Abstract
Background
Rapid, cost-effective tools are needed to estimate the disease burden of acute gastroenteritis (AGE) and norovirus (NoV) in resource-limited settings.
Methods
Households with children (6 weeks–17 years) in rural Guatemala were randomly enrolled into 2 parallel AGE surveillance systems: (1) a prospective cohort, which included an enrollment visit followed by 1 year of prospective observation using a smartphone-based weekly symptom diary; and (2) 2 sequential cross-sectional rapid active sampling (RAS) surveys. Norovirus testing was performed during enrollment (all subjects) and for prospective AGE episodes (prospective cohort only).
Results
The prospective cohort enrolled 207 households (469 children) from April to September 2015 followed by 471 person-years of observation; RAS survey 1 enrolled 210 households (402 children) during October to November 2015, and RAS survey 2 enrolled 210 separate households (368 children) during January to February 2016. The prospective cohort detected a NoV+ AGE prevalence of 11% and a population-attributable fraction (PAF) of −1.6% at enrollment, followed by an incidence of 1.4 episodes/100 person-years. Rapid active sampling surveys 1 and 2 identified a NoV+ AGE prevalence of 14%–21% and a PAF of 3.2%–12.4%.
Conclusions
Rapid active sampling surveys were practical and identified more cases of NoV infection and disease compared with a parallel prospective cohort in rural Guatemala.
Keywords: norovirus, Guatemala, prevalence, surveillance, attributable fraction
Noroviruses (NoVs) are a leading cause of acute gastroenteritis (AGE) in children worldwide, with low- and middle-income countries (LMICs) accounting for the vast majority of morbidity and mortality [1–4]. As was the case with rotavirus, NoV vaccines have the potential, if effective, to significantly reduce childhood morbidity and mortality from AGE [3–9]. Multiple NoV vaccines are in early stages of development, with the most advanced demonstrating immunogenicity and a reduction in disease severity in Phase II trials [10–12]. Before the introduction of NoV vaccines, however, burden of disease data will be needed to guide cost-effectiveness estimates and vaccine prioritization decisions at national and regional levels [13–15].
Most LMICs lack surveillance systems to measure burden of pathogen-specific AGE, relying almost exclusively on passive hospital-based detection of severe AGE. Active cohort surveillance systems are traditionally considered more rigorous, because they detect a greater range of disease severity, but they also require significant time and resources and are seldom used to estimate disease burden in LMICs [3]. Thus, community-based surveillance tools are needed that not only detect a greater spectrum of AGE disease but also can be performed quickly and with limited resources [4, 16, 17]. Cross-sectional epidemiologic surveys are a commonly used tool and have been used to measure the population-level burden of disease as well as the impact of vaccines postlicensure [18–20].
We studied the feasibility and performance of community-based, cross-sectional rapid active sampling (RAS) surveys as a tool to provide timely, low-cost, and practical NoV disease burden estimates in a pediatric population from rural Guatemala. The RAS surveys were performed in parallel to a prospective smartphone-based participatory syndromic surveillance (PSS) cohort for AGE, which was used as a comparator.
METHODS
Two surveillance systems were implemented within the same study catchment area: (1) the PSS system, in which all consenting children from randomly selected households provided baseline clinical data and stool samples at their enrollment visit, and were then followed prospectively for AGE episodes using a weekly smartphone-based interactive symptom diary for 1 year, with home-based stool sample collection by study nurses for self-reported AGE; and (2) 2 sequential RAS surveys among separate households within the same catchment area, in which all enrolled children were asked to provide clinical data and stool samples in a single study visit (Figure 1).
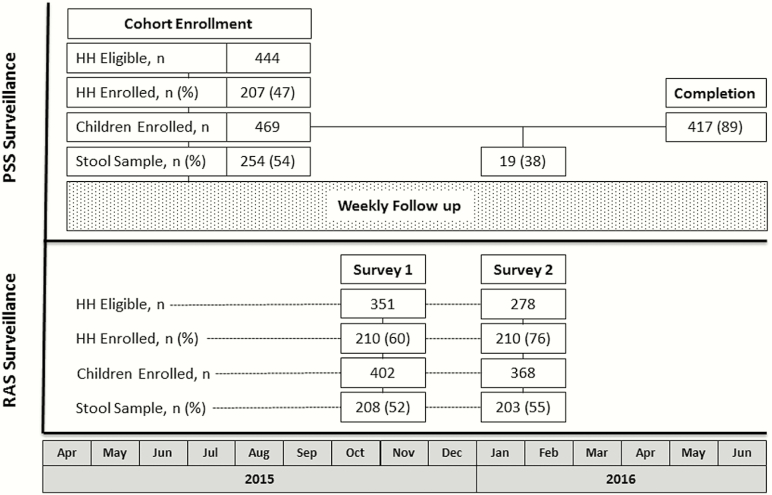
Figure 1.
Study design and CONSORT diagram of study recruitment, enrollment, and completion. The participatory syndromic surveillance (PSS) cohort enrolled children from April to September 2015, followed by prospective observation for acute gastroenteritis episodes (dotted box) for 1 year. The 2 rapid active sampling (RAS) surveys were each conducted on separate households during a single visit from October to November 2015 (RAS survey 1) and January to February 2016 (RAS survey 2). HH, household.
Study Setting and Population
The study was conducted in 25 communities within a 200-km2 catchment area along the coastal lowlands of southwest Guatemala. The populations living in these communities suffer from high levels of food insecurity, poverty, low access to healthcare, and high levels of diarrheal and respiratory disease [21]. Households were selected for screening using a 2-stage cluster randomization framework. Household clusters were sampled from a predetermined grid of the catchment area without replacement. The PSS clusters were chosen first, then the RAS survey 1 (RAS1) clusters, then the RAS survey 2 (RAS2) clusters. Thus, households could only enroll in a single surveillance group (PSS cohort, RAS1, or RAS2), with each group demonstrating similar population density distributions.
Among eligible households, inclusion criteria for participation included age between 6 weeks and 17 years and parental consent (and assent if ≥7 years). Specimen collection was not a requirement for participation. Children were excluded if participating in another research study, and for the PSS cohort only, if the parent was unable to demonstrate proficiency using the smartphone-based symptom diary.
Case Definitions
Before study initiation, diarrhea was defined as the passage of ≥3 unformed stools per day. Acute gastroenteritis was defined as vomiting or diarrhea for ≥3 consecutive days or both for ≥1 day in the preceding week. Norovirus infection was defined as any child (with or without AGE) with a NoV polymerase chain reaction (PCR)-positive stool or rectal swab. Norovirus-associated (NoV+) AGE was defined as AGE with concurrent NoV infection. Asymptomatic children did not meet the case definition of AGE.
Acute Gastroenteritis and Norovirus Surveillance
All households were screened and enrolled using a 2-stage cluster sampling strategy adapted from the World Health Organization (WHO) Lot-Quality Assurance method [22–24]. Thirty separate clusters of 7 households were enrolled into each group, the PSS cohort first and subsequently each of the 2 RAS surveys. Each randomization began by overlaying a grid on a satellite map of the study catchment area and weighting each grid square by its household density. Weight-adjusted squares were then chosen at random, and the household in the southwest corner was selected as the index household for each cluster and confirmed using global positional system (GPS) coordinates. Subsequent households in each cluster were enrolled by continuing to the right of the preceding household until 7 households were enrolled.
Demographic and AGE clinical data were collected at enrollment for all 3 study groups (PSS cohort, RAS1, and RAS2). At the enrollment visit, all subjects, including symptomatic and asymptomatic individuals, were also asked to provide a fresh stool (<2 hours old) or rectal swab sample for NoV testing. Subjects in the PSS cohort were then followed prospectively for AGE. Subjects in the cross-sectional RAS surveys had no follow up, and there was no follow up for subjects with vomiting or diarrhea for less than 3 days (they were considered non-AGE).
For the PSS cohort only, parents were trained at enrollment to use a smartphone with a symptom diary application (Integra IT, Bogota, Colombia) that reminded them to report vomiting or diarrhea for each participating child once weekly. For positive AGE reports, participants were required to provide further clinical data. Reports of AGE were followed by a nurse phone call and a home visit within 72 hours for stool sample collection. If no weekly report was received from a household, a study nurse called the parent to remind them of the submission and inquired about any vomiting or diarrhea in the preceding week.
Laboratory Testing
Specimens were collected at the home using Copan FLOQSwabs (Brescia, Italy) either by rectal swab or on fresh (<2 hours old) stool sample and eluted in eNAT transport solution (Copan, Brescia, Italy) before testing, with both collection techniques previously demonstrating similar molecular viral yield [25, 26]. Samples were stored onsite at −20°C and shipped on dry ice to Universidad del Valle de Guatemala (UVG) for diagnostic testing.
For viral extraction, the specimens were gently mixed with 200 µL of the eNAT transport solution. Viral ribonucleic acid (RNA) was extracted using QIAamp Viral RNA Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. A total of 60 µL of purified viral RNA was obtained and stored at −70°C until reverse-transcriptase-PCR analysis. Molecular testing for NoV genogroup (G)I and GII was performed as previously described [27].
Statistical Analysis
Demographic variables were compared among the 3 study groups (PSS cohort, RAS1, and RAS2) using χ2 tests for categorical variables, Student t test for 2-way comparisons of continuous variables, and generalized linear models for 3-way comparisons of continuous variables across PSS, RAS1, and RAS2. To compare the burden of disease estimates derived from each RAS survey to the PSS prospective cohort, we first divided the data from the PSS study into enrollment (prevalence) and follow-up (incidence). Prevalence of AGE, NoV+ AGE, and any NoV infection (symptomatic or asymptomatic) was calculated, including stratification by age group, from each of the 3 cross-sectional datasets (PSS enrollment, RAS1, and RAS2). The incidence densities of AGE and NoV+ AGE were obtained from the prospective follow-up surveillance in the PSS cohort, excluding children who had been diagnosed with NoV infection at enrollment (already counted for prevalence), given their reduced risk of subsequent NoV+ AGE from possible acquired immunity [3]. To compare the cross-sectional and prospective data, we converted the prevalence of AGE in the cross- sectional datasets into incidence rates using the following formula: Prevalence/(1-Prevalence) = Incidence × Duration of AGE. The population-attributable fraction (PAF), also known as the population-attributable risk [28], was used to estimate the proportion of AGE attributable to NoV. The PAF was calculated by subtracting the prevalence of NoV among asymptomatic subjects from the prevalence of NoV among subjects with AGE for each of the 3 cross-sectional datasets, using both univariate models and multivariate models adjusted for age and sex.
We created a “rapid Vesikari score” as a correlate for AGE severity, adapted from the modified Vesikari score [29], to be performed at the community level instead of a healthcare setting, and therefore excluding the variables for referral to a health center and treatment for dehydration, given low healthcare and rehydration program access in these communities. The score included diarrhea duration: 1–4 days (1 point), 5 days (2 points), ≥6 days (3 points); maximum number of stools/24 hours: 1–3 (1 point), 4–5 (2 points), ≥6 (3 points); vomiting duration: 1 day (1 point), 2 days (2 points), ≥3 days (3 points); maximum number of vomiting episodes/24 hours: 1 (1 point), 2–4 (2 points), ≥5 (3 points); and fever: no (1 point) or yes (2 points). The rapid Vesikari score was compared by Student t test between the cross-sectional and prospective datasets. SAS version 9.4 (SAS Institute, Inc., Cary, NC) was used for all data analysis.
Ethical Oversight
The study was approved by the Colorado Multiple Institutional Review Board, the UVG Institutional Review Board, and the Guatemala Ministry of Health National Ethics Committee. The local southwest Trifinio Community Advisory Board for Research agreed to the study.
RESULTS
The PSS study enrolled 469 children from 207 households from April to September 2015, followed by 471 person-years (PY) of prospective observation through June 2016 (Figure 1, Table 1). The mean weekly symptom reporting rate among PSS households was 78% (range, 58%–89%). The 2 RAS cross-sectional surveys were each completed over 4–6 weeks during October–November 2015 and January–February 2016, enrolling a total of 770 children from 420 households. The most commonly cited reasons for declining study participation (n = 164) included lack of perceived benefit to the child (42%) and discomfort with specimen collection (28%); 18% who declined participation in the PSS study did not want responsibility for the smartphone. Eligible RAS households had a greater enrollment rate than PSS households (67% vs 47%, P < .001), and enrolled RAS households had slightly more people per household (5.4 vs 5.0 people, P = .006), who were also younger (P = .04) and more often nonindigenous (P = .003). There were no significant differences in household cluster density between the PSS, RAS1, and RAS2 study groups (P = .54).
Table 1.
Characteristics of Study Participants in the Prospective Cohort and the Cross-Sectional RAS Surveys
| Characteristic | PSS Cohort (n = 469) | RAS Survey 1 (n = 402) | RAS Survey 2 (n = 368) | P Value a |
|---|---|---|---|---|
| Female, n (%) | 225 (48) | 210 (52) | 188 (51) | .41 |
| Ladino ethnicity, n (%) | 452 (97) | 383 (98) | 367 (100) | .003 |
| Age, years, mean (SD) | 7.3 (4.7) | 7.3 (4.9) | 6.5 (5.0) | .04 |
| No. of people in HH, mean (SD) | 5.0 (1.8) | 5.3 (2.1) | 5.5 (2.2) | .01 |
| No. <18 years per HH, mean (SD) | 2.6 (1.4) | 2.4 (1.5) | 2.6 (1.4) | .19 |
| No. <5 years per HH, mean (SD) | 1.0 (0.8) | 1.0 (0.8) | 1.0 (0.9) | .99 |
| Primary caregiver is literate, n (%) | 183 (89) | 176 (85) | 183 (87) | .59 |
Abbreviations: HH, household; PSS, participatory syndromic surveillance; RAS, rapid active sampling; SD, standard deviation.
a P values for categorical variables calculated using χ2 test, and P values for continuous variable calculated using general linear models, assuming equal variance across the 3 groups.
The rectal swab acceptance rate at enrollment was similar between groups overall (P = .81) and among those with AGE (P = .38). In the PSS cohort, the acceptance rate of rectal swabs decreased with time, from 53% during the first half of the study (April–November 2015) to 11% in the second half of the study (December 2015–June 2016, P < .001). Children who refused to provide a stool sample were significantly older than children who did provide a stool sample (mean 6.8 years vs 3.7 years, P < .001); there were no differences by gender, ethnicity, literacy rate, or number of individuals in the household.
Performance of Surveillance Systems in Acute Gastroenteritis and Noroviruses Detection
The AGE prevalence at PSS enrollment and RAS1 and RAS2 were 10%, 14%, and 8% (P = .31), respectively (Table 2). Of 134 total individuals with AGE, 124 (93%) reported ≥3 days of diarrhea, 10 (8%) reported ≥3 days of vomiting, and 30 (24%) reported ≥1 day of both. Of those children with a sample available, the prevalence of NoV+ AGE during PSS enrollment and RAS1 and RAS2 was 11%, 14%, and 21% (P = .31), respectively, with the greatest prevalence during RAS2 (January–February 2016); the NoV+ prevalence among children without AGE (asymptomatic infection) during the PSS enrollment, RAS1, and RAS2 was 11%, 12%, and 8% (P = .36). Of the NoV+ samples, 10% were GI, 89% GII, and 1% co-GI/GII.
Table 2.
Prevalence, Attributable Fraction, and Incidence of NoV-Associated AGE Among Children in Southwest Guatemala, 2015–2016
| Outcome | PSS Cohort (n = 469) | RAS Survey 1 (n = 402) | RAS Survey 2 (n = 368) | P Value a |
|---|---|---|---|---|
| Cross-sectionalb | April–September 2015 | October–November 2015 | January–February 2016 | |
| AGE, n (%) | 49 (10) | 56 (14) | 29 (8) | .31 |
| Sample available, n (%) | 36 (73) | 42 (75) | 24 (83) | .38 |
| NoV+, n (%) | 4 (11) | 6 (14) | 5 (21) | .31 |
| Asymptomatic NoV, n (%) | 25 (11) | 20 (12) | 15 (8) | .36 |
| NoV+ PAF, %c | −1.6 | 3.2 | 12.4 | <.001 |
| Rapid Vesikari score (SD) | 7.2 (1.7) | 7.5 (1.5) | 7.1 (1.8) | .55 |
| Prospective follow upd | April 2015–August 2016 | |||
| AGE episodes reported | 50 | N/A | N/A | N/A |
| AGE incidencee | 11.4 per 100 PY | 1316/100 PY | 697/100 PY | <.001 |
| NoV+ AGE incidencee | 1.4 per 100 PY | 154/100 PY | 131/100 PY | <.001 |
| Rapid Vesikari score (SD) | 8.1 (1.6) | N/A | N/A | .005 |
Abbreviations: AGE, acute gastroenteritis; N/A, not applicable; NoV, norovirus; PAF, population-attributable fraction; PSS, participatory syndromic surveillance; PY, person-years; RAS, rapid active sampling; SD, standard deviation.
a P values for comparison of cross-sectional AGE, sample available, NoV+, and asymptomatic NoV calculated using the asymptotic Breslow-Day test for homogeneity. P values for comparison of cross-sectional NoV+ PAF, AGE incidence, and AGE NoV+ incidence calculated using pairwise t tests. P value for comparison of cross-sectional rapid Vesikari score calculated using general linear models for continuous variables, assuming equal variance across the 3 groups. Overall mean rapid Vesikari score of the 3 cross-sectional studies (PSS enrollment, RAS survey 1, and RAS survey 2) compared with the mean rapid Vesikari score of the prospective follow-up portion of the PSS study calculated using general linear models for continuous variables.
bIncludes the enrollment visits from the PSS and all visits from the RAS survey 1 and RAS survey 2.
cPopulation-attributable fractions adjusted for age and number of people living in the household.
dChildren who tested positive for NoV at enrollment were excluded from the analysis of the prospective follow-up data.
eTo compare the cross-sectional and prospective data, we converted the prevalence of AGE in the cross-sectional datasets into incidence rates using the following formula: Prevalence/(1-Prevalence) = Incidence × Duration of AGE.
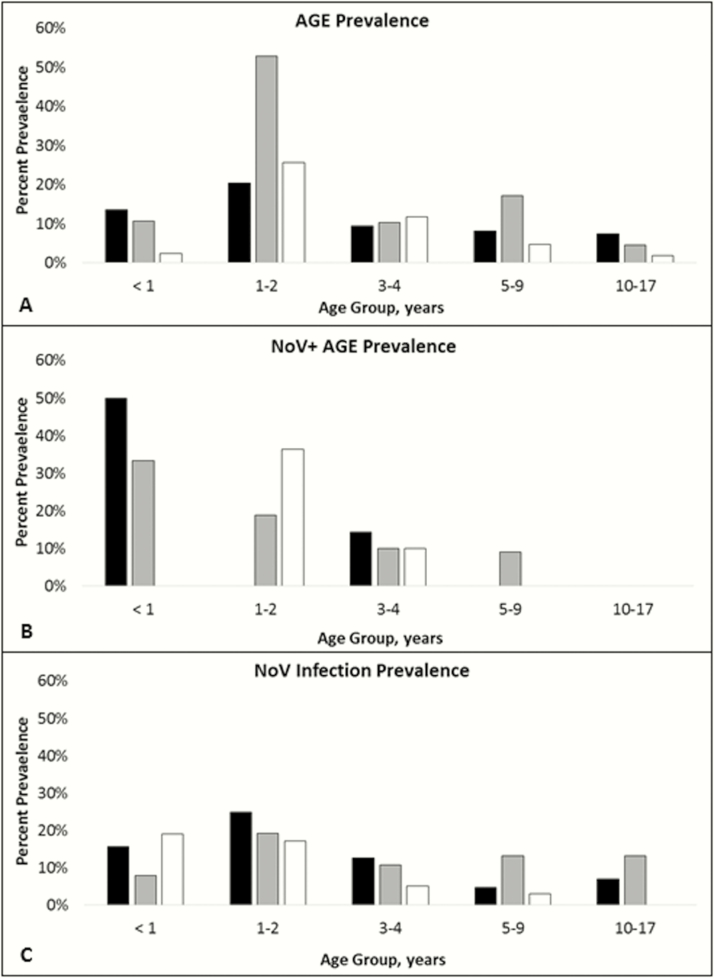
Among children with samples available and positive NoV testing during the PSS enrollment and RAS1 and RAS2, the asymptomatic-to-symptomatic NoV ratio was 6.3:1 vs 3.3:1 and 3.0:1, respectively (P = .29). The overall PAF of AGE adjusted for age and gender was 4.4% (range, −1.6% to 12.4%) with the greatest PAF observed during RAS2 (P < .001). When stratified by age, the prevalence of AGE, NoV+ AGE, and NoV infection was greatest in younger children (Figure 2). Reported AGE episodes were of greater severity according to the rapid Vesikari score in the prospective PSS cohort compared with the 3 cross-sectional datasets (8.1 vs 7.3, P = .005) (Table 2). After converting prevalence data to incidence, the cross-sectional groups (PSS enrollment, RAS1, and RAS2) detected significantly greater incidence rates than the PSS prospective follow-up period for both AGE (PSS enrolment = 858/100 PY, RAS1 = 1316/100 PY, and RAS2 = 697/100 PY; PSS prospective follow up = 11.4/100 PY; P < .001) and NoV+ AGE (PSS enrollment = 83/100 PY, RAS1 = 154/100 PY, and RAS2 = 131/100 PY; PSS prospective follow up = 1.4/100 PY; P < .001). Excluding PSS subjects who refused stool collection led to AGE and NoV+ AGE incidences of 12.1/100 PY and 1.5/100 PY, respectively.
Figure 2.
Prevalence of acute gastroenteritis (AGE), norovirus (NoV)+ AGE, and NoV infection by age and study group, April 2015 to February 2016. Age-stratified prevalence of AGE (A), AGE with concomitant NoV infection (NoV+ AGE) (B), and NoV infection (symptomatic or asymptomatic) (C). Black: prospective participatory syndromic surveillance cohort; gray: rapid active sampling (RAS) survey 1; white: RAS survey 2.
DISCUSSION
Rapid active sampling surveys were a feasible and effective method to estimate NoV disease burden in a resource-limited region of Guatemala. The ability to perform asymptomatic stool testing allowed estimation of the attributable fraction in this setting where multiple pathogens are frequently encountered within the same AGE episode. Each population-based RAS survey was completed in under 6 weeks and consistently identified a high prevalence of NoV infection, AGE, and NoV+ AGE, and they detected seasonal differences in NoV disease burden over a single year, with the greatest burden identified during Guatemala’s peak NoV season [30]. Although no widely accepted “gold standard” exists for enteric disease surveillance, the RAS surveys identified a higher incidence of AGE and NoV+ AGE than the smartphone-based prospective cohort (PSS), which had a high weekly reporting rate (78%) over the same sampling timeframe and population.
Estimating the burden of AGE attributable to NoV in a population is complicated by prolonged viral shedding, asymptomatic infection (especially in young children), and the presence of copathogens [17, 31, 32]. Other prospective studies have used the PAF to account for “background rates” of enteric pathogens in the study population and to adjust the estimation of each pathogen’s contribution to the overall burden of disease [28, 33]. The rigorous methodology and broad diagnostic testing used by these studies is valuable in characterizing overall causality of AGE in LMICs, but their expense and required infrastructure make them impractical. Rapid active sampling surveys, which use a WHO-adapted randomized 2-stage cluster sampling strategy, offer a feasible and affordable alternative.
Rapid active sampling burden of disease estimates were similar to those reported from other studies in LMICs. The 2 RAS surveys identified NoV in 17% (range, 14%–21%) of AGE episodes (all levels of severity and ages) and provided a PAF estimate of 7.6% (range, 3.2%–12.4%). A prior clinic-based enteric surveillance study in Guatemala, which enrolled all ages, identified NoV in 23% of AGE episodes from November to January and 11% of AGE episodes from February to October [30]. The Malnutrition and Enteric Disease (MAL-ED) Study detected NoV in 23.5% of AGE episodes (range, 7.1%–32.8%) and 19% of asymptomatic stool samples (range, 2.2%–30.4%), for an overall adjusted PAF of 5.1% [31, 32]. The Global Enteric Multicenter Study (GEMS) study and several meta-analyses also report similar NoV prevalence and PAF to our study [2, 17, 34–36].
These findings support the utility of RAS surveys in estimating NoV disease burden in resource-limited settings, allowing public health officials to quickly ascertain local prevalence data with limited public resources, especially as an alternative to passive surveillance and more expensive prospective active surveillance. Rapid active sampling surveys may also be useful to quickly and cost-effectively identify NoV vaccine trial or intervention sites, and, when serially repeated, they may be useful to estimate population-level vaccine impact, as they have after hepatitis A [37] Streptococcus pneumoniae [38] and Haemophilus influenzae type b [39] vaccination programs.
Although incidence rates provide a better estimate of the population risk of new infections and disease, our prospective PSS surveillance did not detect as many AGE and NoV cases despite a weekly symptom diary reporting rate of 78%, resulting in a lower incidence rate compared with other prospective studies [9, 31, 40–43]. This may be due to a greater number of older children in our study (mean age = 7.3 years) compared with others, which typically only enroll children <5 years. Reporting bias may have also played a role because individuals were more likely to report an episode of AGE meeting a more stringent case definition during cross-sectional RAS surveys, which asked about symptoms in the preceding week, than during the prospective PSS surveillance period, which sought to identify an ongoing episode of AGE. This is supported by the finding that AGE episodes identified during the prospective PSS period were significantly more severe than the AGE episodes reported during PSS enrollment and the 2 RAS surveys. In addition, individuals may have underreported milder AGE episodes to avoid rectal swab testing, which was a concern to some parents, and acceptance rates of rectal swabs did decrease over the course of the PSS study.
Our study had several limitations. Data collection was limited to a single year in a specific population, and it did not include testing for additional pathogens beyond NoV. We sought to limit sampling bias by performing population-based, randomized cluster sampling within the same catchment area for each study group. Nonetheless, there were some baseline differences in characteristics between study groups, and we adjusted for these differences when feasible. The calculations of incidence densities of NoV infection in the 3 surveys were not adjusted for the seasonality of NoV, due to the small numbers of NoV infection that were identified throughout the year of observation. Our case definition, which included ≥3 days of vomiting or diarrhea, was stricter than other studies, making comparisons more difficult. The stool sample collection rate was lower during the prospective observation period compared with the cross-sectional studies, which was improved by also allowing sampling of fresh stool samples, and may have been improved further by incorporating more rapid diagnostic testing. This was one of the first smartphone-based prospective surveillance studies performed in a LMIC, and, despite training of parents and the high levels of response attained, much needs to be learned on how parents can best report symptoms using this new technology compared with the standard household visit [44, 45].
CONCLUSIONS
In summary, RAS surveys were shown to be an innovative, low-cost, and effective method to quickly estimate the burden of NoV infection and disease in a resource-limited setting. The estimated NoV prevalence and PAF that we observed in the RAS surveys were similar to those previously reported in Guatemala and other multisite studies in LMICs. Future studies should replicate the use of RAS surveys in other settings and for other vaccine preventable diseases, because they offer a promising tool to measure changes in the burden of disease after the introduction preventive interventions, such as NoV vaccine.
Acknowledgments
Author contributions. Study design, study execution strategy, and interpretation of data were done by D. O., M. M. L., C. C.-R., and E. J. A. Data acquisition was coordinated by M. A. P.-A., A. Z., D. O., M. M. L., and E. J. A. under the auspices of Integra (Bogota, Colombia). Laboratory analysis and interpretation were coordinated by M. R. L. and C. C.-R. Data analysis and report generation were performed by M. M. L., D. O., and E. J. A. Preparation of the manuscript was led by D. O., M. M. L., and E. J. A. All authors reviewed and approved the final version.
We thank the following personnel for their significant contributions to this research: CU Trifinio Research Team, including Neudy Rojop, Andrea Chacon, and Carlos Alvarez Guillen; Mirsa Ariano and Erick Mollinedo (Universidad del Valle de Guatemala); and Ricardo Zambrano-Perilla and Sergio Ricardo Rodríguez-Castro (Integra IT Colombia). We also thank Kathryn Edwards for review and comments to our manuscript.
Disclaimer. The contents are the authors’ sole responsibility and do not necessarily represent an official view from the National Institutes of Health (NIH).
Financial support. This study was funded by an Investigator-Initiated Sponsored Research Grant from Takeda Pharmaceuticals (IISR-2014-100647). D. O. is supported by NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Sciences Institute Grant Number UL1 TR001082 and the Children’s Hospital of Colorado Research Scholar Award.
Potential conflicts of interest. E. J. A. has served on an Advisory Board for Takeda Vaccines Inc. and is partially supported by research grants from GlaxoSmithKline Biologicals and Takeda Vaccines Inc. M. M. L. is partially supported by grants from GlaxoSmithKline Biologicals and Pantheryx Inc. D. O. is partially supported a grant from Takeda Vaccines Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pires SM, Fischer-Walker CL, Lanata CF, et al. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS One 2015; 10:e0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopman BA. Global Burden of Norovirus and Prospects for Vaccine Development: Centers for Disease Control and Prevention, 2015. Available at: https://www.cdc.gov/norovirus/downloads/global-burden-report.pdf. Accessed 5 October 2016. [Google Scholar]
- 4. Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med 2016; 13:e1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. [DOI] [PubMed] [Google Scholar]
- 6. Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757–63. [DOI] [PubMed] [Google Scholar]
- 7. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 8. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. [DOI] [PubMed] [Google Scholar]
- 9. Lopman BA, Trivedi T, Vicuña Y, et al. Norovirus infection and disease in an ecuadorian birth cohort: association of certain norovirus genotypes with host FUT2 secretor status. J Infect Dis 2015; 211:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernstein DI, Atmar RL, Lyon GM, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 2015; 211:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riddle MS, Walker RI. Status of vaccine research and development for norovirus. Vaccine 2016; 34:2895–9. [DOI] [PubMed] [Google Scholar]
- 12. Atmar RL, Baehner F, Cramer JP, et al. Rapid responses to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: a randomized controlled trial. J Infect Dis 2016; 214:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu D, Yen C, Yin ZD, et al. The public health burden of rotavirus disease in children younger than five years and considerations for rotavirus vaccine introduction in China. Pediatr Infect Dis J 2016; 35:e392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glass RI, Kilgore PE, Holman RC, et al. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis 1996; 174(Suppl 1):S5–11. [DOI] [PubMed] [Google Scholar]
- 15. Patel MM, Tate JE, Selvarangan R, et al. Routine laboratory testing data for surveillance of rotavirus hospitalizations to evaluate the impact of vaccination. Pediatr Infect Dis J 2007; 26:914–9. [DOI] [PubMed] [Google Scholar]
- 16. Glass RI, Bresee JS, Turcios R, et al. Rotavirus vaccines: targeting the developing world. J Infect Dis 2005; 192(Suppl 1):S160–6. [DOI] [PubMed] [Google Scholar]
- 17. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 18. Bahl S, Estívariz CF, Sutter RW, et al. Cross-sectional serologic assessment of immunity to poliovirus infection in high-risk areas of northern India. J Infect Dis 2014; 210(Suppl 1):S243–51. [DOI] [PubMed] [Google Scholar]
- 19. Lazcano-Ponce E, Conde-Gonzalez C, Rojas R, et al. Seroprevalence of hepatitis A virus in a cross-sectional study in Mexico: implications for hepatitis A vaccination. Hum Vaccin Immunother 2013; 9:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Lu P, Hu Y, et al. Cross-sectional surveys of measles antibodies in the Jiangsu Province of China from 2008 to 2010: the effect of high coverage with two doses of measles vaccine among children. PLoS One 2013; 8:e66771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asturias EJ, Heinrichs G, Domek G, et al. The center for human development in Guatemala: an innovative model for global population health. Adv Pediatr 2016; 63:357–87. [DOI] [PubMed] [Google Scholar]
- 22. Singh J, Jain DC, Sharma RS, Verghese T. Evaluation of immunization coverage by lot quality assurance sampling compared with 30-cluster sampling in a primary health centre in India. Bull World Health Organ 1996; 74:269–74. [PMC free article] [PubMed] [Google Scholar]
- 23. D’Ardenne KK, Darrow J, Furniss A, et al. Use of rapid needs assessment as a tool to identify vaccination delays in Guatemala and Peru. Vaccine 2016; 34:1719–25. [DOI] [PubMed] [Google Scholar]
- 24. Lanata CF, Black RE. Lot quality assurance sampling techniques in health surveys in developing countries: advantages and current constraints. World Health Stat Q 1991; 44:133–9. [PubMed] [Google Scholar]
- 25. Goldfarb DM, Steenhoff AP, Pernica JM, et al. Evaluation of anatomically designed flocked rectal swabs for molecular detection of enteric pathogens in children admitted to hospital with severe gastroenteritis in Botswana. J Clin Microbiol 2014; 52:3922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arvelo W, Hall AJ, Estevez A, et al. Diagnostic performance of rectal swab versus bulk stool specimens for the detection of rotavirus and norovirus: implications for outbreak investigations. J Clin Virol 2013; 58:678–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trujillo AA, McCaustland KA, Zheng DP, et al. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol 2006; 44:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blackwelder WC, Biswas K, Wu Y, et al. Statistical methods in the Global Enteric Multicenter Study (GEMS). Clin Infect Dis 2012; 55(Suppl 4):S246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schnadower D, Tarr PI, Gorelick MH, et al. Validation of the modified Vesikari score in children with gastroenteritis in 5 US emergency departments. J Pediatr Gastroenterol Nutr 2013; 57:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Estévez A, Arvelo W, Hall AJ, et al. Prevalence and genetic diversity of norovirus among patients with acute diarrhea in Guatemala. J Med Virol 2013; 85:1293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rouhani S, Penataro Yori P, Paredes Olortegui M, et al. Norovirus infection and acquired immunity in 8 countries: results from the MAL-ED study. Clin Infect Dis 2016; 62:1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Platts-Mills JA, McCormick BJ, Kosek M, et al. Methods of analysis of enteropathogen infection in the MAL-ED Cohort Study. Clin Infect Dis 2014; 59(Suppl 4):S233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu J, Platts-Mills JA, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016; 388:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Ryan M, Riera-Montes M, Lopman B. Norovirus in Latin America: systematic review and meta-analysis. Pediatr Infect Dis J 2017; 36:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mans J, Armah GE, Steele AD, Taylor MB. Norovirus epidemiology in Africa: a review. PLoS One 2016; 11:e0146280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayorga Perez O, Brinkhof MW, Egger M, et al. Decreasing risk of hepatitis A infection in León, Nicaragua: evidence from cross-sectional and longitudinal seroepidemiology studies. PLoS One 2014; 9:e87643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roca A, Hill PC, Townend J, et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med 2011; 8:e1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hammitt LL, Crane RJ, Karani A, et al. Effect of Haemophilus influenzae type b vaccination without a booster dose on invasive H influenzae type b disease, nasopharyngeal carriage, and population immunity in Kilifi, Kenya: a 15-year regional surveillance study. Lancet Glob Health 2016; 4:e185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Brien SJ, Donaldson AL, Iturriza-Gomara M, Tam CC. Age-specific incidence rates for norovirus in the community and presenting to primary healthcare facilities in the United Kingdom. J Infect Dis 2016; 213(Suppl 1):S15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zambruni M, Luna G, Silva M, et al. High prevalence and increased severity of norovirus mixed infections among children 12-24 months of age living in the suburban areas of Lima, Peru. J Pediatric Infect Dis Soc 2016; 5:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Becker-Dreps S, Bucardo F, Vilchez S, et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: a prospective, population-based study in Nicaragua. Pediatr Infect Dis J 2014; 33:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saito M, Goel-Apaza S, Espetia S, et al. Multiple norovirus infections in a birth cohort in a Peruvian periurban community. Clin Infect Dis 2014; 58:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marcano Belisario JS, Jamsek J, Huckvale K, et al. Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database Syst Rev 2015; MR000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wójcik OP, Brownstein JS, Chunara R, Johansson MA. Public health for the people: participatory infectious disease surveillance in the digital age. Emerg Themes Epidemiol 2014; 11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]