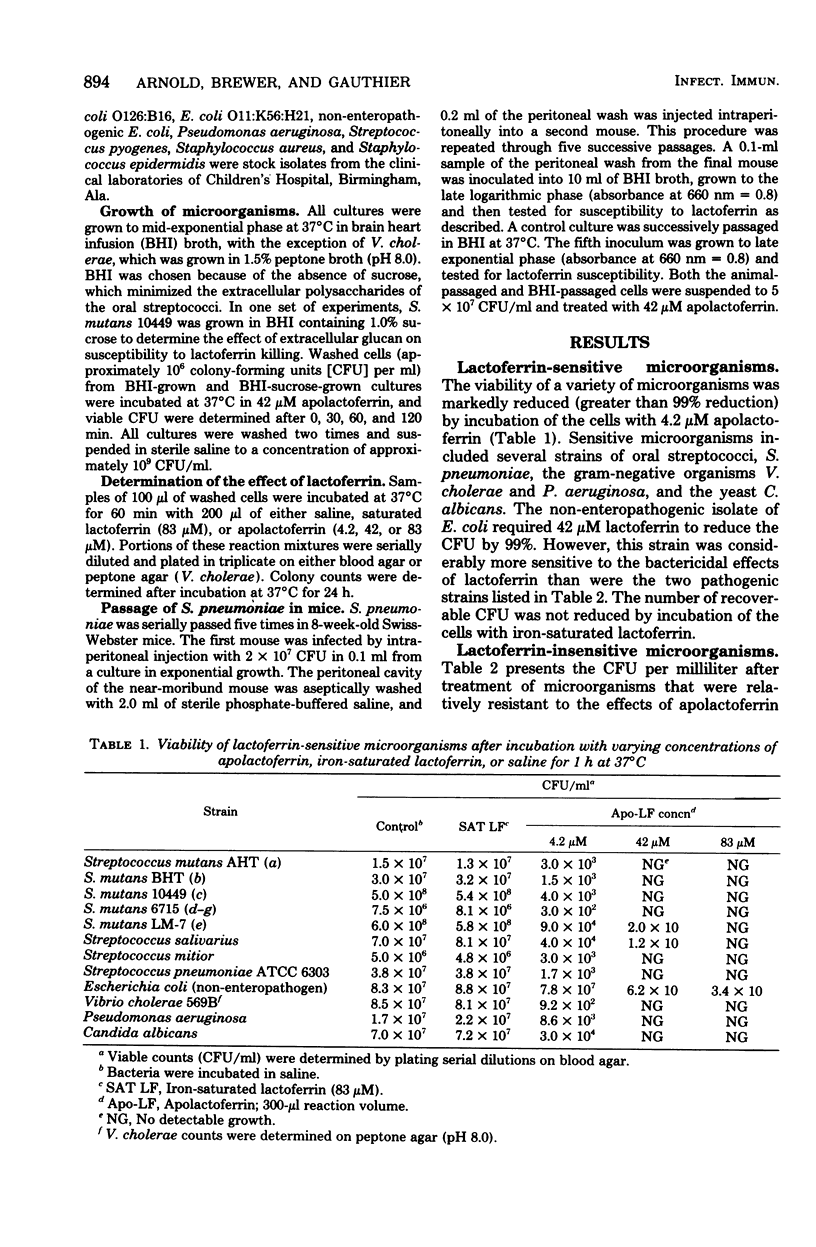
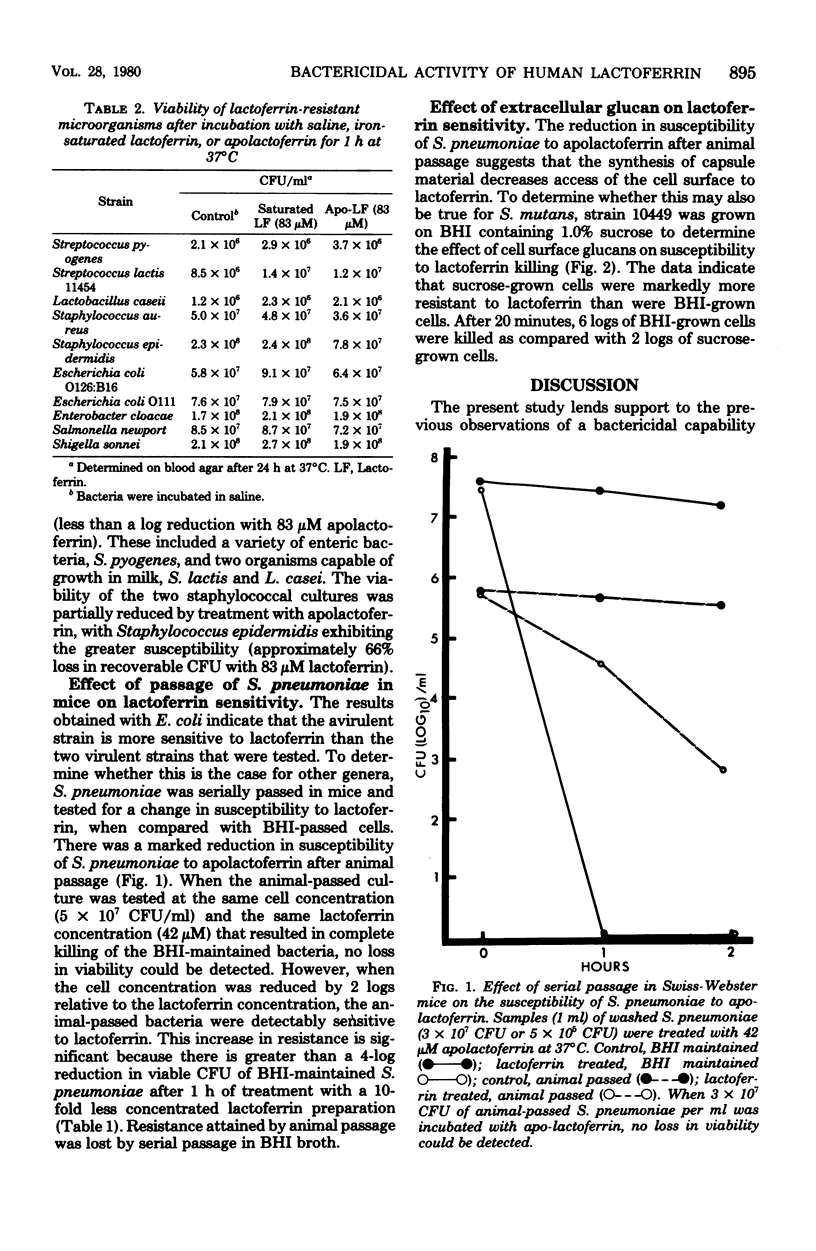
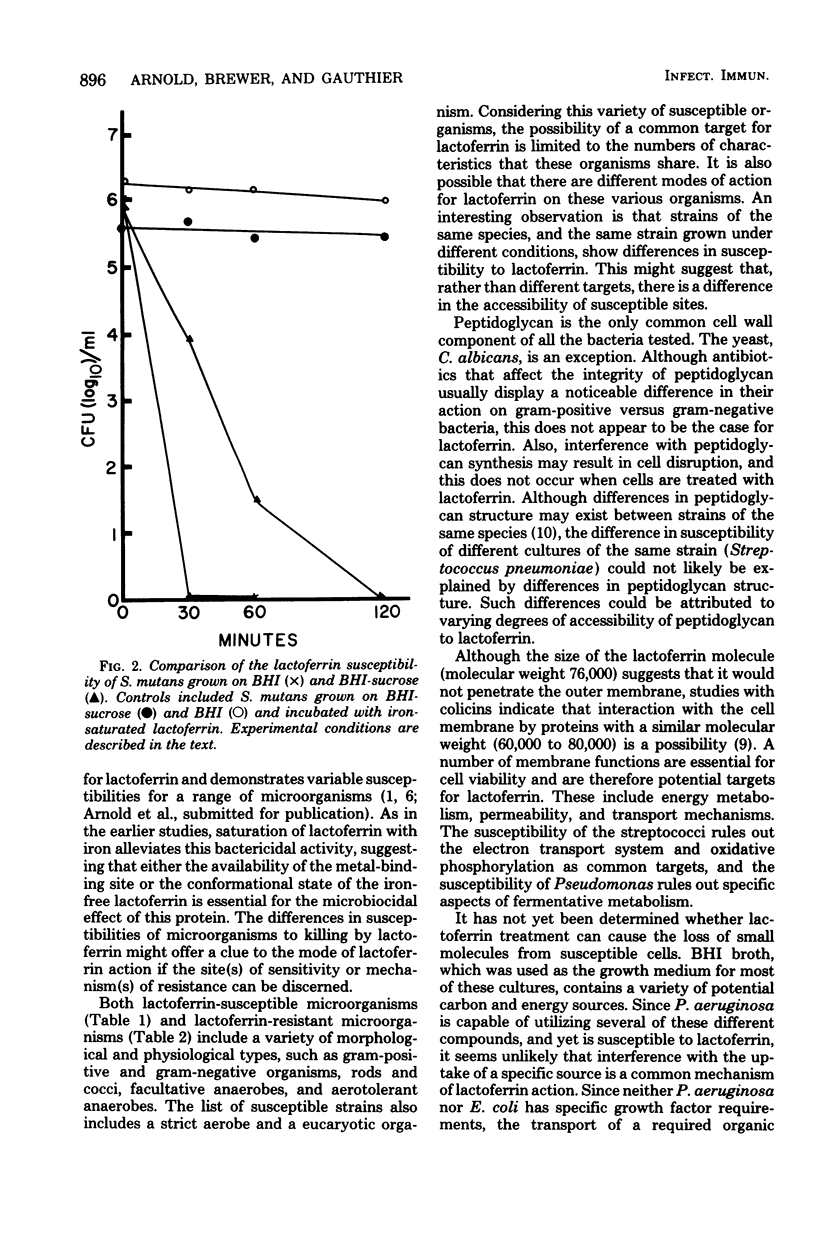
Lactoferrin is an iron-binding protein that has been detected in secretions that bathe human mucosal tissues. Previous studies have shown that, when this protein is in the iron-free state, it is capable of a direct bactericidal effect on Streptococcus mutans and Vibrio cholerae. The present study demonstrates variable susceptibilities for a variety of different microorganisms. The list of susceptible organisms includes gram-positive and gram-negative microbes, rods and cocci, facultative anaerobes, and aerotolerant anaerobes. Similar morphological and physiological types are represented among the lalctoferrin-resistant bacteria. S. mutans was more resistant to lactoferrin when grown on a sucrose-contaning medium than when it was grown on brain heart infusion broth without added scurose. When a lactoferrin-sensitive, avirulent strain of Streptococcus pneumoniae was passed through mice, the resultant virulent culture became lactoferrin resistant. Since organisms of the same species and even of the same strain (S. pneumoniae) can differ in susceptibility to lactoferrin, it appears that accessibility to the lactoferrin target site may account for differences in susceptibility. It appears that there may be a relation between virulence and resistance to lactoferrin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., De Duve C., Masson P. L., Heremans J. F. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970 Mar 1;131(3):559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. Effect of iron on the bactericidal proteins from rabbit polymorphonuclear leukocytes. Nature. 1970 Aug 22;227(5260):849–851. doi: 10.1038/227849a0. [DOI] [PubMed] [Google Scholar]
- Green I., Kirkpatrick C. H., Dale D. C. Lactoferrin--specific localization in the nuclei of human polymorphonuclear neutrophilic leukocytes. Proc Soc Exp Biol Med. 1971 Sep;137(4):1311–1317. doi: 10.3181/00379727-137-35779. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hamada S., Ooshima T., Kotani S., Kato K. Chemical composition of Streptococcus mutans cell walls and their susceptibility to Flavobacterium L-11 enzyme. Microbiol Immunol. 1979;23(5):319–328. doi: 10.1111/j.1348-0421.1979.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Kvach J. T., Wiles T. I., Mellencamp M. W., Kochan I. Use of transferrin-iron enterobactin complexes as the source of iron by serum-exposed bacteria. Infect Immun. 1977 Nov;18(2):439–445. doi: 10.1128/iai.18.2.439-445.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V., Shokrani F. Biological activities of pyochelins: iron-chelating agents of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):878–890. doi: 10.1128/iai.22.3.878-890.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Prignot J. J., Wauters G. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax. 1966 Nov;21(6):538–544. doi: 10.1136/thx.21.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Siderophore production by Vibrio cholerae. Infect Immun. 1978 Apr;20(1):310–311. doi: 10.1128/iai.20.1.310-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



