Abstract
Background
Physiologist-led stress echocardiography (PLSE) services provide potential for expansion of SE services and increased productivity for cardiologists. There are however no published data on the feasibility of PLSE. We sought to assess the feasibility, safety and robustness of PLSE and cardiologist-led stress echocardiography (CLSE) for coronary artery disease (CAD) assessment.
Methods
Retrospective analysis of 898 patients undergoing PLSE or CLSE for CAD assessment using exercise or dobutamine stress over 24 months. PLSE involved 2 cardiac physiologists (exercise) or 1 physiologist plus 1 cardiac nurse (dobutamine). A cardiology registrar was present in the echocardiography department during PLSE in case of medical complications. CLSE involved 1 physiologist and 1 trainee cardiologist who analysed the study and reviewed findings with an imaging cardiologist. Sixteen-segment wall motion scoring (WMS, WMSI) analysis was performed. Feasibility (stressor, image quality, proportion of completed studies, agreement with imaging cardiologist analysis) and safety (complication rate) were compared for PLSE and CLSE.
Results
The majority of studies were CLSE (56.2%) and used dobutamine (68.7%). PLSE more commonly used exercise (69.2%). Overall, 96% of studies were successfully completed (>14 diagnostic segments in 98%, P = 0.899 PLSE vs CLSE). Commencement of PLSE was associated with an increase in annual SE’s performed for CAD assessment. Complication rates were comparably very low for PLSE and CLSE (0.8% vs 1.8%, P = 0.187). There was excellent agreement between PLSE and CLSE WMS interpretation of 480 myocardial segments at rest (κ = 0.87) and stress (κ = 0.70) and WMSI (ICCs and Pearson’s r >0.90, zero Bland–Altman mean bias).
Conclusion
This to our knowledge is the first study of the feasibility of PLSE. PLSE performed by well-trained physiologists is feasible and safe in contemporary practice. PLSE and CLSE interpretation of stress echocardiography for CAD agree very closely.
Keywords: stress echocardiography, physiologist, coronary disease, dobutamine, exercise
Introduction
The expanding responsibilities and skillset of the Highly Specialised Cardiac Echocardiography Physiologist include performance and analysis of exercise and dobutamine stress echocardiography studies (1, 2). Physiologist-led stress echocardiography (PLSE) services are performed independent of the input of a cardiologist and are increasing in prevalence in the United Kingdom. They provide potential for expansion of stress echocardiography services, reductions in waiting times and scope for senior physiologists to increase their impact on the echocardiography department, whilst reducing the time spent by consultant cardiologists in analysing stress echocardiography studies, increasing their productivity.
There are however no published data on PLSEs. We sought to assess the feasibility, safety and robustness of PLSE for exercise and dobutamine stress echocardiography in the assessment of coronary artery disease (CAD) in real-life practice, and compare these measures with conventional cardiologist-led stress echocardiography (CLSE) to demonstrate the non-inferiority and feasibility of PLSE in contemporary practice.
Methods
Study population
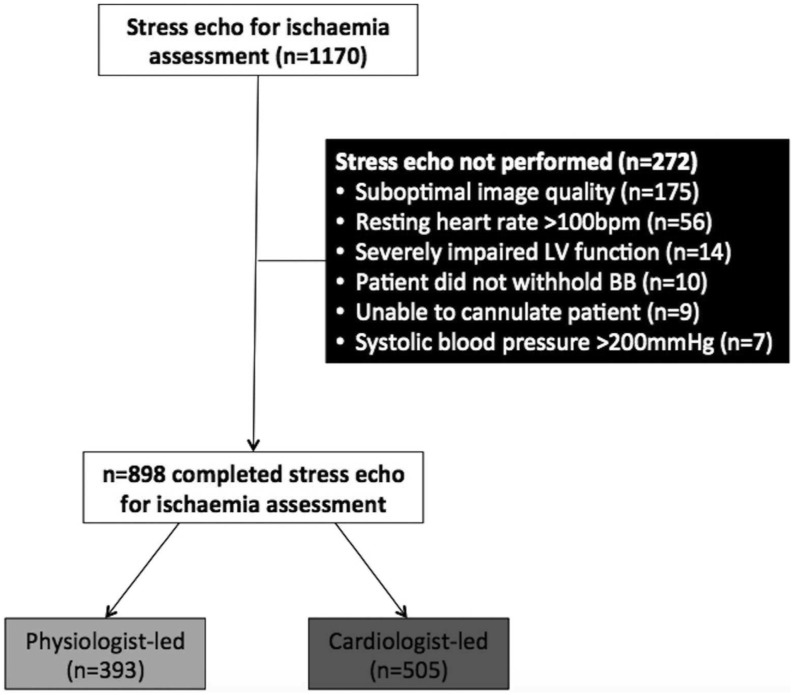
The University Hospital of North Midlands (UHNM) hosts a large, British Society of Echocardiography (BSE)-accredited echocardiography service. Patients undergoing elective outpatient stress echocardiography for the assessment of CAD during the 24-month period of 1st January 2014 to 31st December 2015 at UHNM were included. Those who completed the test with a diagnostic study with ≥14 analysable segments comprised the final cohort in this single-centre, retrospective, observational study. Exclusion criteria included <14 diagnostic myocardial segments, resting heart rate >100 beats per minute (bpm), inability to peripherally cannulate the patient, failure to withhold heart rate-limiting medications for ≥48 h, severe left ventricular systolic dysfunction, severe aortic stenosis or left ventricular outflow tract obstruction, severe uncontrolled hypertension and active endocarditis or myopericarditis (1, 3, 4). Figure 1 illustrates patient selection. The study was approved by the trust’s research and audit department who confirmed that patient consent and ethical approval were not required for this retrospective, observational study.
Figure 1.
CONSORT diagram illustrating selection of the final cohort of patients, subjects who completed the test with a resultant diagnostic, analysable stress echocardiography study for the assessment of ischaemia.
Stress echocardiography
Stress protocols
Two stress protocols were employed. Exercise stress echocardiography (ESE) employed Standard Bruce protocol treadmill exercise using a GE Case 8200W Exercise Testing System with GE T2100 Treadmill (GE Healthcare) with achievement of ≥85% of maximal heart rate defined as the target heart rate (THR) endpoint of adequate stress workload (0.85 × (220 bpm–age) for males and 0.85 × (210 bpm–age) for females) (3, 4). Dobutamine stress echocardiography (DSE) employed a staged peripheral dobutamine infusion (10, 20, 30 and 40 μg/kg/min ± atropine) with achievement of ≥85% of maximal heart rate defined as the THR endpoint of adequate stress (3, 4). Standard criteria for terminating stress included achievement of THR wherever possible. If this was not possible, limiting cardiorespiratory symptoms, sustained arrhythmia, significant vasovagal reaction, induced akinesia in ≥2 adjacent myocardial segments and ≥2 mm horizontal ST-segment shift permitted stress termination (3, 4).
Imaging protocols
Imaging was performed using a standard protocol of rest and peak stress acquisition visualising the 16 American Heart Association left ventricular myocardial segments using a GE Vivid E9 platform (GE Healthcare) for ESE (3, 5, 6). For DSE, images were acquired at rest, low-dose infusion (10 µg/kg/min) and peak stress. Microbubble ultrasound contrast (Sonovue (sulphur hexafluoride), Bracco Pharmaceuticals, Milan, Italy) was used, at low mechanical index (MI 0.1), where >1 myocardial segment was suboptimally visualised.
Stress echocardiography analysis
Segmental image quality was diagnostic where the segment was visualised at all image acquisition stages without significant foreshortening or difference in imaging plane. Left ventricular systolic function was assessed at rest and peak stress using (a) Simpson’s Biplane ejection fraction and (b) visual grading of segmental wall motion score (WMS) as: 1 = normokinetic, 2 = hypokinetic, 3 = akinetic, 4 = dyskinetic and 5 = aneurysmal (6). Segmental ischaemia was defined as an increase in WMS of ≥1 at peak stress in a segment with resting normokinesis or hypokinesis. The endpoint of positivity of the stress test for ischaemia was defined as ischaemia in ≥2 segments at peak stress (3, 4). Wall motion scoring index (WMSI) was defined as the mean of the sum of segmental WMS.
Physiologist- and cardiologist-led stress echocardiography
PLSE was introduced at UHNM in August 2013. The physiologist-led ESE team consisted of 2 cardiac physiologists. TG acquired and interpreted the studies and had BSE Adult Transthoracic Echocardiography accreditation, competence in contrast echocardiography, plus 5-year stress echocardiography experience with specialised training of >500 studies performed and interpreted under the supervision of a cardiologist with >10 years European Association of Echocardiography (EAE) Level 3 stress echocardiography competence (GH) (2, 4, 7). The physiologist-led DSE team consisted of 1 physiologist (TG) plus a cardiology nurse coordinating the dobutamine infusion. A cardiology specialist registrar was present in the echocardiography department during PLSE in case of medical complications. CLSE was defined as acquisition of stress echocardiography images by a cardiac physiologist with stress supervision by a cardiology registrar. Study analysis was performed by the cardiology registrar and reports reviewed by a consultant imaging cardiologist (GH). For both PLSE and CLSE, ≥1 member of the team in the echocardiography lab had Advanced Life Support accreditation and all others had Intermediate Life Support accreditation (2, 3, 4).
Statistical analysis
Normality was assessed using Kolmogorov–Smirnoff testing and Q–Q plots. Normally distributed variables were expressed as mean ± s.d. and compared using independent Student’s t-tests. Non-normally distributed data were expressed as median (1st and 3rd quartiles) and analysed using Mann–Whitney testing. Categorical variables were compared using chi-squared testing. Thirty studies (15 positive, 15 negative) were randomly selected using a random-number generator and analysed by both the physiologist (TG) and imaging cardiologists (GH, SD) for the assessment of interobserver agreement between PLSE and CLSE segmental WMSI analysis using Pearson’s correlation coefficient, two-way mixed-effect intraclass correlation coefficient (ICC) for absolute agreement and Bland–Altman analysis. On ICC, agreement was defined as excellent (ICC ≥0.75), good (ICC 0.6–0.74), fair (ICC 0.4–0.59) or poor (ICC <0.40) (8). Interobserver agreement for the comparison of physiologist and cardiologist-analysed segmental WMS was assessed using weighted kappa coefficient, with agreement defined as excellent (0.81–0.99), strong (0.61–0.80), moderate (0.41–0.60) and fair (0.21–0.40) (9).
Results
Stress echocardiography service at UHNM
During the 24-month study period, 1170 patients attended UHNM for elective stress echocardiography for CAD assessment. Of these, 898 (77%) underwent stress echocardiography, with reasons for not performing the study illustrated in Fig. 1 and Table 1. The majority were CLSE (n = 505 (56.2%) vs n = 393 (43.8%)); however, the proportion of PLSE significantly increased during the study period (2014: 150/389 (38.6%), 2015: 266/509 (47.8%)). Commencement of PLSE was associated with an increase in total annual number of stress echocardiograms performed for CAD assessment (2013: 596, 2014: 804, 2015: 850).
Table 1.
Stress echocardiography baseline findings.
| Variable | Total cohort (n = 898) | Physiologist led (n = 393) | Cardiologist led (n = 505) | P |
|---|---|---|---|---|
| Patients completing SE | 898 | 393 | 505 | |
| Reasons for not performing SE | 272 | |||
| Suboptimal images/windows | 175 | |||
| Resting heart rate >100 bpm | 56 | |||
| Severe LV systolic dysfunction | 9 | |||
| Rate-limiting drug not withheld | 10 | |||
| Severe aortic stenosis | 1 | |||
| Hypertension with SBP >200 | 7 | |||
| Unable to cannulate patient | 9 | 3/1170 (0.3%) | 6/1170 (0.5%) | 0.437 |
| Stressor | ||||
| Exercise (ESE) | 281 (31.3%) | 272 (69.2%) | 9 (1.8%) | <0.0001 |
| Dobutamine (DSE) | 617 (68.7%) | 121 (30.8%) | 496 (98.2%) | <0.0001 |
| Image quality | ||||
| Diagnostic after start | 868 (96.7) | 378 (96.2%) | 490 (97.0%) | 0.302 |
| Non-diagnostic after start | 30 (3.3%) | 15 (3.3%) | 15 (3.0%) | 0.302 |
| Poor stress image quality | 17 (1.9%) | 9 (2.3%) | 8 (1.6%) | 0.299 |
| Test terminated early | 13 (1.4%) | 6 (1.5%) | 7 (1.4%) | 0.538 |
| Number of analysable segments | 16 (16–16) | 16 (16–16) | 16 (16–16) | 0.899 |
| 14 analysable segments | 6 (0.7%) | 4 (1.0%) | 2 (0.4%) | 0.234 |
| 15 analysable segments | 19 (2.1%) | 8 (2.0) | 11 (2.2%) | 0.538 |
| 16 analysable segments | 873 (97.2%) | 381 (96.9%) | 492 (97.4%) | 0.407 |
Data expressed as n (% of total cohort). P < 0.05 is taken as significant.
LV, left ventricular; SBP, systolic blood pressure; SE, stress echocardiogram.
Stress echocardiography baseline findings
Baseline findings for patients undergoing PLSE and CLSE are shown in Table 1. There was no significant difference in gender (PLSE 53.7% males vs CLSE 52.9% males, P = 0.807) or age (PLSE 60.6 ± 12.2 vs CLSE 61.6 ± 11.6, P = 0.225). Overall, dobutamine was the commonly used stressor (68.7%), particular for CLSE (98.2%), whereas the majority of PLSE used exercise (69.2%, P < 0.001 vs PLSE). Over 96% of both PLSE and CLSE studies were successfully completed and of high-image quality (>14/16 diagnostic segments, 98%, P = 0.899 CLSE vs PLSE).
Stress echocardiography safety
There was a similarly very low complication rate during stress echocardiography for both PLSE and CLSE (0.8% vs 1.8%, respectively, P = 0.187). Serious complications were extremely rare, with only 1 acute coronary syndrome (myocardial infarction in CLSE group) and no cases of anaphylaxis, atropine intoxication, cardiac rupture, cerebrovascular accident, death or sustained ventricular arrhythmia. There was no difference in the overall or individual rate of complications or safety for PLSE and CLSE (Table 2).
Table 2.
Stress echocardiography safety outcomes.
| Complication during SE | Total cohort (n = 898) | Physiologist led (n = 393) | Cardiologist led (n = 505) | P |
|---|---|---|---|---|
| No complication | 886 (98.7%) | 390 (99.2%) | 496 (98.2%) | 0.187 |
| All arrhythmias | 8 (0.9%) | 3 (0.8%) | 5 (1.0%) | 0.72 |
| Sustained ventricular arrhythmia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 |
| Acute coronary syndrome/MI | 1 (0.1%) | 0 (0.0%) | 1 (0.2%) | 0.377 |
| Allergic reaction | 4 (0.5%) | 0 (0.0%) | 3 (0.3%) | 0.126 |
| Anaphylaxis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.126 |
| Atropine intoxication | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 |
| Cardiac rupture | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 |
| Cerebrovascular accident | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 |
| Death | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 |
Stress echocardiography interpretation
Stress echocardiography interpretation results
Stress echocardiography interpretation results are shown in Table 3. Eighty-three percent of studies were negative for ischaemia, with similar values for PLSE (83.7%) and CLSE (82.5%, P = 0.331). There was no significant difference in WMSI at rest (P = 0.728) or stress (P = 0.229) in PLSE and CLSE studies. There was a significant increase in left ventricular ejection fraction at peak stress in PLSE and CLSE studies.
Table 3.
Stress echocardiography interpretation results.
| Variable | Total cohort (n = 898) | Physiologist led (n = 393) | Cardiologist led (n = 505) | P |
|---|---|---|---|---|
| SE result | ||||
| Negative | 745 (83%) | 329/393 (83.7%) | 416/505 (82.4%) | 0.331 |
| Positive | 153 (17%) | 64/393 (16.3%) | 89/505 (17.6%) | |
| Wall motion score index | ||||
| Rest | 1 (1–1), 1.04 ± 0.1 | 1 (1–1), 1.05 ± 0.1 | 1 (1–1), 1.04 ± 0.1 | 0.728 |
| Stress | 1 (1–1), 1.05 ± 0.2 | 1 (1–1), 1.05 ± 0.1 | 1 (1–1), 1.04 ± 0.1 | 0.229 |
| LV ejection fraction | ||||
| Rest | 62.2 ± 8.0 | 61.6 ± 7.5 | 62.8 ± 8.4 | 0.031 |
| Stress | 78.2 ± 8.5 | 77.4 ± 8.3 | 78.9 ± 8.6 | 0.023 |
Data expressed as n (% of total cohort), mean ± s.d., median (25th–75th centiles). P < 0.05 is taken as significant.
LV, left ventricle; SE, stress echocardiogram.
Agreement between physiologist and cardiologist stress echocardiography analysis
Data are expressed in Table 4 and Supplementary Figs 1 and 2 (see section on supplementary data given at the end of this article). There was very strong agreement between PLSE and CLSE analysis of 480 myocardial segments. At rest and stress, there was excellent agreement for WMSI analysis with Pearson’s correlation coefficients and ICC values in excess of 0.90. In addition, there was zero mean bias and close levels of agreement for WMSI analysis on Bland–Altman analysis (0.0 (+0.1, −0.1) at rest, 0.0 (+0.2, −0.3) at stress). At rest, there was excellent agreement for WMS with weighted kappa 0.87, and at stress, there was strong agreement for WMS with weighted kappa 0.70.
Table 4.
Agreement between physiologist and consultant-led stress echo analysis.
| Variable | Total cohort (n = 898) |
|---|---|
| WMSI physiologist vs cardiologist (REST) | |
| Pearson’s correlation coefficient (PCC) | 0.960 (P < 0.0001) |
| Intraclass correlation coefficient (ICC) | 0.958 (P < 0.0001) |
| Bland–Altman (LoA, 95% CI) | 0.0 (+0.1, −0.1) |
| WMSI physiologist vs cardiologist (STRESS) | |
| Pearson’s correlation coefficient (PCC) | 0.908 (P < 0.0001) |
| Intraclass correlation coefficient (ICC) | 0.887 (P < 0.0001) |
| Bland–Altman (LoA, 95% CI) | 0.0 (+0.2, −0.3) |
| WMS physiologist vs cardiologist (REST) | |
| Weighted kappa (κ) | 0.87 (0.75–0.96, 95% CI) |
| WMS physiologist vs cardiologist (STRESS) | |
| Weighted kappa (κ) | 0.70 (0.60–0.79, 95% CI) |
Data expressed as n (% of total cohort), mean ± s.d. P < 0.05 is taken as significant.
WMS, wall motion scoring; WMSI, wall motion scoring index.
Discussion
To our knowledge, this is the first study of the feasibility, safety and robustness of PLSE for CAD assessment. We demonstrate that PLSE is as feasible, safe and robust as CLSE in contemporary practice for CAD assessment, and there is excellent agreement between PLSE and CLSE segmental analysis at rest and peak stress.
Stress echocardiography is a well-validated, non-invasive functional test using a variety of stressors for CAD detection and risk stratification (3, 5). Inotropic pharmacological stress using dobutamine or treadmill and bicycle exercise stress form the mainstay of contemporary practice (10).
Stress echocardiography services have traditionally been run by cardiologists with physiologist and nurse support, particularly in the setting of dobutamine stress (3, 11, 12). Recently in the United Kingdom, the development of specialist roles within cardiac physiology has led to the advent of the Advanced Practitioner, Clinical Scientist and Specialist/Consultant Echocardiographer (13, 14). This has been facilitated by Scientific Training Programs (STP) and Higher Specialist Scientific Training programmes (HSST) for cardiac physiologists (15). This will further facilitate the growth of advanced physiologist-led services, including stress echocardiography as an adjunct to traditional consultant-led imaging cardiology.
Feasibility of PLSE
Stress echocardiography is a highly operator-dependent technique necessitating high image quality for diagnostic accuracy. We report a similarly very high proportion of diagnostic, completed studies with 16 analysable myocardial segments with PLSE and CLSE (~97%), in keeping with the literature base (16) and alternative non-invasive functional assessment modalities including vasodilator stress cardiovascular MRI (CMR) (17) and nuclear single-photon emission computed tomography (SPECT) (18). This supports our view on the importance of a robust training period in stress echocardiography image acquisition and optimisation before commencing a PLSE service.
There is a small literature base regarding training in stress echocardiography interpretation. Picano (19) demonstrated significantly improved interpretation accuracy of beginners (<20 studies interpreted with expert) for 50 dipyridamole stress echocardiograms before and after a training period of 100 studies performed alongside a supervising expert. The relative difference in diagnostic accuracy vs invasive coronary angiography between beginners and experts of 28.5% (62% vs 85% accuracy) was reduced to 3.5% (83% vs 86% accuracy) after training (P < 0.001), which has formed the basis of current recommendations of a minimum requirement of performance and interpretation of 100 studies before independent interpretation. The study used dipyridamole stress, which typically results in a relatively low incidence of wall motion abnormalities in the presence of mild or moderate CAD (20). Higher heart rates and greater potential for movement or lung artefact associated with dobutamine and exercise stress could potentially make the learning curve more challenging in contemporary practice (21).
Varga (22) demonstrated improved accuracy of stress echocardiography interpretation of beginners compared with invasive coronary angiography after joint group reporting of 50 studies at an intensive 2-day ‘stress echocardiography training school’. However, even after the training period, mean accuracy was only 64% and interobserver agreement for WMS analysis was only κ = 0.39, reinforcing the importance of high-volume training and joint interpretation with an expert.
Accredited transthoracic echocardiography physiologists are well suited to progress to stress echocardiography. A large proportion of their workload involves assessing left ventricular systolic function, providing a sound foundation to develop expert reading skills in the more demanding role of stress echocardiography. In our study, physiologists performing PLSE had BSE Adult Transthoracic Echocardiography accreditation and ≥5-year training with ≥250 stress echocardiography studies (exercise and dobutamine ± contrast) performed and interpreted under the supervision of an imaging cardiologist with EAE Level 3 stress echocardiography competence and maintained their skills by performing and interpreting ≥100 PLSE studies annually. This far exceeds the supervised performance and interpretation of ≥100 stress echocardiography studies recommended by the EAE and BSE for training in stress echocardiography (2, 3, 4). We demonstrated safe performance, competent image acquisition and excellent interpretation agreement between PLSE and CLSE for the interpretation of studies at both rest and stress. We feel that a total of 100 supervised training studies may potentially be insufficient given the greater degree of complexity of performance and interpretation of stress echocardiography compared with conventional transthoracic echocardiography, the requirement for familiarity with dobutamine and exercise stress and contrast use and importance of experience to both mitigate and deal with potential complications in stress echocardiography. The recent implementation of BSE accreditation in stress echocardiography may further facilitate this.
Safety of PLSE
PLSE (dobutamine and exercise) was incorporated into a well-established cardiologist-led echocardiography service at UHNM in 2013. The safe performance of DSE requires clinician support due to the small but inherent risks of arrhythmia, acute coronary syndromes and death (11, 12). Hence, all dobutamine PLSE lists were performed with a clinician present in the echocardiography department in case of emergencies and ≥1 member of the team in the echocardiography lab had Advanced Life Support accreditation and all others had Intermediate Life Support accreditation.
We encountered a very low incidence of minor and major complications, with only 1 patient experiencing an acute coronary syndrome. The incidence of complications in our study is in keeping with the literature base of comparably large single-centre studies (n ~ 1000) (3, 23, 24, 25, 26, 27), despite our predominance of DSE (68.7%), which is typically associated with higher complication rates compared with exercise and dipyridamole stress. Our complication rates are also comparable with those in larger, multicentre registries, especially when considering that we included all arrhythmias in our study unlike the majority of studies, which only include life-threatening arrhythmias. Indeed none of our patients experienced sustained ventricular arrhythmias. Varga (11) reported a 0.3% incidence of life-threatening arrhythmias or myocardial infarction in a registry of 35,103 dobutamine and 26,295 exercise stress echocardiograms. A large-scale review of 55,071 of dobutamine–atropine stress echocardiography showed the risk of potentially life-threatening complications at 0.2% (12). Our reported coronary event rate is more in keeping with their registry at 0.5% and 0.3% for PLSE and CLSE, respectively. These data support the safe practice of physiologist-led dobutamine and ESE with the appropriate clinical support.
Importantly, there were no differences in the overall or individual rate of complications or safety for PLSE vs CLSE. This confirms that a PLSE service with established safety protocols, appropriately accredited staff (ALS/ILS) and an attending physician in the echocardiography department is as safe in contemporary practice as a CLSE service.
There is to date only one published study assessing the safety of stress echocardiography independent of clinician supervision (27). Bremer found no difference in the safety profile of DSE undertaken with supervision by cardiologists (n = 516) and registered nurse echocardiographers (n = 519), with both cohorts demonstrating only 1 serious complication each (1× sustained ventricular arrhythmia). Our contemporary study demonstrates a safety profile for DSE in keeping with this and builds on this study by also assessing ESE and is more applicable to contemporary European practice since the registered nurse echocardiographer is not a role seen in Europe.
Limitations
The predominant use of exercise stress with its intrinsically lower complication rate may potentially contribute to the excellent safety profile of PLSE. We however feel that is unlikely to be the case given the extremely low absolute complication rate of PLSE (3/393 cases (0.8%)) and fact that in the second year of the study in which 51% of the 242 PLSEs used dobutamine stress, only 1 patient (0.4%) had a complication (atrial arrhythmia). Although this is a single-centre study, the study size is large and in keeping with single-centre published studies in the literature base. For the assessment of interobserver analysis agreement of PLSE vs CLSE, despite the modest sample size of studies (n = 30), this provided a large sample size for segmental WMS analysis (n = 480) with adequate statistical power for Bland–Altman, ICC and weighted kappa analysis, which all consistently demonstrated excellent agreement. The focus of this paper was on the feasibility and safety of PLSE and due to this, the agreement between PLSE/CLSE and invasive coronary angiography as a gold standard of accuracy was not assessed. We however feel that the well powered and closely agreeable segmental comparison between PLSE and surrogate ‘gold standard’ of CLSE by experienced imaging cardiologists provides an insight into the strong diagnostic performance of PLSE. Future work would benefit from assessment of diagnostic performance of PLSE and CLSE against invasive coronary angiography.
Conclusions
This study of 898 stress echocardiograms demonstrates that PLSE is as feasible, safe and robust as CLSE in contemporary practice for CAD assessment using both exercise and dobutamine stress. There is excellent agreement between PLSE and CLSE interpretation of segmental wall motion analysis at rest and peak stress. Adequate training is of paramount importance with a need for the supervision of performance, image acquisition and joint interpretation of studies with a Level 3 accredited expert before independent practice is commenced. If this can be achieved, PLSE is a safe and feasible adjunct to an established stress echocardiography service.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Consent
Patient consent was not required for this retrospective study and there is no identifiable patient information in our raw or presented study data.
Authors contribution statement
J N K and T G conceived the idea for this substudy and oversaw data collection undertaken by T F, L M, A M, Z M, K S and R B. T G analysed stress echocardiograms for the PLSE service. S D and G H analysed studies for CLSE assessment of interobserver variability vs PLSE. J N K performed the data and statistical analysis. J N K and T G and wrote the paper. All authors critically reviewed the manuscript for important intellectual content.
Acknowledgements
The authors are grateful to Daniel Mooney for data identifying the patients to include in our study.
References
- 1.Indrajith M, Garbi M, Monaghan MJ. 2016. Setting up a stress echo service: best practice. Heart 102 1763–1770. ( 10.1136/heartjnl-2015-308165) [DOI] [PubMed] [Google Scholar]
- 2.Popescu BA, Andrade MJ, Badano LP, Fox KF, Flachskampf FA, Lancellotti P, Varga A, Sicari R, Evangelista A, Nihoyannopoulos P,, et al. 2009. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. European Journal of Echocardiography 10 893–905. ( 10.1093/ejechocard/jep151) [DOI] [PubMed] [Google Scholar]
- 3.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL. 2009. Stress echocardiography expert consensus statement – executive summary: European Association of Echocardiography (EAE). European Heart Journal 30 278–289. ( 10.1093/eurheartj/ehn492) [DOI] [PubMed] [Google Scholar]
- 4.Becher H, Chambers J, Fox K, Jones R, Leech GJ, Masani N, Monaghan M, More R, Nihoyannopoulos P, Rimington H, et al. 2004. BSE procedure guidelines for the clinical application of stress echocardiography, recommendations for performance and interpretation of stress echocardiography: a report of the British Society of Echocardiography Policy Committee. Heart 90 (Supplement 6) vi23–vi30. ( 10.1136/hrt.2004.047985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senior R, Monaghan M, Becher H, Mayet J, Nihoyannopoulos P, British Society of Echocardiography 2005. Stress echocardiography for the diagnosis and risk stratification of patients with suspected or known coronary artery disease: a critical appraisal. Supported by the British Society of Echocardiography. Heart 91 427–436. ( 10.1136/hrt.2004.044396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerqueira MD Weissman NJ Dilsizian V Jacobs AK Kaul S Laskey WK Pennell DJ Rumberger JA Ryan T & Verani MS. 2002. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105 539–542. ( 10.1161/hbib402.102975) [DOI] [PubMed] [Google Scholar]
- 7.Popp R, Agatston A, Armstrong W, Nanda N, Pearlman A, Rakowski H, Seward J, Silverman N, Smith M, Stewart W, et al. 1998. Recommendations for training in performance and interpretation of stress echocardiography. Committee on Physician Training and Education of the American Society of Echocardiography. Journal of the American Society of Echocardiography 11 95–96. ( 10.1016/S0894-7317(98)70131-2) [DOI] [PubMed] [Google Scholar]
- 8.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JAC. 2005. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. Journal of Clinical Medicine and Research 7 783–791. ( 10.1080/10976640500295417) [DOI] [PubMed] [Google Scholar]
- 9.Viera AJ, Garrett JM. 2005. Understanding interobserver agreement: the kappa statistic. Family Medicine 37 360–363. [PubMed] [Google Scholar]
- 10.Bhattacharyya S, Chehab O, Khattar R, Lloyd G, Senior R, British Society of Echocardiography 2014. Stress echocardiography in clinical practice: a United Kingdom National Health Service Survey on behalf of the British Society of Echocardiography. European Heart Journal: Cardiovascular Imaging 15 158–163. ( 10.1093/ehjci/jebib82) [DOI] [PubMed] [Google Scholar]
- 11.Varga A, Garcia MA, Picano E, International Stress Echo Complication Registry 2006. Safety of stress echocardiography (from the International Stress Echo Complication Registry). American Journal of Cardiology 98 541–543. ( 10.1016/j.amjcard.2006.02.064) [DOI] [PubMed] [Google Scholar]
- 12.Geleijnse ML, Krenning BJ, Nemes A, van Dalen BM, Soliman OI, Ten Cate FJ, Schinkel AF, Boersma E, Simoons ML. 2010. Incidence, pathophysiology, and treatment of complications during dobutamine-atropine stress echocardiography. Circulation 121 1756–1767. ( 10.1161/CIRCULATIONAHA.109.859264) [DOI] [PubMed] [Google Scholar]
- 13.BCS 2015. Strategic Review of Cardiac Physiology Services in England: Final Report. London, UK: British Cardiovascular Society: (available at: http://www.bcs.com/documents/SRCPS_Final_Report_12052015_2.pdf) [Google Scholar]
- 14.Chambers J, Lloyd G, Rimington HM, Parkin D, Hayes AM, Baldrock-Apps G, Topham A. 2011. Multidisciplinary valve clinics with devolved surveillance: a two-year audit. British Journal of Cardiology 18 231–232. ( 10.5837/bjc.2011.004) [DOI] [Google Scholar]
- 15.DOH 2010. Modernising Scientic Careers: The England Action Plan. London, UK: Department of Health; (available at: https://www.gov.uk/government/publications/modernising-scientific-careers-the-england-action-plan) [Google Scholar]
- 16.Shah BN, Senior R. 2016. Stress echocardiography in patients with morbid obesity. Echo Research and Practice 3 R13–R18. ( 10.1530/ERP-16-0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen BD, Chatterjee N, Ayache J, Freed BH, Lee DC, Carroll T, Markl M, Collins JD, Carr JC. 2015. Stress perfusion cardiac MRI with regadenoson and gadofoveset trisodium. Journal of Clinical Medicine and Research 17 P113 ( 10.1186/1532-429x-17-s1-p113) [DOI] [Google Scholar]
- 18.Duvall WL, Croft LB, Corriel JS, Einstein AJ, Fisher JE, Haynes PS, Rose RK, Henzlova MJ. 2006. SPECT myocardial perfusion imaging in morbidly obese patients: image quality, hemodynamic response to pharmacologic stress, and diagnostic and prognostic value. Journal of Nuclear Cardiology 13 202–209. ( 10.1007/BF02971244) [DOI] [PubMed] [Google Scholar]
- 19.Picano E, Lattanzi F, Orlandini A, Marini C, L’Abbate A. 1991. Stress echocardiography and the human factor: the importance of being expert. Journal of the American College of Cardiology 17 666–669. ( 10.1016/S0735-1097(10)80182-2) [DOI] [PubMed] [Google Scholar]
- 20.Soman P, Lahiri A, Senior R. 2004. Vasodilator stress induces infrequent wall thickening abnormalities compared to perfusion defects in mild-to-moderate coronary artery disease: implications for the choice of imaging modality with vasodilator stress. Echocardiography 21 307–312. ( 10.1111/j.0742-2822.2004.03006.x) [DOI] [PubMed] [Google Scholar]
- 21.Picano E, Lattanzi F, Masini M, Distante A, L’Abbate A. 1987. Comparison of the high-dose dipyridamole-echocardiography test and exercise two-dimensional echocardiography for diagnosis of coronary artery disease. American Journal of Cardiology 59 539–542. ( 10.1016/0002-9149(87)91165-9) [DOI] [PubMed] [Google Scholar]
- 22.Varga A, Picano E, Dodi C, Barbieri A, Pratali L, Gaddi O. 1999. Madness and method in stress echo reading. European Heart Journal 20 1271–1275. ( 10.1053/euhj.1999.1541) [DOI] [PubMed] [Google Scholar]
- 23.Mertes H, Sawada SG, Ryan T, Segar DS, Kovacs R, Foltz J, Feigenbaum H. 1993. Symptoms, adverse effects, and complications associated with dobutamine stress echocardiography. Experience in 1118 patients. Circulation 88 15–19. ( 10.1161/01.CIR.88.1.15) [DOI] [PubMed] [Google Scholar]
- 24.Pellikka PA, Roger VL, Oh JK, Miller FA, Seward JB, Tajik AJ. 1995. Stress echocardiography. Part II. Dobutamine stress echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clinic Proceedings 70 16–27. ( 10.1016/S0025-6196(11)64660-0) [DOI] [PubMed] [Google Scholar]
- 25.Zahn R Lotter R Nohl H Schiele R Bergmeier C Zander M Seidl K & Senges J. 1996. Feasibility and safety of dobutamine stress echocardiography: experiences with 1,000 studies. Zeitschrift für Kardiologie 85 28–34. [PubMed] [Google Scholar]
- 26.Elhendy A, van Domburg RT, Poldermans D, Bax JJ, Nierop PR, Geleijnse ML, Roelandt JR. 1998. Safety and feasibility of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in diabetic patients unable to perform an exercise stress test. Diabetes Care 21 1797–1802. ( 10.2337/diacare.21.11.1797) [DOI] [PubMed] [Google Scholar]
- 27.Bremer ML, Monahan KH, Stussy VL, Miller FA, Seward JB, Pellikka PA. 1998. Safety of dobutamine stress echocardiography supervised by registered nurse sonographers. Journal of the American Society of Echocardiography 11 601–605. ( 10.1016/S0894-7317(98)70035-5) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a