Abstract
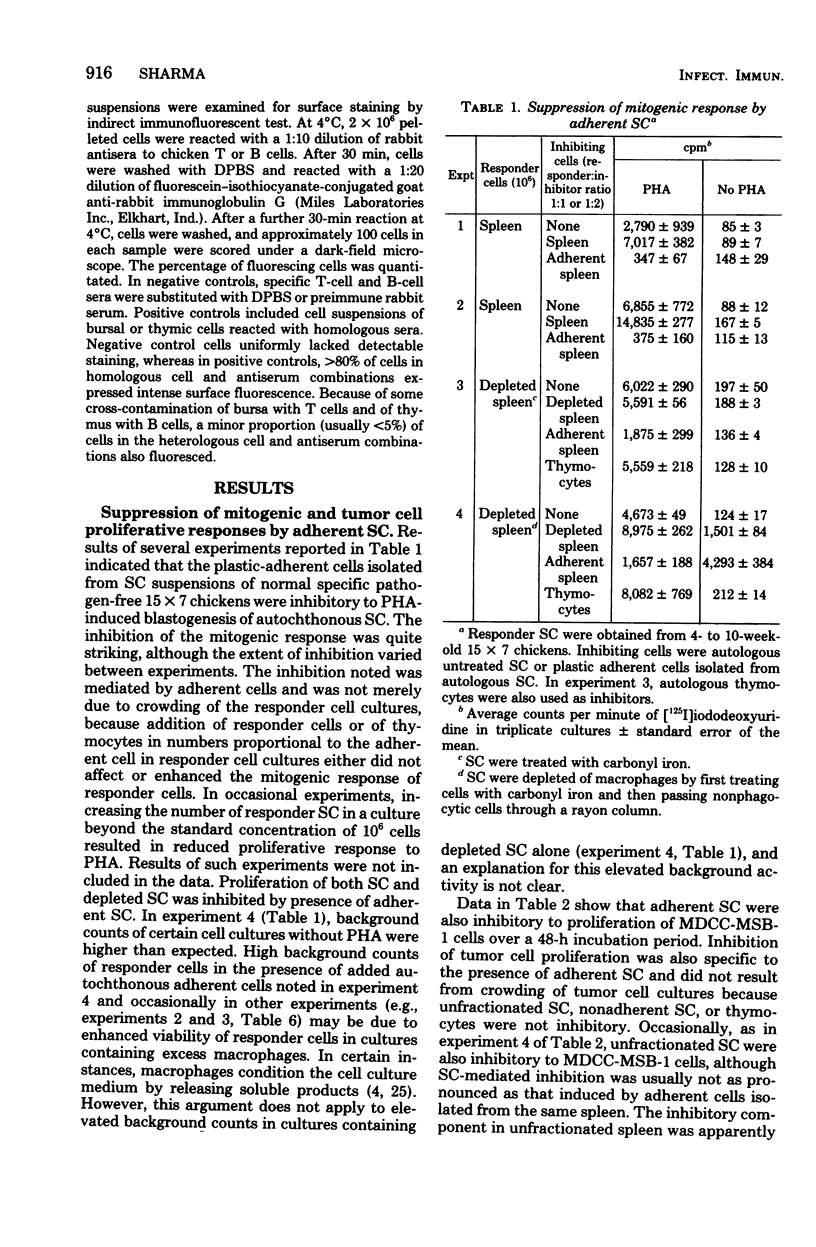
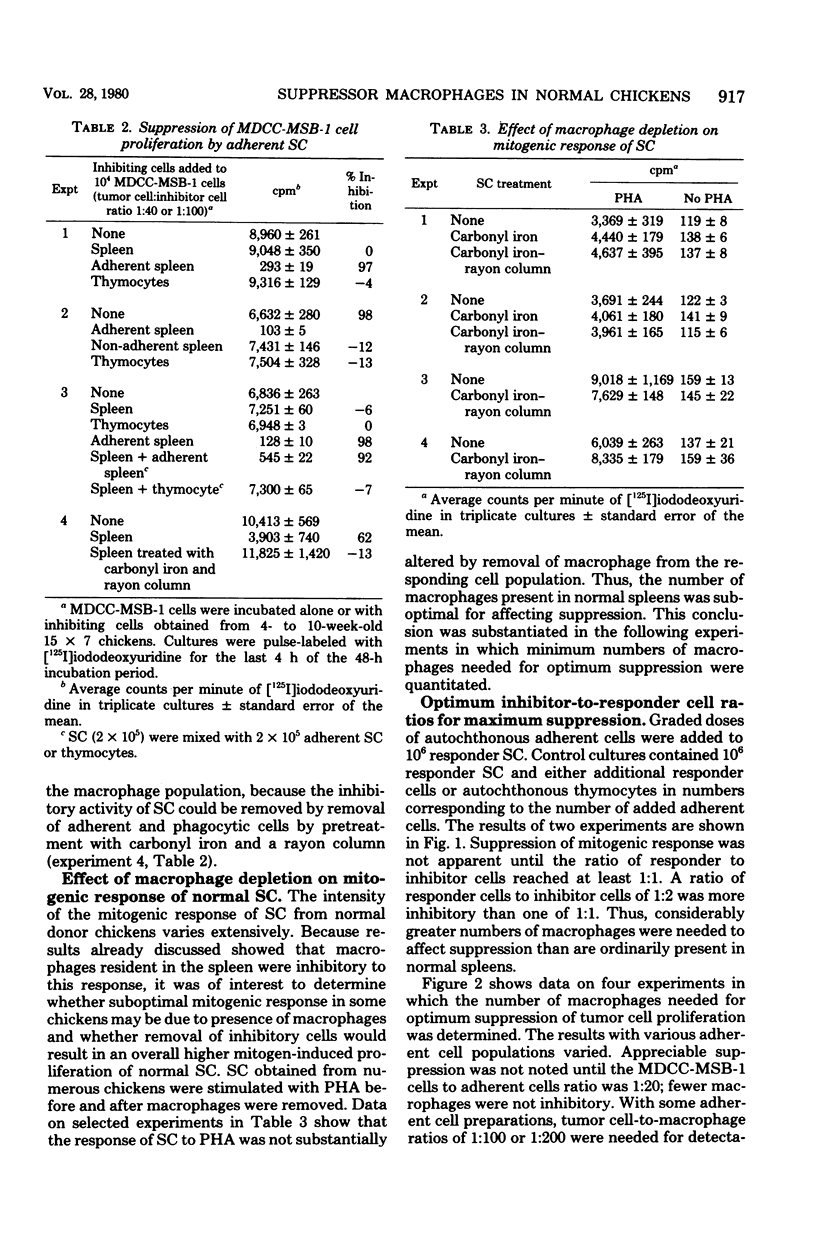
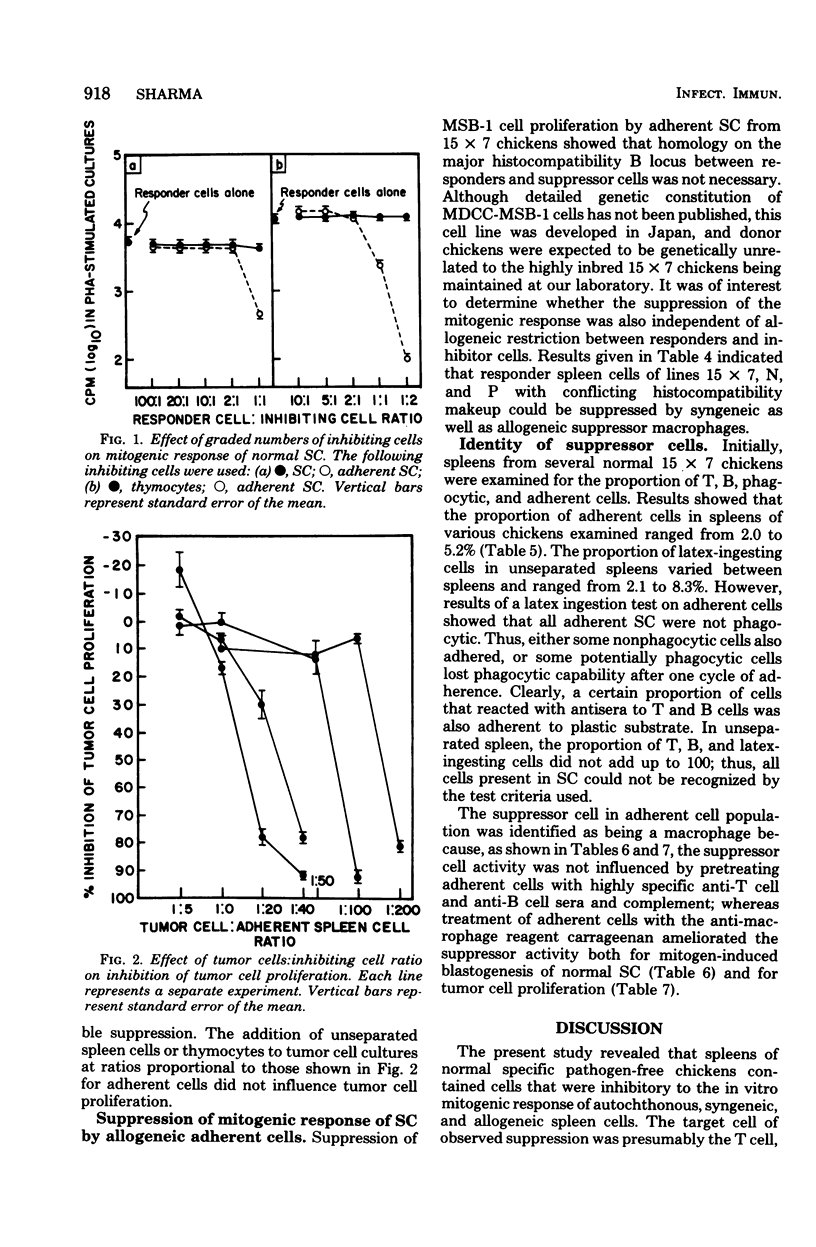
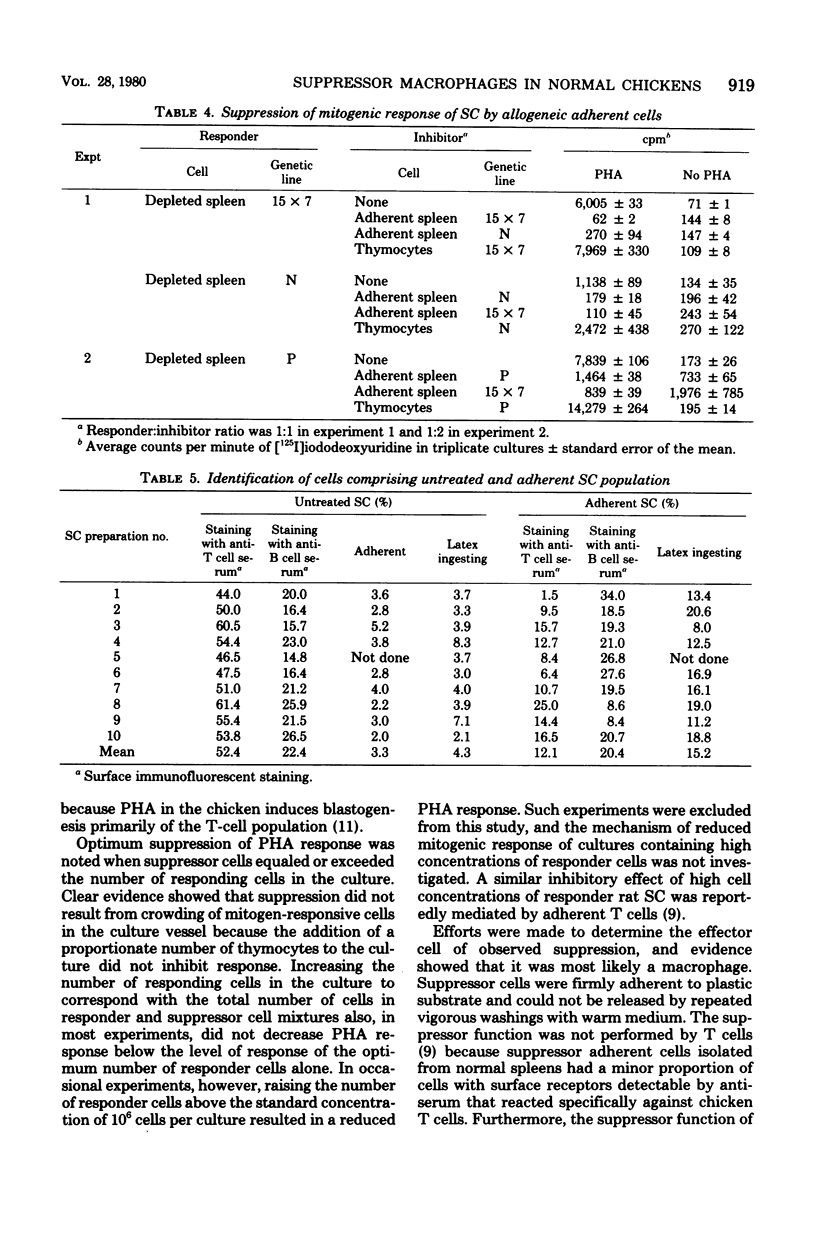
Adherent cells isolated from spleen of normal specific pathogen-free chickens inhibited mitogen-induced blastogenesis of autochthonous, syngeneic, or allogeneic lymphocytes. The adherent cells were also inhibitory to in vitro proliferation of cells of a rapidly dividing tumor line, MDCC-MSB-1, derived from a lymphoma induced by Marek's disease virus. The effector cell of suppression of both lymphoprolifrative functions appeared to be a macrophage because the suppressive activity of adherent cells could be abrogated by pretreatment with carrageenan but not with antisera specific to chicken T or B cells. The proportion of macrophages needed for effective suppression was substantially higher than the proportion of macrophages ordinarily present in spleen of normal, unstimulated chickens. This heretofore unrecognized suppressive capability of normal, presumably resting macrophages have been detected in certain infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm G. V., Siccardi F. J., Peterson R. D. Impairment of the lymphocyte response to phytohaemagglutinin in chickens with Marek's disease. Acta Pathol Microbiol Scand A. 1972;80(1):109–114. doi: 10.1111/j.1699-0463.1972.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Alter B. J., Solliday S., Zoschke D. C., Janis M. Lymphocyte reactivity in vitro. II. Soluble reconstituting factor permitting response of purified lymphocyte. Cell Immunol. 1970 Jul;1(2):219–227. doi: 10.1016/0008-8749(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Burg R. W., Feldbush T., Morris C. A., Maag T. A. Depression of thymus-and bursa-dependent immune systems chicks with Marek's disease. Avian Dis. 1971 Oct-Dec;15(4):662–671. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. Regulation of lymphocyte responses in vitro. V. Suppressor activity of adherent and nonadherent rat lymphoid cells. Cell Immunol. 1973 Oct;9(1):12–24. doi: 10.1016/0008-8749(73)90163-9. [DOI] [PubMed] [Google Scholar]
- Folch H., Yoshinaga M., Waksman B. H. Regulation of lymphocyte responses in vitro. 3. Inhibition by adherent cells of the T-lymphocyte response to phytohemagglutinin. J Immunol. 1973 Mar;110(3):835–839. [PubMed] [Google Scholar]
- Glaser M., Kirchner H., Herberman R. B. Inhibition of in vitro lymphoproliferative responses to tumor-associated antigens by suppressor cells from rats bearing progressively growing Gross leukemia virus-induced tumors. Int J Cancer. 1975 Sep 15;16(3):384–393. doi: 10.1002/ijc.2910160305. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Roitt I. M., Rose M. E. Effect of bursectomy and thymectomy on the responses of chicken peripheral blood lymphocytes to phytohaemagglutinin. Nature. 1968 Oct 19;220(5164):293–295. doi: 10.1038/220293a0. [DOI] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Keller R. Cytostatic elimination of syngeneic rat tumor cells in vitro by nonspecifically activated macrophages. J Exp Med. 1973 Sep 1;138(3):625–644. doi: 10.1084/jem.138.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Susceptibility of normal and transformed cell lines to cytostatic and cytocidal effects exerted by macrophages. J Natl Cancer Inst. 1976 Feb;56(2):369–374. doi: 10.1093/jnci/56.2.369. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Herberman R. B., Glaser M., Lavrin D. H. Suppression of in vitro lymphocyte stimulation in mice bearing primary Moloney sarcoma virus-induced tumors. Cell Immunol. 1974 Jul;13(1):32–40. doi: 10.1016/0008-8749(74)90224-x. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Holden H. T., Herberman Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975 Nov;115(5):1212–1216. [PubMed] [Google Scholar]
- Klaus G. G. Generation of thymus-derived helper cells by macrophage-associated antigen. Cell Immunol. 1974 Mar 15;10(3):483–488. doi: 10.1016/0008-8749(74)90140-3. [DOI] [PubMed] [Google Scholar]
- Lake W. W., Bice D., Schwartz H. J., Salvaggio J. Suppression of in vitro antigen-induced lymphocyte transformation by carrageenan, a macrophage-toxic agent. J Immunol. 1971 Dec;107(6):1745–1751. [PubMed] [Google Scholar]
- Landolfo S., Herberman R. B., Holden H. T. Stimulation of mouse migration inhibitory factor (MIF) production form MSV-immune lymphocytes by soluble tumor-associated antigen: requirement for histocompatible macrophages. J Immunol. 1977 Apr;118(4):1244–1248. [PubMed] [Google Scholar]
- Lee L. F., Sharma J. M., Nazerian K., Witter R. L. Suppression and enhancement of mitogen response in chickens infected with Marek's disease virus and the herpesvirus of turkeys. Infect Immun. 1978 Aug;21(2):474–479. doi: 10.1128/iai.21.2.474-479.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. F., Sharma J. M., Nazerian K., Witter R. L. Suppression of mitogen-induced proliferation of normal spleen cells by macrophages from chickens inoculated with Marek's disease virus. J Immunol. 1978 May;120(5):1554–1559. [PubMed] [Google Scholar]
- Levis W. R., Robbins J. H. Effect of glass-adherent cells on the blastogenic response of 'purified' lymphocytes to phytohemagglutinin. Exp Cell Res. 1970 Jul;61(1):153–158. doi: 10.1016/0014-4827(70)90269-7. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. S., Lapen R. F. Splenic cell mitogenic response in Marek's disease: comparison between noninfected tumor-bearing and nontumor-bearing infected chickens. Am J Vet Res. 1974 Jul;35(7):977–980. [PubMed] [Google Scholar]
- Meltzer M. S., Oppenheim J. J. Bidirectional amplification of macrophage-lymphocyte interactions: enhanced lymphocyte activation factor production by activated adherent mouse peritoneal cells. J Immunol. 1977 Jan;118(1):77–82. [PubMed] [Google Scholar]
- Meltzer M. S., Tucker R. W., Sanford K. K., Leonard E. J. Interaction of BCG-activated macrophages with neoplastic and nonneoplastic cell lines in vitro : quantitation of the cytotoxic reaction by release of tritiated thymidine from prelabeled target cells. J Natl Cancer Inst. 1975 May;54(5):1177–1184. doi: 10.1093/jnci/54.5.1177. [DOI] [PubMed] [Google Scholar]
- Meyers P., Ritts G. D., Johnson D. R. Phytohemagglutinin-induced leukocyte blastogenesis in normal and avian leukosis virus-infected chickens. Cell Immunol. 1976 Nov;27(1):140–146. doi: 10.1016/0008-8749(76)90163-5. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The immunogenic capacity of antigen taken up by peritoneal exudate cells. Immunology. 1969 Jan;16(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Nazerian K., Witter R. L. Properties of a chicken lymphoblastoid cell line from Marek's disease tumor. J Natl Cancer Inst. 1975 Feb;54(2):453–458. [PubMed] [Google Scholar]
- Oehler J. R., Campbell D. A., Jr, Herberman R. B. In vitro inhibition of lymphoproliferative responses to tumor associated antigens and of lymphoma cell proliferation by rat splenic macrophages. Cell Immunol. 1977 Feb;28(2):355–370. doi: 10.1016/0008-8749(77)90118-6. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Dutton R. W. Inhibition of spleen cell DNA synthesis by autologous macrophages. J Immunol. 1966 Nov;97(5):663–669. [PubMed] [Google Scholar]
- Pope B. L., Whitney R. B., Levy J. G., Kilburn D. G. Suppressor cells in the spleens of tumor-bearing mice: enrichment by centrifugation on hypaque-ficoll and characterization of the suppressor population. J Immunol. 1976 May;116(5):1342–1346. [PubMed] [Google Scholar]
- Rode H. N., Gordon J. The mixed leukocyte culture: a three component system. J Immunol. 1970 Jun;104(6):1453–1457. [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Corynebacterium parvum as a therapeutic antitumor agent in mice. I. Systemic effects from intravenous injection. J Natl Cancer Inst. 1974 Sep;53(3):855–860. doi: 10.1093/jnci/53.3.855. [DOI] [PubMed] [Google Scholar]
- Sharma J. M. Cell-mediated immunity to tumor antigen in Marek's disease: susceptibility of effector cells to antithymocyte serum and enhancement of cytotoxic activity by Vibrio cholerae neuraminidase. Infect Immun. 1977 Oct;18(1):46–51. doi: 10.1128/iai.18.1.46-51.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J. M., Coulson B. D. Cell-mediated cytotoxic response to cells bearing Marek's disease tumor-associated surface antigen in chickens infected with Marek's disease virus. J Natl Cancer Inst. 1977 Jun;58(6):1647–1651. doi: 10.1093/jnci/58.6.1647. [DOI] [PubMed] [Google Scholar]
- Sharma J. M., Herberman R. B., Djeu J. Y., Nunn M. E. Production of migration inhibition factor by spleen cells of normal rats upon culture in vitro with tumor cells and cells expressing endogenous virus. J Immunol. 1979 Jul;123(1):222–231. [PubMed] [Google Scholar]
- Sharma J. M., Witter R. L., Coulson B. D. Development of cell-mediated immunity to Marek's disease tumor cells in chickens inoculated with Marek's disease vaccines. J Natl Cancer Inst. 1978 Nov;61(5):1273–1280. doi: 10.1093/jnci/61.5.1273. [DOI] [PubMed] [Google Scholar]
- Theis G. A., McBride R. A., Schierman L. W. Depression of in vitro responsiveness to phytohemagglutinin in spleen cells cultured from chickens with Marek's disease. J Immunol. 1975 Sep;115(3):848–853. [PubMed] [Google Scholar]
- Treves A. J., Schechter B., Cohen I. R., Feldman M. Sensitization of T lymphocytes in vitro by syngeneic macrophages fed with tumor antigens. J Immunol. 1976 Apr;116(4):1059–1064. [PubMed] [Google Scholar]
- Wagner H., Feldmann M., Boyle W., Schrader J. W. Cell-mediated immune response in vitro. 3. The requirement for macrophages in cytotoxic reactions against cell-bound and subcellular alloantigens. J Exp Med. 1972 Aug 1;136(2):331–343. doi: 10.1084/jem.136.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wilton J. M., Rosenstreich D. L., Oppenheim J. J. The role of macrophages in the production of lymphokines by T and B lymphocytes. J Immunol. 1975 Apr;114(4):1296–1301. [PubMed] [Google Scholar]
- Waldron J. A., Jr, Horn R. G., Rosenthal A. S. Antigen-induced proliferation of guinea pig lymphocytes in vitro: obligatory role of macrophages in the recognition of antigen by immune T-lymphocytes. J Immunol. 1973 Jul;111(1):58–64. [PubMed] [Google Scholar]


