Abstract
Aims
Lupus myocarditis occurs in 5–10% of patients with systemic lupus erythematosus (SLE). No single feature is diagnostic of lupus myocarditis. Speckle tracking echocardiography (STE) can detect subclinical left ventricular dysfunction in SLE patients, with limited research on its utility in clinical lupus myocarditis. We report on STE in comparison to conventional echocardiography in patients with clinical lupus myocarditis.
Methods and results
A retrospective study was done at a tertiary referral hospital in South Africa. SLE patients with lupus myocarditis were included and compared to healthy controls. Echocardiographic images were reanalyzed, including global longitudinal strain through STE. A poor echocardiographic outcome was defined as final left ventricular ejection fraction (LVEF) <40%. 28 SLE patients fulfilled the criteria. Global longitudinal strain correlated with global (LVEF: r = −0.808; P = 0.001) and regional (wall motion score: r = 0.715; P < 0.001) function. In patients presenting with a LVEF ≥50%, global longitudinal strain (P = 0.023), wall motion score (P = 0.005) and diastolic function (P = 0.004) were significantly impaired vs controls. Following treatment, LVEF (35–47% (P = 0.023)) and wall motion score (1.88–1.5 (P = 0.017)) improved but not global longitudinal strain. Initial LVEF (34%; P = 0.046) and global longitudinal strain (−9.5%; P = 0.095) were lower in patients with a final LVEF <40%.
Conclusions
This is the first known report on STE in a series of patients with clinical lupus myocarditis. Global longitudinal strain correlated with regional and global left ventricular function. Global longitudinal strain, wall motion score and diastolic parameters may be more sensitive markers of lupus myocarditis in patients presenting with a preserved LVEF ≥50%. A poor initial LVEF and global longitudinal strain were associated with a persistent LVEF <40%. Echocardiography is a non-invasive tool with diagnostic and prognostic value in lupus myocarditis.
Keywords: myocarditis, systemic lupus erythematosus, speckle tracking echocardiography
Introduction
Lupus myocarditis is a serious manifestation of systemic lupus erythematosus (SLE) with clinically evident myocarditis occurring in 5–10% of patients (1, 2). No single clinical or imaging feature is diagnostic of lupus myocarditis. Although endomyocardial biopsy is regarded as the diagnostic gold standard, the invasiveness of the procedure and poor negative predictive value limit its utility (3). The diagnosis is usually based on a clinical impression of cardiac failure or unexplained arrhythmia, supported by non-invasive tests including cardiac imaging (4, 5).
Echocardiography is frequently used to support a diagnosis of lupus myocarditis (6, 7). Accurate assessment of ventricular wall motion (velocity) is essential in the evaluation of regional myocardial function.
Cardiac magnetic resonance imaging is regarded as the non-invasive investigation of choice for the diagnosis of myocarditis, including lupus myocarditis (5, 8, 9). It is however an expensive tool especially in resource-limited settings. Echocardiography on the other hand is cost effective and can be utilized at the bedside, even in the unstable, ventilated patient.
The aim of our study was to give a comprehensive description of STE. Findings in comparison to conventional echocardiography, including tissue Doppler imaging in a group of patients with clinically evident lupus myocarditis and compare the results to that of a healthy control group.
Methods
Patients and controls
A retrospective study was done at Tygerberg Hospital, a tertiary referral center in the Western Cape of South Africa. Our institution renders a tertiary service to a population of approximately 3.6 million people in the Cape Town area. Clinical records of all SLE inpatients and outpatients between January 2008 and January 2014 were screened for inclusion. Adult (13 years and older) patients (fulfilling the 1997 revised American College of Rheumatology criteria) with a diagnosis of lupus myocarditis were included (10). Lupus myocarditis was defined as clinical and echocardiographic evidence of impaired myocardial function (regional and/or global) attributed to active SLE. Patients with a cardiomyopathy attributed to causes other than SLE were excluded. Controls were recruited from health care workers as well as medical students at our institution and matched to our patient group with regard to age, gender and ethnicity. All controls included were healthy, non-lupus individuals with no known cardiac risk factors or history of cardiovascular disease, a normal physical examination and a low pre-test probability of cardiac disease.
Clinical and laboratory data
Data collected included demographics (gender, age, ethnicity and co-morbid conditions); duration of SLE at diagnosis of lupus myocarditis; SLE Disease Activity Index (SLEDAI) at the time of diagnosis of lupus myocarditis; detail of systemic involvement; symptoms and signs of lupus myocarditis (11). Relevant laboratory data were documented including auto-antibody results and chemistry (serum-creatinine, cardiac enzymes and urine analysis). Chest radiographs, electrocardiograms and angiogram reports were included where available.
Conventional two-dimensional echocardiography and two-dimensional STE analysis
Standard two-dimensional echocardiograms were originally performed on all patients with a M4S probe using a Vivid 7 Dimension ultrasound system (General Electric Medical Systems, South Africa). All the available original echocardiographic images were retrieved form a digital image archive (EchoPAC platform (2DS-software package, version 3.3), General Electric Medical Systems) and reanalyzed by a clinician experienced in echocardiography. Serial images were described in relation to the time of lupus myocarditis diagnosis. Structural and functional measurements, including pulse wave and tissue Doppler imaging were done in accordance with international echocardiography guidelines (12, 13, 14). Global left ventricular function was obtained using the Simpsons biplane method or visual estimation if the endocardial definition was inadequate. Right ventricular function and hemodynamic changes were assessed by determining the tricuspid annular plane systolic excursion (TAPSE) and tricuspid regurgitation maximal velocity (TR Vmax) (15). Diastolic dysfunction was assessed in terms of mitral annular velocity in early diastole (MA E′ave) (average of lateral and septal measurement) as a marker of active, early left ventricular relaxation and the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity (MA E/E′) as marker of left ventricular filling pressures.
Regional left ventricular function was described with regard to regional wall motion abnormalities based on the 16-segment model. The wall motion score index for an individual patient was derived as the sum of all scores divided by the number of segments visualized (13).
STE analysis was not included in the original echocardiographic assessment of the study population. Cine-loops that were stored in DICOM digital format were selected from three apical views (3-chamber, 4-chamber and 2-chamber views). The images were downloaded from a central archive to a computer workstation and analyzed offline using customized software within a personal computer workstation (EchoPAC platform). Longitudinal segmental strain was measured in the basal, mid and apical segments (according to the 17-segment model) while global peak longitudinal strain or peak systolic longitudinal strain rate was averaged from all 3 apical views. Only studies with images of sufficient quality were used for speckle tracking analysis. All controls underwent standard echocardiography, tissue Doppler imaging and STE analyses. Analyses were done in accordance with international guidelines (14, 16, 17, 18).
Outcomes
Follow-up was concluded on 31 October, 2014. Where follow-up echocardiograms were available, functional and structural parameters were described in terms of change from the time of diagnosis. A poor echocardiographic outcome was defined as a final left ventricular ejection fraction (LVEF) <40%.
Statistical analysis
Descriptive analysis was done using frequency tables with numerical variables summarized as means and a standard deviation with 95% confidence intervals (normally distributed) and median, range and interquartile range (not normally distributed). Comparisons between the patient and control group were made with the Fisher’s Exact Test (independent groups, binary), the Pearson chi-square test (various ethnic groups) and the independent samples test (normally distributed means).
The paired Wilcoxon signed-rank test (data not normally distributed) was used to compare the initial and final echocardiograms. The Mann–Whitney U test (data not normally distributed) was used to compare echocardiographic data in patients with a poor outcome to those without as well as in comparing echocardiographic data in patient with a preserved LVEF at diagnosis to those without.
Variables in the control group and the two different categories of LVEF were compared using the Kruskal–Wallis test (omnibus-). Dunnet’s post hoc test with adjustment for multiple testing was used to determine significant differences between the three groups.
Spearman’s correlations were used to determine the relationships among continuous variables (nonparametric, Spearman’s correlation coefficient) while Pearson two-tailed correlations were used to determine the relationships between global longitudinal strain and clinical parameters. A P < 0.05 was considered as statistically significant.
Ethical consideration
The study was approved by the Health Research Ethics Committee of Stellenbosch University, South Africa. Research was conducted according to the ethical guidelines and principles of the International Declaration of Helsinki, South African Guidelines for Good Clinical Practice and the Medical Research Council Ethical guidelines for research. In view of the retrospective nature of the study, the difficulty in tracing individual subjects and the absence of risk to the subjects, the Health Research Ethics Committee, Stellenbosch University granted a waiver of informed consent for the patients included into the study. Informed consent has been obtained from each healthy volunteer after full explanation of the purpose and nature of all procedures used.
Results
Clinical and demographic features
A total of 457 SLE patients’ clinical records were screened. Fifty-five patients were considered to have had possible lupus myocarditis of which 27 patients were excluded due to a cardiomyopathy attributed to causes other than SLE. Twenty-eight patients (6.1%) fulfilled inclusion criteria. Twenty-eight healthy non-lupus controls were included. There were no significant differences between the patient and control group with regard to gender, ethnicity and age. Details of the demographics, clinical and laboratory features of patients at the time of diagnosis are summarized in Table 1.
Table 1.
Demographic and clinical features of patients at the time of diagnosis of lupus myocarditis compared to a healthy non-lupus control group.
| Lupus myocarditis group | Healthy control group | |
|---|---|---|
| n/total (%) | n/total (%) | |
| Female gender | 26/28 (92.9) | 26/28 (92.9) |
| Ethnicity: mixed racial ancestry | 25/28 (89.3) | 25/28 (89.3) |
| Age (years) mean ± s.d. | 28.32 ± 11.35 | 28.48 ± 11.33 |
| Duration of SLE (weeks) median (IQR) | 11.5 (IQR: 0–119) | |
| SLEDAI median (IQR) | 17.5 (IQR: 12.3–24) | |
| Lupus nephritis | 19 (67.9) | |
| Co-morbidities | ||
| Antiphospholipid syndrome | 1/28 (3.6) | 0/28 (0) |
| Hypertension | 7/28 (25) | 0/28 (0) |
| Diabetes mellitus | 1/28 (3.6) | 0/28 (0) |
| Dyslipidemia | 2/28 (7.1) | 0/28 (0) |
| Clinical features of lupus myocarditis | ||
| Symptoms | ||
| New York Heart Association class 3/more dyspnea | 21/23 (91.3) | |
| Orthopnoea/paroxysmal nocturnal dyspnea | 6/28 (21.4) | |
| Palpitations | 1/28 (3.6) | |
| Chest pain | 2/28 (7.1) | |
| Signs | ||
| Respiratory crackles/pulmonary edema | 24/28 (85.7) | |
| Pleural effusion | 9/28 (32.1) | |
| Raised jugular venous pressure | 7/28 (25) | |
| Displaced apex | 8/28 (28.6) | |
| Tachycardia | 26/28 (92.9) | |
| New murmur | 1/28 (3.6) | |
| S3 gallop | 11/28 (39.3) | |
| Pedal edema | 12/28 (42.9) |
IQR, inter quartile range; s.d., standard deviation; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index.
The anti-nuclear antibody titer was positive in all patients while antiphospholipid antibodies were present in two patients. Forty percent of patients had a raised creatine kinase (median: 105 µg/L; interquartile range (IQR): 45–778) compared to a raised troponin-I (normal range <0.04 µg/L) in 72.7% (median: 0.109 µg/L; IQR: 0.04–2.77). Although 67.9% of patients had concomitant lupus nephritis the median glomerular filtration rate was 122 mL/min/1.73 m2 (IQR: 56–168).
Eighty-six per cent of patients presented with congestive cardiac failure while three patients (11%) presented in cardiogenic shock. The most frequent electrocardiogram findings were sinus tachycardia (75%) and non-specific ST segment and T-wave abnormalities (78%). Fourteen per cent of patients developed arrhythmias including ventricular extra systoles, atrial fibrillation and ventricular tachycardia. Chest radiographs had features of pulmonary congestion (78.6%) and pleural effusions (64.3%). One patient underwent angiography confirming normal coronary arteries.
Treatment of lupus myocarditis
The majority of patients were treated with corticosteroids including intravenous solu-medrol pulse therapy (67.9% of patients) and/or oral prednisone (96.4%). Further immunosuppressive therapy used as induction or maintenance therapy included cyclophosphamide, azathioprine, mycophenolate mofetil, intravenous immunoglobulin and rituximab. Four patients received more than one form of immunosuppression for either resistant lupus myocarditis or a relapse. Anti-failure therapy included angiotensin-converting enzyme inhibitors (71.4%), diuretics (75%), beta-blockers (60.7%) and inotropes (14.3%).
Echocardiographic features
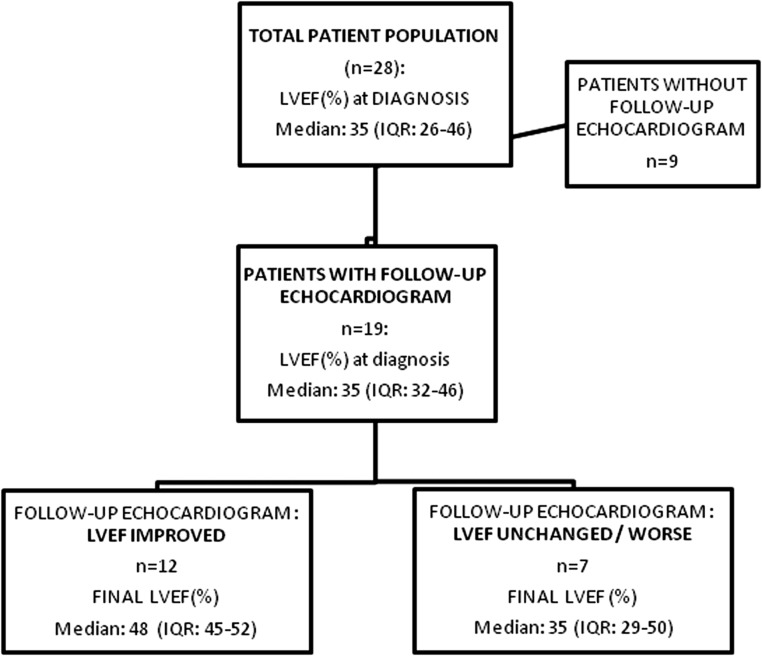
Initial echocardiographic characteristics in patients compared to controls are summarized in Table 2. Left ventricular chamber size was preserved in 60.7% of patients at diagnosis. Seventeen patients (17/27; 63%) presented with a severely impaired LVEF (≤35%) while 25.9% of patients had a normal to only mildly impaired LVEF (≥45%). In seven patients (36.8%), the median LVEF remained unchanged or deteriorated further despite treatment (Fig. 1).
Table 2.
Echocardiographic findings of lupus myocarditis group at diagnosis (initial) compared to those of a healthy control group.
| Initial echocardiogram in lupus myocarditis group | Initial echocardiogram in healthy control group | ||
|---|---|---|---|
| Total n = 28 | Median (IQR)/ratio (%) of test done | Median (IQR)/ratio (%) of test done | P value |
| Structural parameter | |||
| LAa diameter (cm) | 3.2 (2.8–3.9) | 3.0 (2.8–3.3) | 0.105 |
| LVIDb (cm) | 5.2 (4.4–5.6) | 4.6 (4.3–4.8) | 0.046 |
| RVIDc (cm) | 3.2 (2.7–3.7) | 3.0 (2.9–3.2) | 0.176 |
| Valvular dysfunction (mild/moderate) | 13/27 (48.2) MR | 0/28 MR | <0.001 |
| 7/27 (25.9) TR | 0/28 TR | 0.007 | |
| Pericardial effusion | Small 7/27 (25.9) | All: 0/28 | 0.005 |
| Moderate: 2/27 (7.4) | |||
| Large: 1/27 (3.7) | |||
| Regional function parameter | |||
| RWMA present | 24/24 | 0/28 | |
| Wall motion scored | 2.0 (1.8–2.6) | 1 (1–1) | <0.001 |
| Global function parameter | |||
| MA E′avee (cm/s) | 8.0 (6.0–11.0) | 12.0 (11.0–14.5) | <0.001 |
| MA E/E′f | 11.6 (10.3–16.2) | 7.3 (5.3–8.0) | <0.001 |
| LVEFg: numerical (%) | 35 (26–46) | 63.5 (58.0–68.0) | <0.001 |
| LVEF: categorical | |||
| ≥55% | 2/27 (7.4) | 28/28 | |
| 45–54% | 5/27 (18.5) | ||
| 36–44% | 3/27 (11.1) | ||
| ≤35% | 17/27 (63.0) | ||
| TAPSEh (cm) | 1.7 (1.6–2.1) | 2.0 (1.8–2.3) | 0.006 |
| GLSi (%) | −10.9 (−13.7 to −7.8) | −22.1 (−23.5 to −20.8) | <0.001 |
Figure 1.
Flow chart depicting the improvement/deterioration in left ventricular function (LVEF) from the time of diagnosis to the final echocardiogram in 19 patients where a follow-up echocardiogram was available. IQR, interquartile range; LVEF, left ventricular ejection fraction.
Left ventricular filling pressures were normal (MA E/E′ <8) in 2/21 patients (9.5%) and increased (MA E/E′ >15) in 7/21 patients (33.3%). Left ventricular relaxation was impaired (MA E′ave <8 cm/s) in 11/23 patients (47.8%) (22). None of the control patients had evidence of impaired left ventricular relaxation or increased left ventricular filling pressures. Other parameters of regional and global left ventricular function (wall motion score and global longitudinal strain) were significantly reduced in comparison to the control group (P < 0.001).
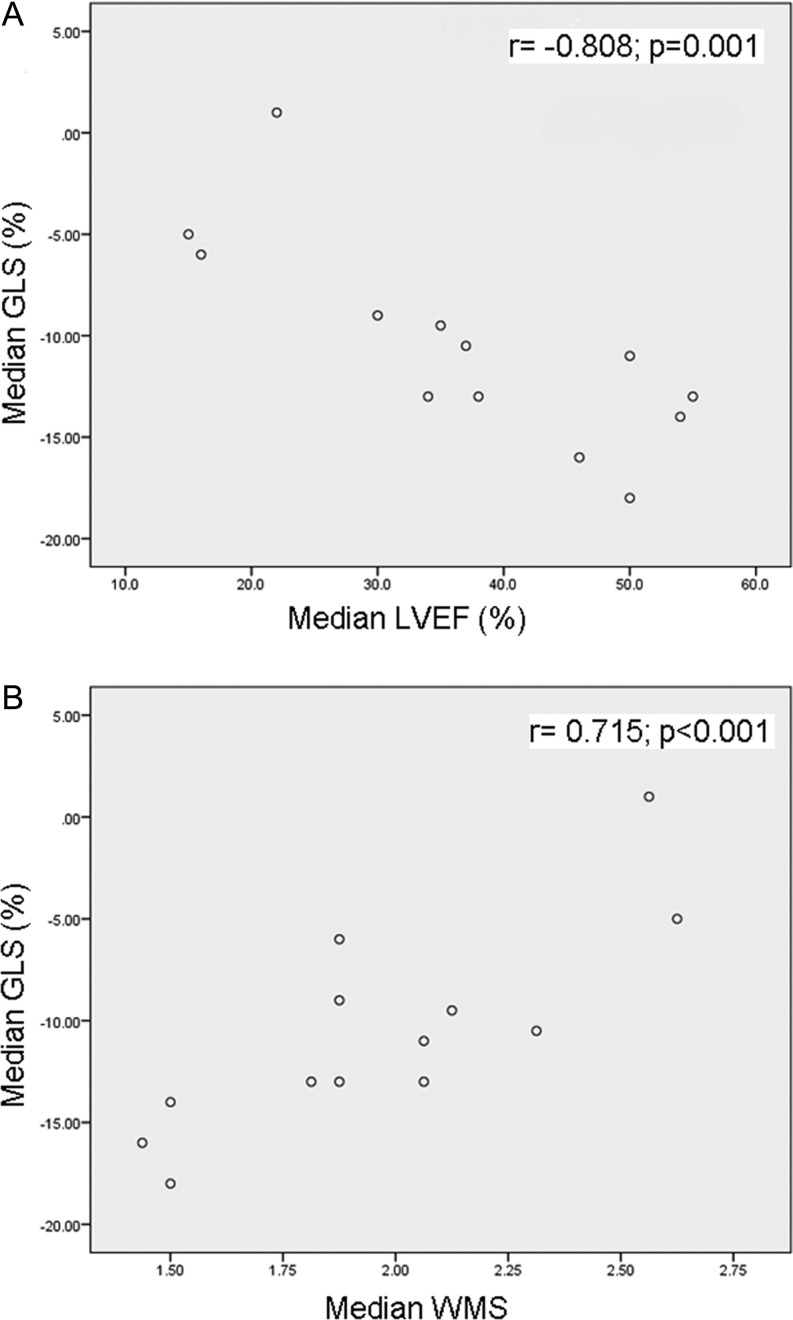
At diagnosis, global longitudinal strain correlated well with other parameters of global left ventricular function (LVEF: r = −0.808; P = 0.001; Fig. 2A) and regional left ventricular function (wall motion score: r = 0.715; P < 0.001; Fig. 2B). No correlation was demonstrated between global longitudinal strain and parameters of diastolic left ventricular function (MA E/E′ (r = 0.205; P = 0.523); MA E′ave (r = −0.41; P = 0.165)) nor right ventricular function and – hemodynamics ((TAPSE): r = −0.039; P = 0.905; right ventricular systolic pressure (RSVP): r = 0.068; P = 0.841).
Figure 2.
Correlation between the median global longitudinal strain (%) and (A) median left ventricular ejection fraction (%) and (B) median wall motion score at diagnosis. GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; WMS, wall motion score.
A weaker correlation was seen between global longitudinal strain and renal function (glomerular filtration rate: r = −0.502; P = 0.081). No other clinical parameters including age (r = −0.263; P = 0.386), SLEDAI (r = −0.277; P = 0.359) and duration of SLE (r = 0.304; P = 0.312), nor laboratory parameters (C-reactive protein, creatine kinase, troponin) showed any significant correlations with global longitudinal strain.
Echocardiographic features of patients with a preserved left ventricular function at diagnosis
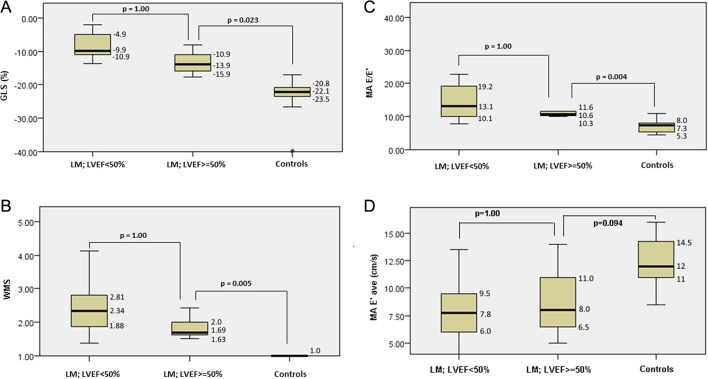
Six patients (6/27) presented with a relatively preserved LVEF of ≥50%. In this subgroup of patients, other measures of left ventricular function including global longitudinal strain, wall motion score and measures of diastolic left ventricular function (MA E/E′ and MA E′ave) were significantly impaired in comparison to the control group while measures of right ventricular function (TAPSE) were not significantly different (Fig. 3A, B, C and D).
Figure 3.
Box and whisker plots show the comparison between patients who presented with either impaired (LVEF <50%) or preserved left ventricular systolic function (LVEF ≥50%) and normal controls by analysis of variance for GLS (A), WMS (B) and parameters of diastolic function, MA E/E′ (C) and MA E′ave (D). The numeric values reported denote the median (horizontal line of the box) and the inter quartile range (top and bottom line). E′, early diastolic mitral annular velocity, average of lateral and septal measurement; E/E′, ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E′); GLS, global longitudinal strain; LM, lupus myocarditis; LVEF, left ventricular ejection fraction; MA, mitral annular; WMS, wall motion score.
Follow-up data
Clinical follow-up data were available for a median period of 563 days (range 4–1740) after diagnosis. Although one patient was lost to follow-up, the latest available clinical detail (555 days since initial presentation) as well as a follow-up echocardiogram (283 days after presentation) were obtained from the patient’s medical records. At the time, the patient had no clinical signs of lupus myocarditis and the LVEF had recovered to 45%.
Nineteen patients (67.9%) had one or more follow-up echocardiogram following the diagnosis of lupus myocarditis (median 390 days; IQR: 93–680). Repeat echocardiograms were not routinely done but requested at the discretion of the treating clinician. Of the nine patients who did not undergo follow-up imaging, seven died (three due to lupus myocarditis) while the remaining two patients showed a full clinical recovery without recurrence of cardiac manifestations (data available at 639 and 750 days, respectively after their lupus myocarditis diagnosis).
Table 3 provides a detailed summary of the structural and functional echocardiographic findings of these 19 patients at diagnosis as well as at follow-up (latest available echocardiogram). Following treatment for lupus myocarditis, both the median LVEF and wall motion score significantly improved (P = 0.023 and P = 0.017, respectively) in contrast to global longitudinal strain (P = 0.47) and parameters of diastolic function (MA E′ave: P = 0.649 and MA E/E′: P = 0.281).
Table 3.
Echocardiographic findings at diagnosis (initial) and most recent echocardiogram (latest) following treatment for lupus myocarditis.
| Initial echocardiogram in lupus myocarditis group | Latest echocardiogram in lupus myocarditis group | ||
|---|---|---|---|
| Total n = 19 | Median (IQR)/ratio (%) of test done | Median (IQR)/ratio (%) of test done | P value |
| Structural parameter | |||
| LAa diameter (cm) | 3.3 (2.8–3.9) | 3 (2.5–3.4) | 0.088 |
| LVIDb (cm) | 5.3 (4.5–5.6) | 4.8 (4.0–5.6) | 0.106 |
| RVIDc (cm) | 3.1 (3.0–3.9) | 3 (2.6–3.2) | 0.071 |
| Valvular dysfunction (mild/moderate) | 10/19 (52.6) MR | 5/19 (26.3) MR | |
| 5/17 (29.4) TR | 5/17 (29.4) TR | ||
| Pericardial effusion | 6/18 (33.3) small | Small 2/18 (11.1) | |
| 1/18 (5.6) large | |||
| Regional function parameter | |||
| RWMA present | 17/17 (100) | 16/17 (94.1) | |
| Wall motion scored | 1.88 (1.69–2.38) | 1.50 (1.31–2.00) | 0.017 |
| Global function parameter | |||
| MA E′avee (cm/s) | 8.0 (6.0–11.0) | 8.8 (5.8–10.0) | 0.649 |
| MA E/E′f | 11.6 (10.0–16.2) | 10 (7.75–15.8) | 0.281 |
| LVEFg: numerical (%) | 35 (32–46) | 47 (37–50) | 0.023 |
| LVEF: categorical | |||
| ≥55% | 0/19 (0) | 3/19 (15.8) | |
| 45–54% | 5/19 (26.3) | 10/19 (52.6) | |
| 36–44% | 4/19 (21.1) | 2/19 (10.5) | |
| ≤35% | 10/19 (52.6) | 4/19 (21.1) | |
| TAPSEh (cm) | 1.9 (1.6–2.1) | 1.7 (1.6–2.0) | 0.395 |
| Impaired GLSi | 13/13 (100) | 13/13 (100) | |
| GLS(%) | −13.0 (−13.5 to −10.3) | −15 (−14 to −5) | 0.47 |
LA diameter: normal ≤3.8 cm; bLVID: normal ≤5.3 cm; cRVID: normal ≤4.2 cm; dWall motion score increased if >1; eMA E′ average: normal <8 cm/s; fMA E/E′: normal <8; increased LV filling pressure >15; gLVEF: normal ≥55%; mild impairment: 45–54%; moderate impairment: 36–44%; severe impairment: ≤35%; hTAPSE: normal ≥1.6 cm; iGLS: normal −19.7% (95% CI, −20.4 to −18.9%).
E′ave, early diastolic mitral annular velocity, average of lateral and septal measurement; E/E′, ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E′); GLS, global longitudinal strain; IQR, interquartile range; LA, left atrium; LVEF, left ventricular ejection fraction; LVID, left ventricular internal diameter; MA, mitral annular; MR, mitral regurgitation; RVID, right ventricular internal diameter; RWMA, regional wall motion abnormalities; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Associations with a poor echocardiographic outcome
Following immunosuppressive therapy, five out of 19 patients (26.3%) had a final LVEF <40%. A lower initial (at diagnosis) LVEF (P = 0.046) and global longitudinal strain (P = 0.095) were found in patients with a final LVEF of <40% compared to those patients where the LVEF recovered to ≥40% (Table 4).
Table 4.
Initial echocardiographic parameters (at time of diagnosis; total n = 19) in patients with a final LVEF <40% (poor echocardiographic outcome) compared to those with a final LVEF >40%.
| Parameter at diagnosis | Patients with a final LVEF <40% (n = 5) median (IQR) | Patients with a final LVEF ≥40% (n = 14) median (IQR) | P value |
|---|---|---|---|
| MA E′ave (cm/s) | 11.5 (8.0–12.0) | 7.5 (6.5–9.5) | 0.221 |
| MA E/E′ | 10.0 (9.6–12.9) | 13.3 (10.3–16.2) | 0.267 |
| LVID (cm) | 5.6 (5.4–5.7) | 5.2 (4.5–5.5) | 0.343 |
| LVEF (%) | 34.0 (30–35) | 38 (35–50) | 0.046 |
| GLS (%) | −9.5 (−13 to −9) | −13.5 (−16 to −11) | 0.095 |
| Wall motion score | 2.06 (1.88–2.13) | 1.81 (1.5–2.19) | 0.506 |
E′ave, early diastolic mitral annular velocity, average of lateral and septal measurement; E/E′, ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E′); GLS, global longitudinal strain; IQR, interquartile range; LVEF, left ventricular ejection fraction; LVID, left ventricular internal diameter; MA, mitral annular.
Discussion
We have recently reported the clinical features and outcome of lupus myocarditis in the Western Cape, South Africa where we found a high mortality of 17.9% among our patients (19). To the best of our knowledge, this is the first report on the use of STE in a series of SLE patients with clinically evident lupus myocarditis. Our patients were predominantly young females with a recent onset of SLE and a high SLEDAI.
Huang and coworkers demonstrated the ability of STE to detect early impairment in left ventricular function in asymptomatic SLE patients (20). Abnormalities occurred in the absence of changes on conventional echocardiography and global longitudinal strain was independently associated with SLE disease activity. The relevance and clinical implications of these findings in asymptomatic SLE patients have not been clarified.
Echocardiographic findings
The majority of our patients (63%) presented with severe left ventricular dysfunction (LVEF ≤35%). Regional wall motion abnormalities were present in all patients while global longitudinal strain was significantly impaired in comparison to the control group (P < 0.001). Parameters of diastolic function, left ventricular filling pressure and relaxation were impaired in 33.3% and 47.8% of patients, respectively.
It is well described that the subendocardial region is more sensitive to myocardial disease. Early loss of diastolic longitudinal relaxation (MA E′ave) is associated with elevated left ventricular filling pressures (MA E/E′) with predominantly diastolic dysfunction, while the LVEF may still be preserved. Diastolic function is often an early, sensitive marker of pathology in a variety of conditions affecting the left ventricle (16, 17, 21). Longitudinal strain or deformation, measured with STE, represents shortening of longitudinal myocardial fibers during systole, again an earlier, more sensitive marker of left ventricular dysfunction compared to LVEF (22). The midmyocardial and epicardial function may therefore remain relatively unaffected, with circumferential strain and twist showing compensation in order to preserve left ventricular systolic function (16).
We demonstrated a significant improvement in both the LVEF and wall motion score following treatment for myocarditis, in contrast to global longitudinal strain and diastolic parameters (MA E/E′ and MA E′), which did not improve significantly (Table 3).
Correlation between global longitudinal strain and other lupus myocarditis parameters
A strong correlation was demonstrated between global longitudinal strain and parameters of both global (LVEF) and regional (wall motion score) left ventricular function at the time of diagnosis. We did however not find a correlation between global longitudinal strain and parameters of diastolic function. The unexpected absence of a correlation between global longitudinal strain and these markers of early left ventricular dysfunction may be due to the relatively advanced left ventricular dysfunction found in the majority of our patients. This should be further explored in a larger cohort of SLE patients in the absence of clinical myocarditis or myocarditis with a relatively preserved systolic left ventricular function.
In contrast to the findings of Huang and coworkers, global longitudinal strain did not correlate with SLE disease activity (20). The patients from our study population did however present with significantly higher lupus activity (median SLEDAI of 17.5, IQR 2.3–24) in comparison to that of Huang’s study population (SLEDAI 10.5 ± 7.6). Whether this correlation between global longitudinal strain and lupus disease activity is only evident in patients without clinically evident lupus myocarditis or in patients with a lower disease activity can only be speculated.
We found a weak correlation between renal function and global longitudinal strain (r = −0.502; P = 0.081). Although 67.9% of our patients had concomitant lupus nephritis, this was of recent onset and in the absence of advanced renal dysfunction (median glomerular filtration rate 122 mL/min/1.73 m2 (IQR: 56–168)). Left ventricular dysfunction (uremic cardiomyopathy) is well described in end-stage renal disease (23). Impaired global longitudinal strain has been shown to be of diagnostic and prognostic value in this subset of patients (24). The possible correlation between mild, recent-onset renal impairment and left ventricular dysfunction, specifically abnormal global longitudinal strain has not previously been described and should be studied prospectively.
Lupus myocarditis in patients presenting with a preserved LVEF
Although severe left ventricular dysfunction (LVEF ≤35%) was found in 63% of patients, a significant proportion (22.2%) of patients presented with a relatively preserved LVEF of ≥50%. In patients with non-lupus myocarditis with a LVEF ≥50% on conventional echocardiogram, Hsiao and coworkers demonstrated significantly impaired global longitudinal strain in comparison to that of a healthy control group (25). Our results supported these findings with various other parameters of left ventricular function, including global longitudinal strain, wall motion score, MA E/E′ and MA E′ave being significantly impaired in this subgroup of patients compared to our control group.
Our findings also highlight the limitations of using the LVEF in isolation when assessing patients for possible myocarditis, in particular, before deterioration in left ventricular function.
Associations with a poor outcome
Out of 19 patients who had a follow-up echocardiogram, five had a poor echocardiographic outcome. We found a lower initial LVEF as well as global longitudinal strain in this subgroup of patients. An earlier diagnosis of lupus myocarditis, before significant left ventricular functional impairment occurs is likely to play a central role in an improved echocardiographic outcome.
Limitations
Our study had a retrospective design and we relied on the accuracy of clinical records. Despite the relatively small sample size, this is the largest reported series of patients with lupus myocarditis. Our patients were hospitalized, symptomatic SLE patients. The results would therefore not be applicable in asymptomatic SLE patients with possible subclinical myocardial dysfunction. None of our patients had histological confirmation of their myocarditis. We are therefore not able to exclude other causes of cardiomyopathy including undiagnosed antiphospholipid syndrome with microthrombosis or microvascular occlusion with 100% certainty. Patients included into the study had a known diagnosis of lupus myocarditis, which could have led to expectation bias or diagnostic suspicion bias in the reanalysis of the echocardiographic data.
Conclusion
STE is a non-invasive, cost effective tool with diagnostic and prognostic value in patients with clinically evident lupus myocarditis. At the time of diagnosis, we demonstrated strong correlations between STE (global longitudinal strain) and other parameters of left ventricular function, including LVEF and wall motion score. Both a poor LVEF and global longitudinal strain at presentation were associated with a poor echocardiographic outcome (final LVEF <40%). In lupus myocarditis patients who presented with a relatively preserved LVEF (≥50%), global longitudinal strain, wall motion score and diastolic functional parameters were significantly impaired compared to a control group. The diagnostic role of these parameters as earlier, more sensitive markers in clinical lupus myocarditis should be defined more clearly through prospective studies. Future research is also needed to define the significance of echocardiographic evidence of subclinical left ventricular dysfunction in asymptomatic SLE patients in comparison to clinically evident lupus myocarditis. Such research could aid in determining optimal cut-off values for global longitudinal strain supporting a diagnosis of clinical lupus myocarditis.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgement
Tonya Esterhuizen from Stellenbosch University, Biostatistics Unit for the statistical analysis of this manuscript.
References
- 1.Apte M, McGwin G, Vila LM, Kaslow RA, Alarcon GS, Reveille JD. 2007. Associated factors and impact of myocarditis in patients with SLE from LUMINA, a multiethnic US cohort. Rheumatology 47 362–367. ( 10.1093/rheumatology/kem371) [DOI] [PubMed] [Google Scholar]
- 2.Wijetunga M, Rockson S. 2002. Myocarditis in systemic lupus erythematosus. American Journal of Medicine 113 419–423. ( 10.1016/S0002-9343(02)01223-8) [DOI] [PubMed] [Google Scholar]
- 3.Chow LH, Radio SJ, Sears TD, Mcmanus BM. 1989. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. Journal of the American College of Cardiology 14 915–920. ( 10.1016/0735-1097(89)90465-8) [DOI] [PubMed] [Google Scholar]
- 4.Sagar S, Liu PP, Cooper LT. 2012. Myocarditis. Lancet 379 738–747. ( 10.1016/S0140-6736(11)60648-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al. 2009. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. Journal of the American College of Cardiology 53 1475–1487. ( 10.1016/j.jacc.2009.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinamonti B, Alberti E, Cigalotto C, Dreas L, Salvi A, Silvestri F, Camerini F. 1988. Echocardiographic findings in myocarditis. American Journal of Cardiology 62 285–291. ( 10.1016/0002-9149(88)90226-3) [DOI] [PubMed] [Google Scholar]
- 7.Giunta A, Picillo U, Maione S, Migliaresi S, Valentini G, Arnese M, Losardo L, Marone G, Tirre G, Condorelli M. 1993. Spectrum of cardiac involvement in systemic lupus erythematosus: echocardiographic, echo-Doppler observations and immunological investigation. Acta Cardiologica 2 183–197. [PubMed] [Google Scholar]
- 8.Mavrogeni S, Bratis K, Markussis V, Spargias C, Papadopoulou E, Papamentzelopoulos S, Constadoulakis P, Matsoukas E, Kyrou L, Kolovou G. 2013. The diagnostic role of cardiac magnetic resonance imaging in detecting myocardial inflammation in systemic lupus erythematosus. Differentiation from viral myocarditis. Lupus 22 34–43. ( 10.1177/0961203312462265) [DOI] [PubMed] [Google Scholar]
- 9.Mavrogeni S. 2014. Heart failure imaging patterns in systemic lupus erythematosus. Evaluation using cardiovascular magnetic resonance. International Journal of Cardiology 176 557–559. ( 10.1016/j.ijcard.2014.07.019) [DOI] [PubMed] [Google Scholar]
- 10.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester J. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatology 25 1271–1277. ( 10.1002/art.1780251101) [DOI] [PubMed] [Google Scholar]
- 11.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. 1992. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis and Rheumatology 35 630–640. ( 10.1002/art.1780350606) [DOI] [PubMed] [Google Scholar]
- 12.Wharton G, Steeds R, Allen J, Phillips H, Jones R, Kanagala P, Lloyd G, Masani N, Mathew T, Oxborough D, et al. 2015. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Research and Practice 2 G9–G24. ( 10.1530/ERP-14-0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang R, Bierig M, Devereux R, Flachskampf F, Foster E, Pellikka P, Picard MH, Roman MJ, Seward J, Shanewise J, et al. 2006. Recommendations for chamber quantification. European Journal of Echocardiography 7 79–108. ( 10.1016/j.euje.2005.12.014) [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF Smiseth OA Appleton CP Byrd BF Dokainish H, Edvardsen T Flachskampf FA,Gillebert TC Klein AL, Lancellotti P et al. 2016. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 29 277–314. ( 10.1016/j.echo.2016.01.011) [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. 2010. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography Journal of the American Society of Echocardiography 23 685–713. ( 10.1016/j.echo.2010.05.010) [DOI] [PubMed] [Google Scholar]
- 16.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula , Sengupta PP. 2010. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. Journal of the American Society of Echocardiography 23 351–369. ( 10.1016/j.echo.2010.02.015) [DOI] [PubMed] [Google Scholar]
- 17.Blessberger H, Binder T. 2010. Two dimensional speckle tracking echocardiography: basic principles. Heart 96 716–722. ( 10.1136/hrt.2007.141002) [DOI] [PubMed] [Google Scholar]
- 18.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. 2013. Normal ranges of left ventricular strain: a meta-analysis. Journal of the American Society of Echocardiography 26 185–191. ( 10.1016/j.echo.2012.10.008) [DOI] [PubMed] [Google Scholar]
- 19.Du Toit R, Herbst PG, van Rensburg A, du Plessis LM, Reuter H, Doubell AF. 2017. Clinical features and outcome of lupus myocarditis in the Western Cape South Africa. Lupus 26 38–47. ( 10.1177/0961203316651741) [DOI] [PubMed] [Google Scholar]
- 20.Huang B-T, Yao H-M, Huang H. 2014. Left ventricular remodelling and dysfunction in systemic lupus erythematosus: a three-dimensional speckle tracking study. Echocardiography 31 1085–1094. ( 10.1111/echo.12515) [DOI] [PubMed] [Google Scholar]
- 21.Paulus WJ, Tshope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, et al. 2007. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. European Heart Journal 28 2539–2550. ( 10.1093/eurheartj/ehm037) [DOI] [PubMed] [Google Scholar]
- 22.Brown J, Jenkins C, Marwick TH. 2009. Use of myocardial strain to assess global left ventricular function: a comparison with cardiac magnetic resonance and 3-dimensional echocardiography. American Heart Journal 157 102.e1–102.e5. ( 10.1016/j.ahj.2008.08.032) [DOI] [PubMed] [Google Scholar]
- 23.Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG. 2006. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney International 69 1839–1845. ( 10.1038/sj.ki.5000249) [DOI] [PubMed] [Google Scholar]
- 24.Kramann R, Erpenbeck J, Schneider RK, Rohl AB, Hein M, Brandenburg VM, Van Diepen M, Dekker F, Marx N, Floege J, et al. 2014. Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. Journal of the American Society of Nephrology 25 2351–2365. ( 10.1681/ASN.2013070734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsiao J-F, Koshino Y, Bonnichsen CR, Yu Y, Miller FA, Pellikka PA, Cooper LT, Villarraga HR. 2013. Speckle tracking echocardiography in acute myocarditis. International Journal of Cardiovascular Imaging 29 275–284. ( 10.1007/s10554-012-0085-6) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a