Abstract
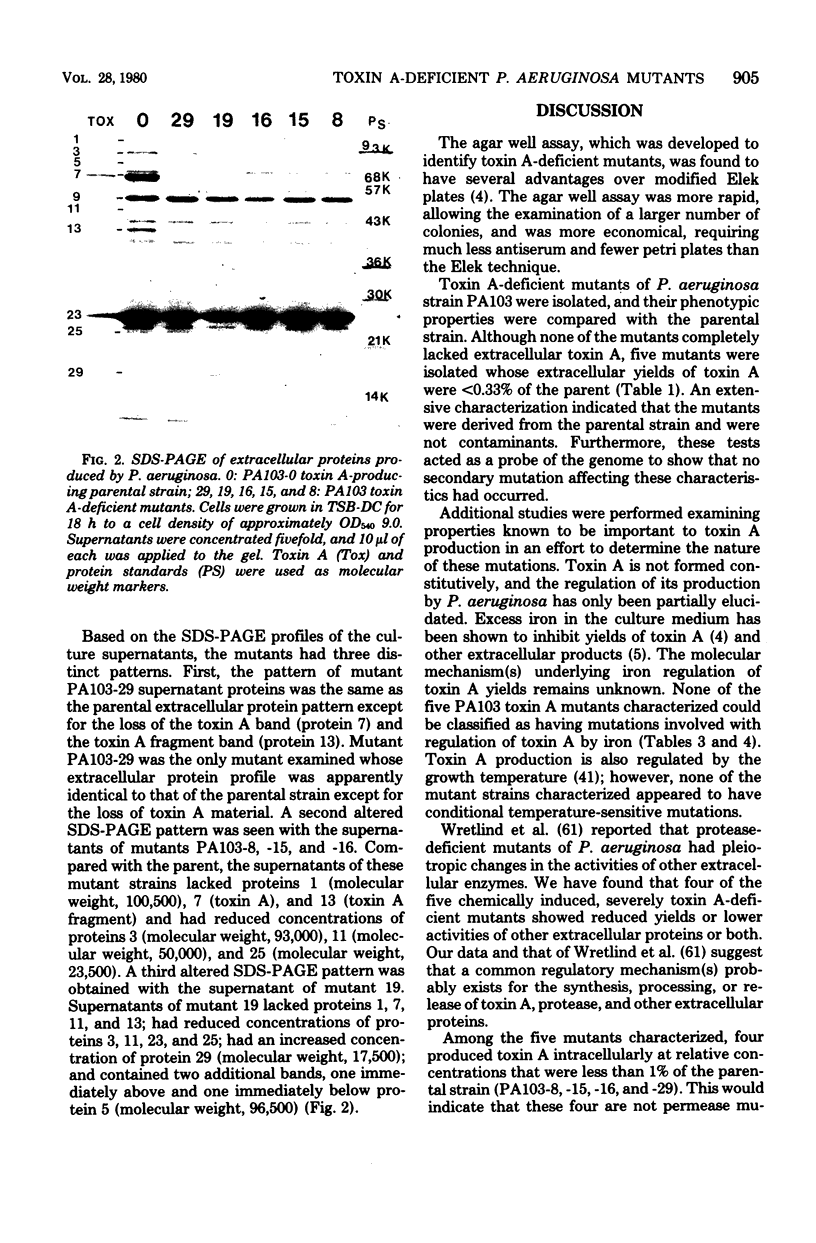
An immunological assay utilizing double-diffusion principles was developed for identification of Pseudomonas aeruginosa mutants deficient in toxin A. Mutations were chemically induced, and mutants were isolated from P. aeruginosa strain PA103. Quantitative assays, both enzymatic and immunological, indicated that five mutants produced toxin A in vitro at levels of 0.3% or less of parental strain levels. Characterization indicated that the mutants fell into four classes and suggested that multiple genes are involved in the regulation of toxin A yields. Classes 1 to 3 produced less than 1% of parental levels of extracellular toxin A. Class 1 mutants are apparently specific for toxin A. Class 2 mutants are pleotropic and produced toxin A, protease, and other extracellular proteins at reduced yields. Class 3 mutants are pleotropic and in addition have relatively high levels of cell-bound toxin A. Class 4 mutants produce toxin A at levels greater than 1% of parental yields. Of 16 toxin A-deficient mutants examined, only 1 was a class 1 mutant. This mutant (PA103-29) was shown to be identical to the parental strain in all respects tested except for its marked deficiency in toxin A. The suitability of this class 1 mutant for use in virulence studies is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAMMAR W. J., CLARKE P. H. INDUCTION AND REPRESSION OF PSEUDOMONAS AERUGINOSA AMIDASE. J Gen Microbiol. 1964 Dec;37:307–319. doi: 10.1099/00221287-37-3-307. [DOI] [PubMed] [Google Scholar]
- BUTLER W. T. HEMAGGLUTINATION STUDIES WITH FORMALINIZED ERYTHROCYTES. EFFECT OF BIS-DIAZO-BENZIDINE AND TANNIC ACID TREATMENT ON SENSITIZATION BY SOLUBLE ANTIGEN. J Immunol. 1963 May;90:663–671. [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Vasil M. L., Sadoff J. C., Iglewski B. H. Incidence of exotoxin production by Pseudomonas species. Infect Immun. 1977 Apr;16(1):362–366. doi: 10.1128/iai.16.1.362-366.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown V. I., Lowbury E. J. Use of an improved cetrimide agar medium and other culture methods for Pseudomonas aeruginosa. J Clin Pathol. 1965 Nov;18(6):752–756. doi: 10.1136/jcp.18.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Burke M. E., Pattee P. A. Purification and characterization of a staphylolytic enzyme from Pseudomonas aeruginosa. J Bacteriol. 1967 Mar;93(3):860–865. doi: 10.1128/jb.93.3.860-865.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- DeBell R. M. Production of exotoxin A by Pseudomonas aeruginosa in a chemically defined medium. Infect Immun. 1979 Apr;24(1):132–138. doi: 10.1128/iai.24.1.132-138.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Herman L. G. Epidemiological fingerprinting of Pseudomonas aeruginosa by the production of and sensitivity of pyocin and bacteriophage. Appl Microbiol. 1969 Nov;18(5):760–765. doi: 10.1128/am.18.5.760-765.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- GABY W. L., FREE E. Differential diagnosis of Pseudomonas-like microorganisms in the clinical laboratory. J Bacteriol. 1958 Oct;76(4):442–444. doi: 10.1128/jb.76.4.442-444.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Baine W. B., Vasil M. L. Quantitative measurements of cholera enterotoxin in cultures of toxinogenic wild-type and nontoxinogenic mutant strains of Vibrio cholerae by using a sensitive and specific reversed passive hemagglutination assay for cholera enerotoxin. Infect Immun. 1978 Jan;19(1):101–106. doi: 10.1128/iai.19.1.101-106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. J. The detection of bacterial esterases and lipases. Med Lab Technol. 1971 Apr;28(2):208–210. [PubMed] [Google Scholar]
- Iglewski B. H., Burns R. P., Gipson I. K. Pathogenesis of corneal damage from pseudomonas exotoxin A. Invest Ophthalmol Vis Sci. 1977 Jan;16(1):73–76. [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- JEFFRIES C. D., HOLTMAN D. F., GUSE D. G. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957 Apr;73(4):590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kawaharajo K., Homma J. Y. Pathogenesis of the mouse keratitis produced with Pseudomonas aeruginosa. Jpn J Exp Med. 1975 Dec;45(6):515–524. [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Callahan L. T., 3rd, Taylor N. S. Neutralizing antibody to Pseudomonas aeruginosa exotoxin in human sera: evidence for in vivo toxin production during infection. Infect Immun. 1976 Oct;14(4):942–947. doi: 10.1128/iai.14.4.942-947.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann W. Formation and isolation of leucocidin from Pseudomonas aeruginosa. J Gen Microbiol. 1976 Apr;93(2):283–291. doi: 10.1099/00221287-93-2-283. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Willett N. P. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968 Sep;16(9):1434–1436. doi: 10.1128/am.16.9.1434-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Ohman D. E., Iglewski B. H. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J Clin Microbiol. 1979 Apr;9(4):538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Hayden C. Secretion of phospholipase C by Pseudomonas aeruginosa. Infect Immun. 1979 Aug;25(2):558–564. doi: 10.1128/iai.25.2.558-564.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L. Identification of Pseudomonas species isolated from hospital environment and human sources. Appl Microbiol. 1968 Oct;16(10):1532–1538. doi: 10.1128/am.16.10.1532-1538.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Pollack M. Purification of Pseudomonas aeruginosa exotoxin by affinity chromatography. Infect Immun. 1978 Jan;19(1):66–70. doi: 10.1128/iai.19.1.66-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Kabat D., Iglewski B. H. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Liu P. V., Iglewski B. H. Temperature-dependent inactivating factor of Pseudomonas aeruginosa exotoxin A. Infect Immun. 1976 May;13(5):1467–1472. doi: 10.1128/iai.13.5.1467-1472.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B., Hedén L., Sjöberg L., Wadström T. Production of enzymes and toxins by hospital strains of Pseudomonas aeruginosa in relation to serotype and phage-typing pattern. J Med Microbiol. 1973 Feb;6(1):91–100. doi: 10.1099/00222615-6-1-91. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Sjöberg L., Wadström T. Protease-deficient mutants of Pseudomonas aeruginosa: pleiotropic changes in activity of other extracellular enzymes. J Gen Microbiol. 1977 Dec;103(2):329–336. doi: 10.1099/00221287-103-2-329. [DOI] [PubMed] [Google Scholar]