Abstract
The typical environmental enrichment (EE) paradigm, which consists of continuous exposure after experimental traumatic brain injury (TBI), promotes behavioral and histological benefits. However, rehabilitation is often abbreviated in the clinic and administered in multiple daily sessions. While recent studies have demonstrated that a once daily 6-hr bout of EE confers benefits comparable to continuous EE, breaking the therapy into two shorter sessions may increase novelty and ultimately enhance recovery. Hence, the aim of the study was to test the hypothesis that functional and histological outcomes will be significantly improved by daily preclinical neurorehabilitation consisting of two 3-hr periods of EE vs. a single 6-hr session. Anesthetized adult male rats received a controlled cortical impact of moderate-to-severe injury (2.8 mm tissue deformation at 4m/s) or sham surgery and were then randomly assigned to groups receiving standard (STD) housing, a single 6-hr session of EE, or two 3-hr sessions of EE daily for 3 weeks. Motor function (beam-balance/traversal) and acquisition of spatial learning/memory retention (Morris water maze) were assessed on post-operative days 1–5 and 14–19, respectively. Cortical lesion volume was quantified on day 21. Both EE conditions improved motor function and acquisition of spatial learning, and reduced cortical lesion volume relative to STD housing (p < 0.05), but did not differ from one another in any endpoint (p > 0.05). The findings replicate previous work showing that 6-hr of EE daily is sufficient to confer behavioral and histological benefits after TBI and extend the findings by demonstrating that the benefits are comparable regardless of how the 6-hrs of EE are accrued. The relevance of the finding is that it can be extrapolated to the clinic and may benefit patients who cannot endure a single extended period of neurorehabilitation.
Keywords: behavioral outcome, controlled cortical impact, environmental enrichment, functional recovery, learning and memory, Morris water maze, traumatic brain injury
Introduction
The World Health Organization predicts that traumatic brain injuries (TBI) will become the leading cause of death and disability by the year 2020 (Hyder et al., 2007). Through many convoluted mechanisms including both the injury caused by the primary initial impact and the damaging secondary and tertiary pathophysiological cascades that follow (Kline et al., 2016; Adelson et al., 1998; Bayir et al., 2009; Bramlett et al., 2016; Carlson et al., 2017), TBIs induce a wide variety of long-lasting symptoms including motor deficiencies (Kline et al., 1994; Blaya et al., 2014; Shear et al., 2015), disrupted cognitive function (Horneman and Emanuelson, 2009; Levin et al., 2010; Barry and Tomes, 2015; Richter et al., 2015), and fatigue (LaChapelle and Finlayson, 1998). While public awareness has increased regarding the dangers of TBI, the affective and economic costs of TBIs remain astronomical and effective treatment strategies are scarce (Doppenberg et al., 2004; Menon, 2009). However, promoting recovery of motor and cognition and managing fatigue continue to be primary concerns of health specialists as they strive to assimilate patients back into society.
Neurorehabilitation and its rodent analogue environmental enrichment (EE) have both been successful in conferring robust benefits after TBI (Chua et al., 2007, de Witt et al., 2011, Bondi et al., 2014; Radabaugh et al., 2016; Hart et al., 2016). Clinical rehabilitative treatments employ a transdisciplinary team of specialists to target multiple different aspects of each unique injury resulting in the patient being exposed to a wide variety of stimuli (Chua et al., 2007). However, with 29% to 47% of TBI patients reporting abnormal levels of fatigue within the first month after injury (Minderhoud et al., 1980), longer rehabilitation sessions may prove to be less productive as the fatigue becomes distracting or unbearable (Keshaven et al., 1981, LaChapelle and Finlayson 1998). Hence, multiple shorter sessions of rehabilitation may ease fatigue and afford more productive rehabilitation efforts as well as increased interaction with the stimuli.
EE mimics many of the stimuli patients are exposed to in the clinic by encouraging voluntary exercise, increasing social interaction, and providing cognitive stimulation. While the stimuli are analogous to the clinic, the typically used paradigm, which consists of continuous exposure after TBI (Radabaugh et al., 2016, de Witt et al., 2011; Monaco et al., 2014; Sozda et al., 2010; Kline et al., 2007; Bondi et al., 2014, 2015) is quite dissimilar as patients do not receive rehabilitation on a continual basis. Previous studies have observed the effects of abbreviated EE after experimental TBI and reported that both male (de Witt et al., 2011) and female (Radabaugh et al., 2016) rats benefit from 6-hrs of EE exposure per day. Indeed, the benefits conferred by the 6-hr EE paradigm are not significantly different from the continuous approach. While patients in the clinic may spend up to 6-hrs participating in neurorehabilitation each day, it is often in multiple, shorter sessions that target different aspects of the recovery process (i.e., speech therapy, vocational therapy, exercise) (Chua et al., 2007). Therefore, progressive experimental manipulations to the typical EE paradigm could continue to refine EE and advance rehabilitation based research.
This study aimed to evaluate the effects generated by two 3-hr sessions of EE exposure each day compared to one 6-hr session. We hypothesized that the group receiving two shorter sessions of EE per day would recover more fully, as measured by well-established motor and cognitive tasks, than the one receiving one 6-hr session of EE. The rationale for the hypothesis is that rats placed into the EE cage twice per day and given a rest period will not be as negatively impacted by fatigue and may acclimate less to the environment thus promoting more interaction with the “novel” stimuli when reintroduced for the second session. This increased amount of engagement could provide further benefit from the rehabilitation and potentially lead to a greater degree of cognitive flexibility and motor ability. Support for this thesis comes from the work of Sozda and colleagues (2010) who showed that atypical EE groups, which had one of the critical components of EE (i.e., physical space, cognitive stimuli, or social interaction) excluded, did not recover to the same degree as those in the typical EE groups, indicating that exploration and engagement are important.
Materials and methods
Subjects and pre-surgical procedures
Fifty-one adult male Sprague-Dawley rats (Harlan, Indianapolis, Indiana) were paired in ventilated polycarbonate rat cages and maintained in a temperature (21 ± 1°C) and light (on 0700-1900 h) controlled environment with food and water available ad libitum. During the week of acclimatization the rats were pre-trained on the beam-walk task to ensure that they could traverse the entire length of the beam effortlessly, typically in under 5 sec. On the morning of surgery, the rats were pre-assessed on the beam-balance and beam-walk tasks to determine baseline performance.
Surgery and acute neurological evaluation
Rats weighing 300–325 g on the day of surgery were subjected to a controlled cortical impact (CCI) injury as previously described (Dixon et al., 1991, Kline et al., 2000, 2007, 2010, 2012; Hoffman et al., 2008; Bondi et al., 2014; Leary et al., 2017). Briefly, a surgical level of anesthesia was induced with 4% isoflurane in 2:1 N2O:O2 and then the rats underwent an endotracheal intubation and secured in a stereotaxic frame and ventilated mechanically. The surgical plane of anesthesia was maintained with 2% isoflurane. Core temperature was maintained at 37 ± 0.5°C with a heating pad. Utilizing aseptic procedures a midline scalp incision was made, the skull was exposed, and a craniectomy (6-mm in diameter) was made in the right hemisphere with a hand held trephine. The bone flap was removed and the craniectomy was enlarged further to accommodate the impact tip (6 mm, flat), which was centered and lowered through the craniectomy until it touched the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a moderate-to-severe brain injury (2.8 mm tissue deformation at 4 m/sec). Anesthesia was discontinued immediately after the impact and the incision was promptly closed. Once sutured, the rats were extubated and assessed for acute neurological outcome. Sham rats underwent all surgical procedures, except the impact.
Acute neurological evaluation
Hind limb reflexive ability was assessed immediately following the cessation of anesthesia and removal from the stereotaxic apparatus by gently squeezing the rats’ paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to prone position on three consecutive trials. These neurological indices are used to determine the level of injury severity (Kline et al., 2007, 2010; Hoffman et al., 2008; Monaco et al., 2013, 2014). Following the acute neurological assessment the rats were randomly assigned to three TBI (n=12 per condition) and three sham control (n=5 per condition) groups.
Housing conditions: standard and environmental manipulation
After the effects of surgical anesthesia abated (as evidenced by spontaneous movement in the holding cage), all the rats were returned to the colony and placed in typical laboratory shoebox cages (37×25×18 cm, 2 rats per cage) with ad libitum food and water. Beginning at 9:00 a.m. the following morning, the rats randomized to a single 6-hr enrichment session were removed from the STD cages and placed in the EE cages until 3:00 p.m., at which time they were returned to STD housing. The rats assigned to two 3-hr sessions were also removed from STD conditions at 9:00 a.m. and returned to the STD cages at 12:00 p.m. where they remained until 3:00 p.m. and then placed back into enrichment until 6:00 p.m. These rehabilitative manipulations occurred each day for 21 days. Briefly, the EE is a specifically designed steel-wire cage (91×76×50 cm) that consists of three levels with ladders to ambulate from one level to another and contains various toys (e.g., balls, blocks, and tubes), nesting materials (e.g., paper towels), and ad libitum food and water (Kline et al., 2007; Sozda et al., 2010). Ten to twelve rats, which included TBI and sham controls, were housed in the EE together to minimize variability among the groups.
Motor performance
Well-established beam-balance and beam-walk tasks were utilized to assess motor function (Kline et al., 2010, 2012; Cheng et al., 2008, 2012, 2016). Briefly, the beam-balance task consisted of placing the rat on an elevated narrow beam (1.5 cm wide) and recording the time it remained on for a maximum of 60 sec. The beam-walk test consisted of recording the elapsed time to traverse the beam (2.5 cm wide × 100 cm long). Baseline performance was assessed 1 hr prior to surgery. Following surgery, testing was conducted on days 1–5, and consisted of three trials (60 sec allotted time with an inter-trial interval of 30 sec) per day on each task. The average daily scores for each subject were used in the statistical analyses.
Cognitive function: acquisition of spatial learning
Spatial learning was assessed in a Morris water maze task (Morris 1984) that has been shown to be sensitive to cognitive function after TBI (Kline et al., 2002a,b, 2007, 2010, 2012; Olsen et al., 2012; Hamm et al., 1996; Lyeth et al., 1990; Bramlett et al., 2016). Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) positioned 26 cm from the maze wall in the southwest quadrant. Training began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the submerged platform (2 cm below the water surface). On day 19 the platform was raised 2 cm above the water line and marked with white tape, which made it visible to the rats and served as a control procedure to determine the contributions of non-spatial factors (e.g., sensory-motor performance, motivation, and visual acuity) on cognitive performance. For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the escape platform within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
Cognitive function: probe trial (memory retention)
On day 19 post-injury, the rats were subjected to a probe trial to test their memory retention. Briefly, the test consisted of a single 30-sec trial where the platform was removed and the rat was allowed to swim freely throughout the pool. The percentage of time spent in the quadrant where the platform was situated on days 14–18 (i.e. target quadrant) was recorded using a spontaneous motor activity and tracking system. Swim speed was also recorded during this testing period. The percent time spent in the target quadrant was used in the statistical analysis.
Histology: cortical lesion volume
After completion of behavioral testing (i.e., day 21), the rats were anesthetized with Fatal-Plus® (0.25 ml, intraperitoneally) and then perfused transcardially with 200 mL 0.1M phosphate-buffered saline (pH 7.4), followed by 300mL 4% paraformaldehyde (PFA). The brains were extracted, post-fixed in 4% PFA for 1 week, dehydrated with alcohols, and embedded in paraffin. Seven-micrometer-thick coronal sections were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on Superfrost®/Plus glass microscope slides. After drying at room temperature, the sections were deparaffinized in xylenes, rehydrated, and stained with cresyl violet. Cortical lesion volumes (mm3) were assessed by an observer blinded to experimental conditions using a Nikon Eclipse 90i microscope (Nikon Corporation, Tokyo, Japan). The area of the lesion (mm2) was first calculated by outlining the inferred area of missing cortical tissue for each section (typically 5–7) (Nikon NIS-Elements AR 3.22.14 software Nikon Corp, Tokyo, Japan), and then by summing the lesions obtained, as previously reported (Monaco et al., 2013, 2014; Olsen et al., 2012).
All experimental procedures were preapproved by the University of Pittsburgh’s Institutional Animal Care and Use Committee. All efforts were made to limit the number of rats used as well as to minimize their discomfort.
Data analysis
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statview 5.0.1 software. The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). Acute neurological assessments, swim speed, visible platform performance, and cortical lesion volumes were analyzed by one-factor ANOVAs. When the overall ANOVAs revealed a significant effect, the Newman-Keuls post-hoc test was employed to determine specific group differences. The data are presented as the mean ± standard error of the mean (S.E.M.) and are considered significant when corresponding p values are ≤ 0.05.
Results
There were no significant differences in any assessment among the sham control groups, regardless of housing condition, and thus the data were pooled into a single SHAM group.
Acute neurological evaluation
No significant differences were observed among the TBI groups for return of hind limb reflex ability after a brief paw pinch (range for right: 149.4 ± 2.4 sec to 153.7 ± 3.6 sec; left range = 154.4 ± 2.7 sec to 157.7 ± 3.3 sec) or righting reflex latency (range = 326.50 ± 12.4 sec to 351.1 ± 22.0 sec,) following the termination of anesthesia (p’s > 0.05). The lack of neurological differences among the TBI groups indicates that all rats received similar levels of anesthesia and injury severity.
Motor function: beam-balance
No baseline differences were observed among the groups as all rats were capable of balancing on the beam for the allotted 60 sec (Fig. 1). However, after the TBI, the repeated-measures ANOVA revealed significant Group [F3,47 = 13.891, p < 0.0001] and Day [F5,235 = 63.749, p < 0.0001] differences, as well as a significant Group × Day interaction [F15,235 = 9.28, p < 0.0001]. The post-hoc analysis revealed that all TBI groups were significantly impaired relative to the SHAM group, which was able to balance for the full 60 sec [p’s < 0.05]. Over the course of the 5 days of testing, all TBI groups showed improvement, however, the post-hoc analysis revealed that both of the EE groups recovered significantly faster than the STD group (p’s < 0.05), and did not differ from one another regardless of how EE was accrued (p > 0.05).
Fig. 1.
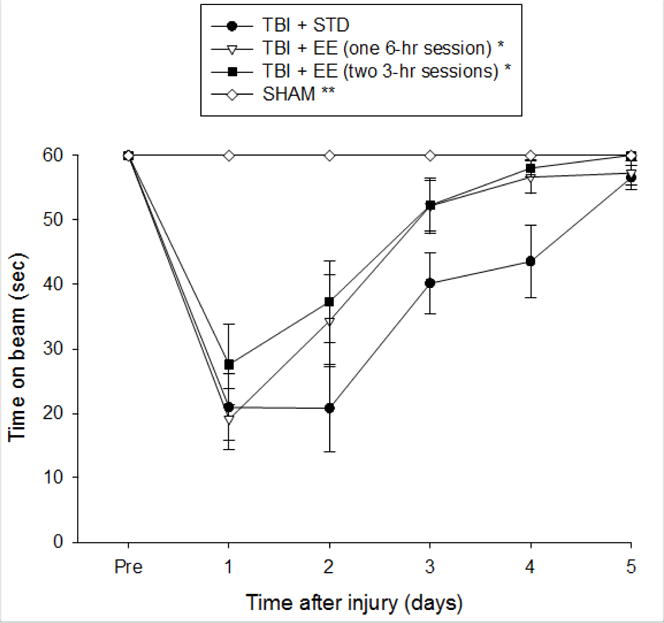
Mean (± S.E.M.) time (sec) balancing on a beam prior to, and after surgery. * p < 0.05 vs. TBI + STD. ** p < 0.05 vs. all TBI groups. No other comparisons were significant (p > 0.05).
Motor assessment: beam-traversal
Prior to surgery, there were no significant differences among any of the groups in the time to fully traverse the beam (Fig. 2). After surgery, the repeated measures ANOVA revealed significant Group [F3, 47 = 70.829, p < 0.0001] and Day [F5,235 = 158.814, p < 0.0001] differences, as well as a significant Group × Day [F15,235 = 20.628, p < 0.0001] interaction. The post-hoc analysis indicated that all TBI groups were significantly impaired compared to the SHAM control [p’s < 0.05]. Moreover, the EE group that was provided two 3-hr sessions was significantly better than the STD group [p < 0.05], but did not differ from the EE group receiving a single 6-hr session [p > 0.05].
Fig. 2.
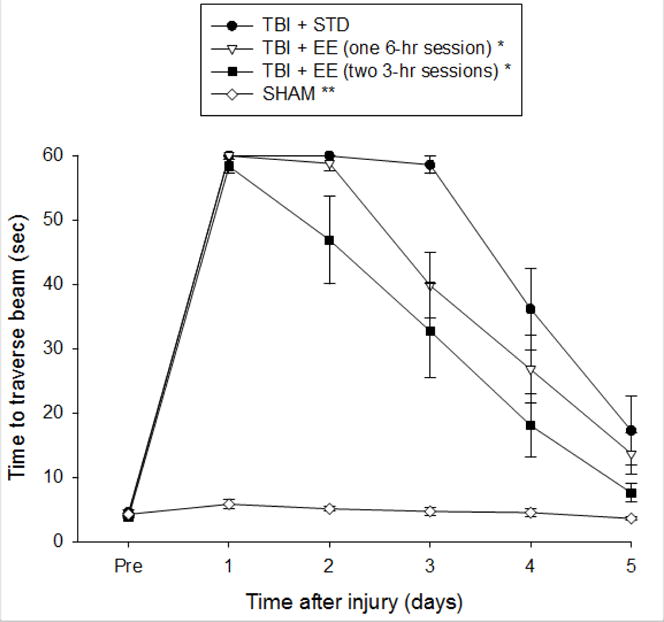
Mean (± S.E.M.) time (sec) to traverse beam after surgery. * p < 0.05 vs. TBI + STD. ** p < 0.05 vs. all TBI groups. No other comparisons were significant (p > 0.05).
Cognitive function: acquisition of spatial learning
Analysis of the spatial learning data revealed significant Group [F3,47 = 20.012, p < 0.0001] and Day [F4,188 = 27.112, p < 0.0001] differences. The post-hoc analysis specified that the SHAM group was significantly better than all TBI groups [p’s < 0.05]. The TBI + EE (one 6-hr session) and TBI + EE (two 3-hr sessions) groups were able to locate the escape platform significantly quicker over time vs. the TBI + STD group [p < 0.05; Fig. 3], and did not differ from one another [p > 0.05]. No significant differences in swim speed (range = 27.9 ± 1.3 cm/sec to 32.2 ± 1.2 cm/sec) or time to reach the visible platform were observed among the TBI or SHAM groups [p > 0.05].
Fig. 3.
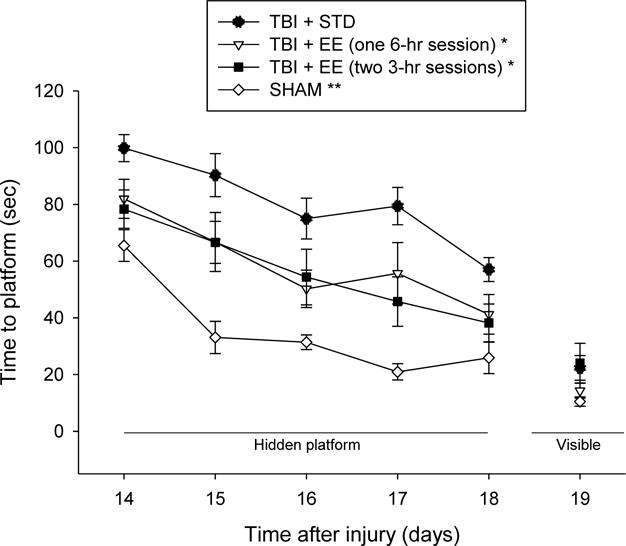
Mean (± S.E.M.) time (sec) to locate a hidden platform in the water maze. * p < 0.05 vs. TBI + STD. ** p < 0.05 vs. all TBI groups. No other comparisons were significant (p > 0.05). No visible platform differences were observed among the groups.
Cognitive function: probe trial
Analysis of the memory retention data revealed a significant Group effect [F3,47 = 9.399, p < 0.0001]. The post-hoc analysis revealed that the SHAM, TBI + EE (one 6-hr session), and TBI + EE (two 3-hr sessions) groups spent a greater percentage of the allotted time in the target quadrant (40.7 ± 1.2 %, 36.7 ± 2.1 %, and 37.7 ± 1.9 %, respectively) vs. the TBI + STD (28.8 ± 1.5 %) group [p’s < 0.05], but did not differ from one another [p’s > 0.05; Fig. 4].
Fig. 4.
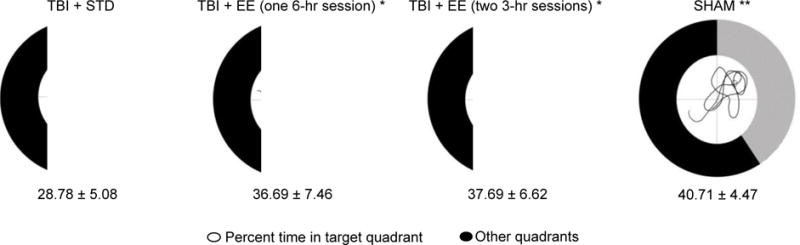
Mean (± S.E.M.) percent time spent in the target quadrant. The lined area shows the percentage of time that each group spent in the target quadrant and the black area represents the percent time spent in the non-target quadrants. The smaller circle in the middle of each chart illustrates a representative swim path of the respective group with the top right quadrant denoting the target. * p < 0.05 vs. TBI + STD. ** p < 0.05 vs. all TBI groups. No other comparisons were significant (p > 0.05).
Histology: quantification of cortical lesion volume
Analysis of the histological data revealed a significant difference among the conditions that was attributed to the TBI + STD group exhibiting a bigger lesion volume (41.8 ± 2.4 mm3) relative to the TBI + EE (one 6-hr session) and TBI + EE (two 3-hr sessions) groups (29.8 ± 3.2 mm3 and 27.3 ± 3.1 mm3, respectively) [p < 0.05]. No difference was revealed between the two EE groups, regardless of how EE was accrued [p > 0.05; Fig. 5].
Fig. 5.
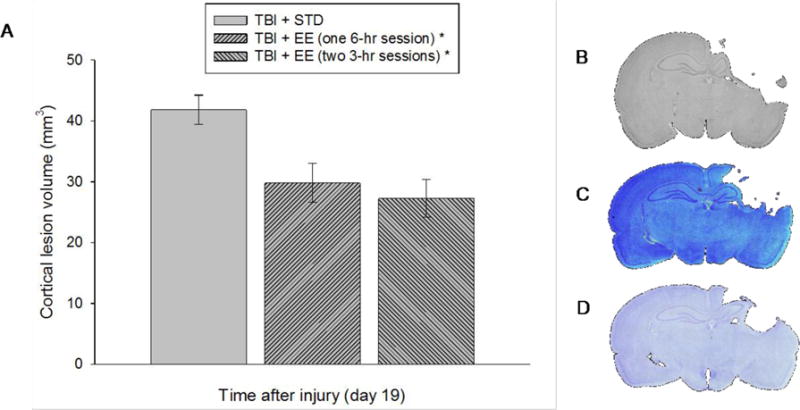
A. Mean (±S.E.M.) cortical lesion volume (mm3) 3 weeks after cortical impact injury. Panels B–D depict average size lesions at the level of the dorsal hippocampus stained with cresyl violet. * p < 0.05 vs. TBI + STD. No other comparisons were significant (p > 0.05).
Discussion
Numerous studies have shown that environmental enrichment (EE), a rodent analogue of rehabilitation, confers significant motor, cognitive, and histological benefits after experimental brain injury in both male and female rodents (Hamm et al., 1996; Passineau et al., 2001; Hicks et al., 2002; Kline et al., 2007; Sozda et al., 2010; de Witt et al., 2011; Bondi et al., 2014; Monaco et al., 2014; Radabaugh et al., 2016; Zeleznikow-Johnston et al., 2017). While the majority of the EE studies have focused on the traditional approach of providing enrichment early and continuously after brain injury, we have steadily been making significant strides in developing EE into a viable preclinical model of neurorehabilitation. We have progressed from a paradigm that does not closely parallel the clinic, to one that comprises some of the crucial clinical facets, such as delayed administration and abbreviated amounts of EE, both of which conform to the rehabilitation model as patients will typically not engage in rehabilitation until discharged from critical care and then will likely receive limited amounts of physiotherapy (Blackerby et al., 1990; Shiel et al., 2001; Zhu et al., 2007; Vanderploeg et al., 2008).
While the changes made thus far provide further support for EE as a preclinical model of neurorehabilitation, there is still much opportunity for refinement. Hence, the goal of the current study was to determine whether breaking up EE into two daily sessions instead of a single longer duration session would provide more robust benefits. We utilized a 6-hr EE paradigm that we have shown to be as beneficial as continuous EE after TBI, and compared that with two 3-hr sessions of EE (morning and afternoon). The rationale was that rats in the dual session rehabilitation program (i.e., EE) would benefit more because they would not acclimate to the EE as quickly and furthermore would re-engage with the environment when placed back after being away for a few hours. Clinically, this break in rehabilitation is to minimize fatigue so that effort does not wane. Experimentally, the renewed and robust interaction with the environment could lead to increased motor activity and a greater cognitive flexibility (De Bartolo et al., 2008; Zeleznikow-Johnston et al., 2017).
The data showed that both EE groups performed significantly better than the STD-housed controls in beam-balance ability, but did not differ from one another. The dual session EE group also performed significantly better on beam-traversal versus the STD-housed rats, but did not differ from the single session EE group. No statistical difference was revealed between the single session EE and STD groups. Moreover, EE, regardless of how it was accrued, also facilitated the acquisition of spatial learning in a well-developed Morris water maze task, relative to the STD controls. Once again, however, there were no differences between the two EE conditions. Mirroring the findings of the neurobehavioral and cognitive tasks, both EE groups had significantly smaller cortical lesions compared to than the STD-housed controls. These findings were observed despite all TBI groups displaying the same acute neurological scores, which is indicative of injury severity (Kline et al., 2007, 2010; Monaco et al., 2013, 2014; Hoffman et al., 2008) and similar baseline performance in the motor tasks. The data replicate previous work from our laboratory showing that abbreviated EE confers significant benefits after TBI (de Witt et al., 2011; Radabaugh et al., 2016), but only partially supports the hypothesis that shorter EE, albeit provided more often would lead to greater outcomes than a single longer session.
The partial support is based on the data from the beam-traversal task, where it appears that a respite from EE in the middle of the day may have restored the novelty of the environment, which translated into greater engagement and subsequent increased motor activity that may have resulted in improved locomotor function. While the rats were unfortunately not observed continuously, it cannot be definitively stated that the rested dual session rats explored the cages more often and longer than the single session rats. Thus, it may be that the statistically significant difference with the dual session EE group compared to the STD and the lack of statistical difference between the single session EE group and the STD condition may be no more than variability among the groups. However, this finding does raise the question of whether humans who are forced more directly by their physiotherapists may indeed benefit more from shorter bouts of rehabilitation due to decreased fatigue and perhaps even boredom with routine exercises. It is also possible that there is an overall maximum benefit that rehabilitation can confer regardless of the novelty of the environment or the amount provided. Indeed, this type of observation is not unique to the experimental setting. A clinical study comparing the outcome of patients in two countries (i.e. Denmark and the United States) with markedly different rehabilitation practices found that recovery was similar amongst patients of comparable injury severities regardless of the intensity of their rehabilitation (Hart et al., 2016).
In conclusion, upon closer inspection of the motor and cognitive data, interesting findings can be appreciated. On both the beam-balance and beam-traversal tasks, the TBI groups receiving EE recovered more rapidly, as noted by significantly better performance on post-operative days 2–4 than the non-enriched STD-housed TBI group. Additionally, both EE groups performed significantly better on the first training day in the water maze task relative to the STD group. These findings suggest that early rehabilitation may lead to an increased ability to learn and perform tasks, which could translate to the clinic as a faster assimilation to normal life. However, many patients experience abnormal levels of fatigue early after injury (Minderhoud et al., 1980; LaChapelle and Finlayson 1998) which could impact the ability to administer and/or successfully complete early rehabilitation. Hence, breaking the treatment into multiple sessions could potentially allow these patients to receive optimal benefits from rehabilitation despite being unable to complete one, long session. Clearly, there is an urgent need to continue to refine the EE paradigm so that it mimics the real world and reinforces translatable research.
Highlights.
Abbreviated EE produces robust cognitive benefits after experimental TBI
Abbreviated EE significantly reduces cortical lesion volume
No differences were observed whether enrichment was accrued in one long or two short sessions
Two short rehabilitation sessions may benefit patients who cannot endure a single extended period.
Acknowledgments
This work was supported, in part, by NIH grants HD069620, HD069620-S1, NS060005, NS084967 (AEK), NS094950, NS099683 (COB), the University of Pittsburgh Physicians /UPMC Academic Foundation, and the UMPC Rehabilitation Institute (COB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelson PD, Whalen MJ, Kochanek PM, Robichaud P, Carlos TM. Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir Suppl. 1998;71:104–106. doi: 10.1007/978-3-7091-6475-4_31. [DOI] [PubMed] [Google Scholar]
- Barry NC, Tomes JL. Remembering your past: the effects of concussion on autobiographical memory recall. J Clin Exp Neuropsychol. 2015;37:994–1003. doi: 10.1080/13803395.2015.1038981. [DOI] [PubMed] [Google Scholar]
- Bayir H, Adelson PD, Wisniewski SR, Shore P, Lai Y, Brown D, Janesko-Feldman KL, Kagan VE, Kochanek PM. Therapeutic hypothermia preserves antioxidant defenses after severe traumatic brain injury in infants and children. Crit Care Med. 2009;37:689–695. doi: 10.1097/CCM.0b013e318194abf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackerby WF. Intensity of rehabilitation and length of stay. Brain Inj. 1990;4:167–173. doi: 10.3109/02699059009026162. [DOI] [PubMed] [Google Scholar]
- Blaya MO, Bramlett HM, Naidoo J, Pieper AA, Dietrich WD. Neuroprotective efficacy of proneurogenic compound after traumatic brain injury. J Neurotrauma. 2014;31:476–486. doi: 10.1089/neu.2013.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Klitsch KC, Leary JB, Kline AE. Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J Neurotrauma. 2014;31:873–888. doi: 10.1089/neu.2014.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Semple BD, Noble-Haeusslein LJ, Osier ND, Carlson SW, Dixon CE, Giza CC, Kline AE. Found in translation: understanding the biology and behavior of experimental traumatic brain injury. Neurosci Biobehav Rev. 2015;58:123–146. doi: 10.1016/j.neubiorev.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD, Dixon CE, Shear DA, Schmid KE, Mondello S, Wang KK, Hayes RL, Povlishock JT, Tortella FC, Kochanek PM. Erythropoietin treatment in traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2016;33:538–552. doi: 10.1089/neu.2015.4116. [DOI] [PubMed] [Google Scholar]
- Carlson SW, Hong Y, Dixon CE. Lithium increases hippocampal SNARE protein abundance after traumatic brain injury. Exp Neurol. 2017;289:55–63. doi: 10.1016/j.expneurol.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Hoffman AN, Zafonte RD, Kline AE. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J Neurotrauma. 2012;29:2684–2688. doi: 10.1089/neu.2012.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Leary JB, Sembhi A, Edwards CM, Bondi CO, Kline AE. 5-hydroxytryptamine1A (5-HT1A) receptor agonists: a decade of empirical evidence supports their use as an efficacious therapeutic strategy for brain trauma. Brain Res. 2016;1640:5–14. doi: 10.1016/j.brainres.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua KS, Ng YS, Yap SG, Bok CW. A brief review of traumatic brain injury rehabilitation. Ann Acad Med Singapore. 2007;36:31–42. [PubMed] [Google Scholar]
- De Bartolo P, Leggio MG, Mandolesi L, Foti F, Ferlazzo F, Petrosini L. Environmental enrichment mitigates the effects of basal forebrain lesions on cognitive flexibility. Neuroscience. 2008;154:444–453. doi: 10.1016/j.neuroscience.2008.03.069. [DOI] [PubMed] [Google Scholar]
- de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghaven PV, Skidmore ER, Kline AE. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous enrichment after traumatic brain injury. Neurorehabil Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Doppenberg EMR, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- Hart T, Whyte J, Poulsen I, Krestensen KS, Nordenbo AM, Chervoneva I, Vaccaro MJ. How do intensity and duration of rehabilitation services affect outcomes from severe traumatic brain injury? A natural experiment comparing health care delivery systems in 2 developed nations. Arch Phys Med Rehabil. 2016;97:2045–2053. doi: 10.1016/j.apmr.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, O’Dell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Zhang L, Atkinson A, Stevenon M, Veneracion M, Seroogy KB. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience. 2002;112:631–637. doi: 10.1016/s0306-4522(02)00104-5. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Cheng JP, Zafonte RD, Kline AE. Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 2008;83:602–607. doi: 10.1016/j.lfs.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneman G, Emanuelson I. Cognitive outcome in children and young adults who sustained a severe and moderate traumatic brain injury 10 years earlier. Brain Inj. 2009;23:907–914. doi: 10.1080/02699050903283239. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–355. [PubMed] [Google Scholar]
- Keshaven MS, Channabasavanna SM, Reddy GN. Post-traumatic psychiatric disturbances: patterns and predictors of outcome. Br J Psychiatry. 1981;138:157–160. doi: 10.1192/bjp.138.2.157. [DOI] [PubMed] [Google Scholar]
- Kline AE, Chen MJ, Tso-Olivas DY, Feeney DM. Methylphenidate treatment following ablation-induced hemiplegia in rat: experience during drug action alters effects on recovery of function. Pharmacol Biochem Behav. 1994;48:773–779. doi: 10.1016/0091-3057(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280:163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- Kline AE, Bolinger BD, Kochanek PM, Carlos TM, Yan HQ, Jenkins LW, Marion DW, Dixon CE. Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res. 2002a;937:22–31. doi: 10.1016/s0006-8993(02)02458-7. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002b;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN. Evaluation of a combined therapeutic regimen of 8-OHDPAT and environmental enrichment after experimental traumatic brain injury. J Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Olsen AS, Sozda CN, Hoffman AN, Cheng JP. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J Neurotrauma. 2012;29:1960–1969. doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: is more better? Progress in Neurobiol. 2016;142:45–67. doi: 10.1016/j.pneurobio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaChapelle DL, Finlayson MA. An evaluation of subjective and objective measures of fatigue in patients with brain injury and healthy controls. Brain Inj. 1998;12:649–659. doi: 10.1080/026990598122214. [DOI] [PubMed] [Google Scholar]
- Leary JB, Bondi CO, LaPorte MJ, Carlson LJ, Radabaugh HL, Cheng JP, Kline AE. The therapeutic efficacy of environmental enrichment and methylphenidate alone and in combination after controlled cortical impact injury. J Neurotrauma. 2017;34:444–450. doi: 10.1089/neu.2016.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, Radaideh M, Wu T, Yallampalli R, Chu Z, Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Max W, Mackenzie EJ, Rice DP. Head injuries: costs and consequences. J Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- Menon DK. Unique challenges in clinical trials in traumatic brain injury. Crit Care Med. 2009;37:S129–S135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- Minderhoud JM, Boelens ME, Huizenga J, Saan RJ. Treatment of minor head injuries. Clin Neurol Neurosurg. 1980;82:127–140. doi: 10.1016/0303-8467(80)90007-4. [DOI] [PubMed] [Google Scholar]
- Monaco CM, Mattiola VV, Folweiler KA, Tay JK, Yelleswarapu NK, Curatolo LM, Matter AM, Cheng JP, Kline AE. Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp Neurol. 2013;247:410–418. doi: 10.1016/j.expneurol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco CM, Gebhardt KM, Chlebowski SM, Shaw KE, Cheng JP, Henchir JJ, Zupa MF, Kline AE. A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J Neurotrauma. 2014;31:1934–1941. doi: 10.1089/neu.2014.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Olsen AS, Sozda CN, Cheng JP, Hoffman AN, Kline AE. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A receptor agonist buspirone. J Neurotrauma. 2012;29:1898–1907. doi: 10.1089/neu.2012.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passineau MJ, Green EJ, Dietrich WD. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp Neurol. 2001;168:373–384. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- Radabaugh HL, Carlson LJ, O’Neil DA, LaPorte MJ, Monaco CM, Cheng JP, de la Tremblaye PB, Lajud N, Bondi CO, Kline AE. Abbreviated environmental enrichment confers neurobehavioral, cognitive, and histological benefits in brain-injured female rats. Exp Neurol. 2016;286:61–68. doi: 10.1016/j.expneurol.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KM, Mödden C, Hanken K, Hildebrant H. Recovery after brain damage: is there any indication for generalization between different cognitive functions? J Clin Exp Neuropsychol. 2015;37:571–580. doi: 10.1080/13803395.2015.1030358. [DOI] [PubMed] [Google Scholar]
- Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Shear DA, Dixon CE, Bramlett HM, Mondello S, Dietrich WD, Deng-Bryant Y, Schmid KE, Wang KKW, Hayes RL, Povlishock JT, Kochanek PM, Tortella FC. Nicotinamide treatment in traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2015;33:523–537. doi: 10.1089/neu.2015.4115. [DOI] [PubMed] [Google Scholar]
- Shiel A, Burn JP, Henry D, Clark J, Wilson BA, Burnett ME, McLellan DL. The effects of increased rehabilitation therapy after brain injury: results of a prospective controlled trial. Clin Rehabil. 2001;15:501–514. doi: 10.1191/026921501680425225. [DOI] [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on function and histological outcome after experiment traumatic brain injury. J Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Schwab K, Walker WC, Fraser JA, Sigford BJ, Date ES, Scott SG, Curtiss G, Salazar AM, Warden DL. Defense and Veterans Brain Injury Center Study. Rehabilitation of traumatic brain injury in active duty military personnel and veterans: Defense and Veterans Brain Injury Center randomized controlled trial of two rehabilitation approaches. Arch Phys Med Rehabil. 2008;89:2227–2238. doi: 10.1016/j.apmr.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Zeleznikow-Johnston A, Burrows EL, Renoir T, Hannan AJ. Environmental enrichment enhances cognitive flexibility in C57BL/6 on a touch screen reversal learning task. Neuropharmacology. 2017;117:219–226. doi: 10.1016/j.neuropharm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Zhu XL, Poon WS, Chan CC, Chan SS. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial Brain Inj. 2007;21:681–690. doi: 10.1080/02699050701468941. [DOI] [PubMed] [Google Scholar]


