Abstract
Background
The presence of morphometric abnormalities of the lateral ventricles, which can reflect focal or diffuse atrophic changes of nearby brain structures, is not well characterized in methamphetamine dependence. The current study was aimed to examine the size and shape alterations of the lateral ventricles in methamphetamine-dependent subjects.
Methods
High-resolution brain structural images were obtained from 37 methamphetamine-dependent subjects and 25 demographically matched healthy individuals. Using a combined volumetric and surface-based morphometric approach, the structural variability of the lateral ventricles, with respect to extent and location, was examined.
Results
Methamphetamine-dependent subjects had an enlarged right lateral ventricle compared with healthy individuals. Morphometric analysis revealed a region-specific pattern of lateral ventricular expansion associated with methamphetamine dependence, which was mainly distributed in the areas adjacent to the ventral striatum, medial prefrontal cortex, and thalamus.
Conclusions
Patterns of shape decomposition in the lateral ventricles may have relevance to the structural vulnerability of the prefrontal-ventral striatal-thalamic circuit to methamphetamine-induced neurotoxicity.
Keywords: Methamphetamine, Lateral ventricle, Surface-based morphometry, Neuroimaging
1. Introduction
Multiple lines of preclinical evidence suggest that chronic methamphetamine use may cause a series of cellular effects that are local to dopamine and serotonin synapses (Kuczenski et al., 2007; Ricaurte et al., 1980) and eventually lead to neurotoxic damages resulting from the pathological mechanisms that include oxidative stress (Cubells et al., 1994) and mitochondrial dysfunction (Wu et al., 2007). These cellular changes may be associated with macroscopic structural abnormalities of both cortical and subcortical brain regions in methamphetamine-dependent subjects (Berman et al., 2008; Chang et al., 2007; Salo and Fassbender, 2012).
Enlargement of the lateral ventricles has been identified as a surrogate marker of cerebral atrophic changes in psychiatric illness including Alzheimer’s dementia (Frisoni et al., 2010; Nestor et al., 2008), schizophrenia (Kempton et al., 2010; Wright et al., 2000), and substance dependence (Pfefferbaum et al., 2001; Thompson et al., 2004; Wobrock et al., 2009). A recent trend to assess morphometric variability of the lateral ventricles has enabled the detection of more subtle changes in shape composition, which could be imperceptible upon volumetric measurement (Styner et al., 2005; Apostolova et al., 2012; Jackson et al., 2011). Further, specific anatomical information with respect to regional changes in the lateral ventricles by surface-based morphometric analysis may also permit evaluation of structural change in surrounding brain structures (Apostolova et al., 2012; Jackson et al., 2011). However, to the best of our knowledge, structural changes in the lateral ventricles have not been studied in methamphetamine-dependent subjects in terms of shape deformation. In this study, we assessed volume differences in the lateral ventricles between cohorts of methamphetamine-dependent subjects and healthy individuals. By using a three-dimensional surface-based morphometric approach, we further evaluated the location of methamphetamine use-related structural abnormalities of the lateral ventricles. Based on previous knowledge of aging-dependent ventricular enlargement (Pfefferbaum et al., 2004) we also examined whether aging effects might accelerate the localized ventricular shape alterations associated with methamphetamine dependence.
2. Methods
2.1. Participants
Study participants were 37 methamphetamine-dependent subjects (mean age=34.8 years, range = 19.0–46.9 years, hereafter referred to as the methamphetamine group) and 25 healthy individuals (mean age = 32.8 years, range = 24.0–44.2 years, hereafter referred to as the control group).
Methamphetamine dependence was diagnosed using the Structured Clinical Interview for DSM-IV (SCID-IV). All subjects in the methamphetamine group had used over 50.0 g of cumulative methamphetamine and were recently abstinent at least 4 weeks. Exclusion criteria for the methamphetamine group were lifetime significant illness including hypertension, hepatitis, and diabetes mellitus, current Axis I diagnoses other than nicotine or methamphetamine dependence as determined using the SCID-IV, antisocial or borderline personality disorders as identified by the Personality Disorder Questionnaire-4, lifetime exposure to any other substances, except nicotine, caffeine, or social alcohol drinking, current and past history of alcohol abuse or dependence, or contraindications to magnetic resonance (MR) scanning. Alcohol history was taken and social alcohol drinking was defined as more than 8g of ethanol per week. Pack-years of cigarette smoking were calculated. The same criteria, except for a diagnosis of methamphetamine dependence, were applied to the control group.
The Addiction Severity Index was administered to assess severity and complications of methamphetamine dependence. Although the screen for seropositivity of human immunodeficiency virus (HIV) infection was not performed because of legal and ethical issue, subjects who had positive results on hepatitis virus c (HCV) antibody and hepatitis virus b surface (HBs) antigen were excluded. The prevalence of HIV infection in South Korea is substantially low as compared with many other countries (Min et al., 2013; UNAIDS, 2012). Furthermore, the recent study examining 318 injecting drug users in South Korea has reported that HIV infections were not found while the prevalence of HCV and HBV infections was 48.4% and 6.6%, respectively (Min et al., 2013). Very few HIV transmission (lower than 1.1%) has been reported to be attributed to injecting equipment in South Korea because of easy access to safe and disposable syringes in South Korea (Kim et al., 2003).
The institutional review board of the Seoul National University Hospital approved the study protocol and all study participants provided written informed consent after a complete description of the study.
2.2. Magnetic resonance image acquisition
High-resolution structural data were obtained from study participants on a 3.0-T GE whole body imaging system (GE VH/i, USA). Using a three-dimensional spoiled gradient echo pulse sequence, contiguous sagittal T1-weighted images were produced with the following parameters: echo time (TE) = 1.4 ms; repetition time (TR) = 5.7 ms; inversion time = 400 ms; 256 × 256 matrix; field of view(FOV) = 22 cm; flip angle = 20°; slice thickness = 0.7 mm, and no skip. Axial T2-weighted images (TE=118 ms, TR = 3500 ms, matrix = 256× 192, FOV = 22cm, FA = 90°, slice thickness =5 mm, 1.5 mm skip) and fluid-attenuated inversion recovery (FLAIR) axial images (TE =145 ms, TR= 9900 ms, inversion time = 2250 ms, matrix= 256× 192, FOV= 22 cm, FA= 90°, slice thickness= 5 mm, 1.5 mm skip) were also acquired in order to screen for gross structural abnormalities. All images were examined for incidental structural abnormalities by a board-certified neuroradiologist who was blind to subject status.
2.3. Image analysis: segmentation and shape analysis of the lateral ventricles
The segmentation of the lateral ventricles was performed using a rater-independent, automated atlas-based tissue segmentation method implemented in FreeSurfer software (http://surfer.nmr.mgh.harvard.edu) (Fischl et al., 2002). This methodology has been validated for accuracy and reproducibility of lateral ventricle segmentation through comparison with manually traced segmentation (Kempton et al., 2011a,b). In brief, the FreeSurfer pipeline (Fischl et al., 2002) processed high-resolution T1-weighted images by performing motion correction, intensity inhomogeneity correction, and skull stripping followed by gray and white matter segmentation. Subcortical structures including the lateral ventricles were segmented using an atlas based approach by assigning each voxel of the preprocessed volume to the corresponding ventricle labels. Information regarding voxel intensity relative to a probabilistic training atlas was utilized for these assignments and subsequent comparisons to neighboring voxel labels obtained from the atlas were performed (Fischl et al., 2002). Among two outputs from the FreeSurfer processing of each hemisphere, which were labeled as ‘lateral ventricle’ and ‘inferior lateral ventricle,’ the label of ‘lateral ventricle’ was used for volume and shape analyses. Because connections with the temporal horn could not occasionally be resolved even in high-resolution MR images (Styner et al., 2005), the anterior, main body, and posterior portion of the lateral ventricles were analyzed in the analysis excluding the temporal horn which was segmented as ‘inferior lateral ventricle.’ Anatomical locations of the lateral ventricle analyzed for this study are presented in Fig. 1. The final segmented images were visually inspected by an expert who judged the appropriateness of images in the volume and shape analysis. Intracranial volume (ICV) and volumes of the lateral ventricles were also measured.
Fig. 1.
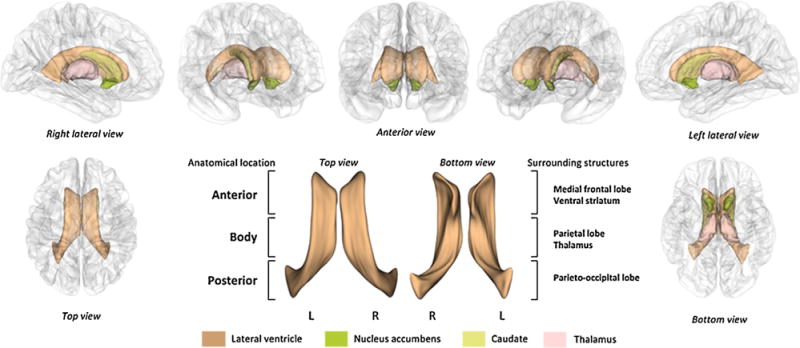
Anatomical location of the lateral ventricles and adjacent subcortical structures.
Surface modeling of the lateral ventricles was performed based on a spherical harmonic-based shape description. The semi-automated processing for shape description was performed with the use of the University of North Carolina shape analysis toolkit (Styner et al., 2003, 2005). Each step for the generation of lateral ventricular surfaces has been described in detail elsewhere (Styner et al., 2003, 2005). In brief, binary segmented images of the lateral ventricles were converted to surface meshes and spherical parameterization was computed (Brechbuhler et al., 1995). Using the first order ellipsoid from the spherical harmonic coefficients, parameter-based rotation was applied to establish spherical harmonic correspondence among vertices of the surface meshes (Gerig et al., 2001). This step was taken to eliminate the effects of rotation and translation. The spherical harmonic description was then uniformly sampled into 2252 triangulated surface points. The surfaces of the lateral ventricles were spatially aligned to an averaged template surface using a rigid-body transformation (Bookstein, 1991).
2.4. Statistical analysis
For comparison of demographic characteristics, independent t-tests or chi-square tests were used for continuous and categorical variables, respectively.
To test for differences in volume measurements of the lateral ventricles, multiple linear regression analysis was used to examine the main diagnostic effects on ventricular volumes adjusting for age, sex, and ICV. Effect sizes for volume differences in lateral ventricles are given by Cohen’s d.
For the statistical analysis of surface coordinates (x, y, and z) at 2252 corresponding points in each left and right lateral ventricle, multivariate analysis of covariance (MANCOVA) with Wilk’s lambda was used to assess diagnostic effects on surface coordinates adjusting for age, sex, and ICV. Repeated analyses including potential confounding variables that could affect changes in ventricular systems as additional covariates such as alcohol history, pack-years of cigarette smoking, and intelligence quotient (IQ) were performed.
In order to correct for multiple comparisons in the imaging data, surface-based cluster size exclusion was used with an initial surface-vertexwise threshold of p < 0.005. The current sample of 62 subjects could detect difference in surface coordinates at initial surface-vertexwise threshold of p < 0.005 (effect size of 0.78, standard deviation [sd] of ±50%) with a power of 0.85. Any difference in surface multivariate metric within a cluster containing less than 20 vertices was excluded. This threshold was ensured to result in a corrected p value of <0.05 across the surface of each ventricle by using the method of AFNI’s AlphaSim (Ward, 2000), which was adapted for use with surface-based statistics (Hagler et al., 2006).
To examine the relationship between age and the magnitude of surface alteration, Euclidian distances of individual surface points that belonged to the clusters showing significant diagnostic effects were calculated. Pearson correlation analysis was used to analyze the associations between the mean scaled distance of surface coordinates in each cluster and age in the methamphetamine and the control groups.
All statistical analyses were performed using Stata SE, v11.0 (Stata Corp., College Station, TX).
3. Results
Both the methamphetamine and control groups were well-matched in terms of age (t = −1.28, df=60, p = 0.21), sex (χ2 = 0.57, df = 1, p = 0.45) and IQ (t = 1.55, df = 60, p = 0.13). Prevalence of social alcohol drinking was not different between groups (χ2 = 0.99, df = 1, p = 0.32). However, pack-years of cigarette smoking were longer in methamphetamine-dependent subjects than in healthy subjects (t = −2.38, df=60, p = 0.02). Mean ICV of the methamphetamine group (1465.1 cm3, sd = 162.6) did not differ from that of the control group (1524.7 cm3, sd = 146.4) (t = 1.47, df = 60, p = 0.15).
Mean duration of regular methamphetamine use, which was calculated by summing up the time when methamphetamine was used more frequently than weekly, was 66.3 months (sd = 48.5) and the average daily doses of methamphetamine were 0.55 g (sd = 0.46). All methamphetamine-dependent subjects did not have any major psychotic disorders including schizophrenia, bipolar disorder, and other psychotic disorders as was determined by the SCID-IV, although some subjects reported episodic intoxication-related hallucination. There was no concomitant DSM-IV diagnosis of depressive or anxiety disorders requiring psychotropic medications in study participants.
Demographic and clinical characteristics of study participants are presented in Table 1.
Table 1.
Demographic and clinical characteristics of methamphetamine-dependent and healthy subjects.
| Characteristics | Methamphetamine group (n = 37) | Control group (n = 25) |
|---|---|---|
| Demographic characteristics | ||
| Age, years: mean (sd) | 34.8 (6.0) | 32.8 (6.0) |
| Gender, male:female | 27:10 | 16:9 |
| Social alcohol drinkinga: no. (%) | 28 (75.7) | 16(64.0) |
| Pack-years of cigarette smoking: mean (sd) | 8.92(10.8) | 3.25 (6.15) |
| Methamphetamine use-related characteristics | ||
| Intravenous use, n | 37 | |
| Age at the first use of methamphetamine,b years: mean (sd) | 22.7 (5.6) | |
| Average daily dose of methamphetamine,c g: mean (sd) | 0.55 (0.46) | – |
| Duration of regular methamphetamine use,d months, mean (sd) | 63.3 (48.5) | – |
| Addiction severity indexe: mean (sd) | ||
| Medical | 0.21 (0.29) | – |
| Employment | 0.64 (0.32) | – |
| Alcohol | 0.15(0.21) | – |
| Drug | 0.07 (0.06) | – |
| Legal | 0.18(0.20) | – |
| Family | 0.19(0.16) | – |
| Psychosocial | 0.21 (0.22) | – |
Social alcohol drinking was defined as more than 8 g of ethanol per week. None of subjects had a history of alcohol abuse or dependence.
Data from 4 subjects were not available.
Data from 1 subject was not available.
Duration of regular methamphetamine use was calculated by summing up the time when methamphetamine was used more frequently than weekly. Data from 4 subjects were not available.
Data from 6 subjects were not available.
3.1. Volume analysis of the lateral ventricles
The methamphetamine group demonstrated a significant volume enlargement in the right lateral ventricle relative to the control group after adjusting for age, sex, and ICV (6549.1 mm3 vs. 5980.7 mm3; β = 0.24, p = 0.039, Cohen’s d = 0.39). Volumes of the left lateral ventricle were not different between groups (methamphetamine group vs. control group, 7680.2 mm3 vs. 7341.0 mm3; β = 0.13, p = 0.23, Cohen’s d = 0.18). Repeated analyses including additional covariates, the presence of social alcohol drinking, pack-years of cigarette smoking, or IQ produced similar results (Supplementary Table 1).
3.2. Shape analysis of the lateral ventricles
Statistical maps demonstrating comparisons of surface coordinates for the lateral ventricles are presented in Fig. 2. All results were adjusted for age, sex, and ICV.
Fig. 2.
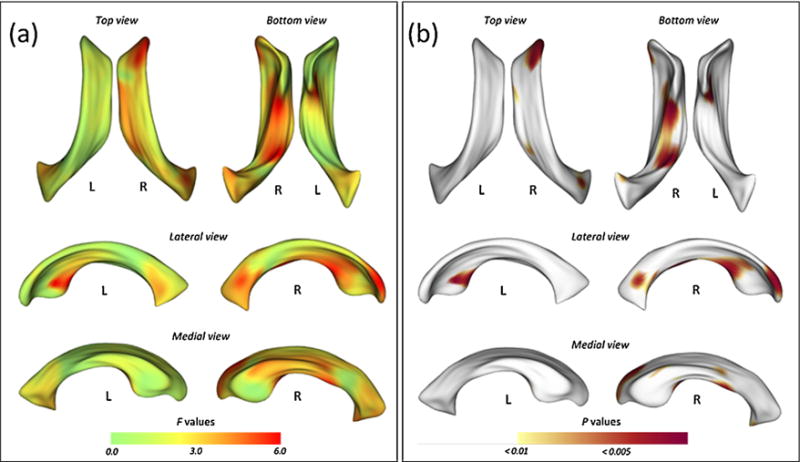
Statistical maps illustrating the location of shape differences in the lateral ventricles between the methamphetamine and control groups. The F statistic (a) and probability (b) maps show the results of multivariate analysis of covariance (MANCOVA) for estimating group differences in surface coordinates after adjusting for age, sex, and intracranial volume.
After the correction for multiple comparisons, 4 clusters showed a significant surface expansion in the methamphetamine group relative to the control group (Fig. 3). There were no significant clusters showing a surface deflation in the methamphetamine group compared with the control group. The locations of lateral ventricular expansion in the methamphetamine group are illustrated in Fig. 3. The first two clusters were located bilaterally in the anteroinferior portion of the lateral ventricles, which are adjacent to the ventral striatum (Fig. 3a). A significant surface expansion in the methamphetamine group was also observed in the cluster located in the anterosuperior portion of the right lateral ventricle, which is surrounded by the medial frontal lobe (Fig. 3b). Inferior body portion of the right lateral ventricle, which is located near the thalamus, was also expanded in the methamphetamine group relative to the control group (Fig. 3c).
Fig. 3.
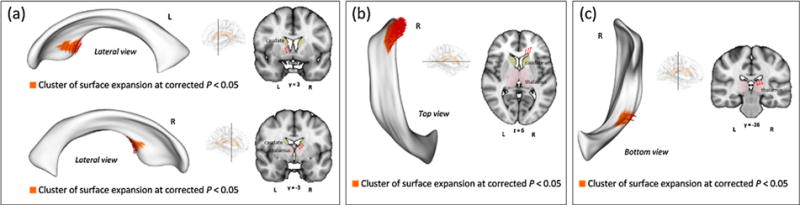
Clusters showing significant surface expansion of the lateral ventricles in the methamphetamine group. Clusters illustrated in the figure denote the significant surface expansion in the methamphetamine group relative to the control group. In order to correct for multiple comparisons, surface-based cluster-size exclusion with the initial threshold of p < 0.005 was used. Any inflation or deflation in surface metrics within a cluster containing less than 20 vertices was excluded. Four clusters of surface expansion related to methamphetamine dependence (red arrows) were found while there was no significant deflated region. (a) Clusters of surface expansion in regions adjacent to the bilateral ventral striatum. (b) A cluster of surface expansion in regions adjacent to the right medial frontal lobe. (c) A cluster of surface expansion in regions adjacent to the right thalamus.
To ensure the robustness of findings, repeated analyses of MANCOVA for surface coordinates including additional covariates (the presence of social alcohol drinking, pack-years of cigarette smoking, or IQ) were performed. Similar results were found and this is likely to show that the current results were not likely to be modulated by these potential confounding factors (Supplementary Table 2).
Within the methamphetamine group, the effects of aging on mean scaled surface distances were prominent in the bilateral anteroinferior clusters (left, r = 0.35, p = 0.032; right, r =0.41, p = 0.011; Fig. 4). Age-related surface expansions were not observed in other clusters (right anterosuperior cluster, r = −0.05, p = 0.76; right inferior cluster, r =0.22, p = 0.18).
Fig. 4.
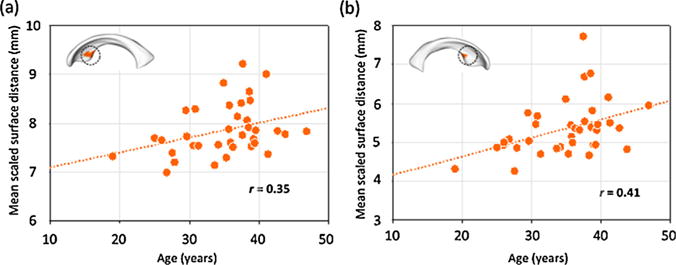
Relationships between mean scaled surface distances of clusters and aging in the methamphetamine group. The mean scaled surface distance of each cluster of significant diagnostic effects was calculated by averaging the Euclidian distances of individual points belonging to each cluster. Mean scaled surface distances of bilateral anteroinferior clusters of showing surface expansion related to methamphetamine dependence (Fig. 3a) were associated with age in the methamphetamine group ((a) left, r=0.35, p = 0.03; (b) right, r =0.41, p = 0.01). There were no correlations observed in the control group (left, r =0.18, p = 0.37; right, r =0.31, p = 0.13).
In contrast, there was no significant relationship between age and mean scaled surface distance of clusters in the control group.
The relationships between mean scaled surface distances in each cluster and methamphetamine use pattern including the duration of regular methamphetamine use and the average daily dose of methamphetamine were not significant (Supplementary Table 3).
4. Discussion
This is the first study to demonstrate morphometric abnormalities of the lateral ventricles associated with methamphetamine dependence using three-dimensional shape analysis, which complements the conventional volumetric analysis. In the volumetric approach, we found right lateral ventricular enlargement in methamphetamine-dependent subjects relative to healthy individuals. Shape analysis revealed that methamphetamine dependence was associated with bilateral surface expansion of the lateral ventricles, which were primarily distributed in areas surrounded by the medial prefrontal cortex, ventral striatum, and thalamus. These cortical and subcortical regions have been implicated in the pathophysiology of methamphetamine dependence (Berman et al., 2008; Chang et al., 2007; Salo and Fassbender, 2012).
Since the lateral ventricle is a relatively well-defined brain structure and one of the easiest regions to segment reliably (Buckley et al., 1999; Kremen et al., 2012), the lateral ventricle has been a major region-of-interest in neuroimaging studies of psychiatric and neurological disorders. For example, enlargement of the lateral ventricles has been one of the most robust and stable findings in schizophrenia (Kempton et al., 2010; Wright et al., 2000) and bipolar disorder (Kempton et al., 2008). Furthermore, an increased rate of ventricular enlargement might be a neurobiological marker of the progression in Alzheimer’s dementia (Frisoni et al., 2010) and mild cognitive impairment (Carmichael et al., 2007).
Given that lateral ventricular expansion can occur ubiquitously during normal aging process (Pfefferbaum et al., 2004), it has been assumed that accumulated environmental insults over the lifespan, in addition to heritability, can determine the structural variability of lateral ventricles (Kremen et al., 2012). For example, excessive alcohol or cannabis use has been reported to be a potential factor to accelerate cerebral atrophic changes followed by enlargement of the lateral ventricles (Pfefferbaum et al., 2001; Wobrock et al., 2009; Welch et al., 2011a,b).
Another issue to consider in interpreting lateral ventricular enlargement is whether it reflects diffuse cerebral atrophy or focal changes of the nearby brain parenchyma. Due to the adaptive capacity of the fluid-filled ventricular system, the shape of the lateral ventricles can change depending on alterations in the surrounding parenchymal structures (Gaser et al., 2004; Preul et al., 2006). For example, lateral ventricular expansion observed in schizophrenia is thought to be related to volume changes in the thalamus, striatum, and superior temporal cortex rather than simply reflecting diffuse cerebral atrophy (Gaser et al., 2004). The current findings of highly localized shape decomposition of the lateral ventricles observed in the methamphetamine group argue in favor of a specific regional vulnerability of the brain to chronic methamphetamine use. Further, a more sensitive measurement obtained using shape analysis also discerned localized surface expansion of the lateral ventricles even without total volume changes.
Striatal structures, regions with the highest density of dopaminergic synapses, have been the focus of preclinical research examining the neurotoxic effects of methamphetamine in that these regions are primary action sites of methamphetamine (Chang et al., 2007). Converging neuroimaging evidence has also indicated that chronic methamphetamine use might lead to structural and metabolic changes in the human striatum (Chang et al., 2007). Our findings suggest that the surface expansion of the lateral ventricles toward the ventral striatum, consistent with the regional vulnerability of this region to the neurotoxic effects of methamphetamine use. Existing knowledge based on human neuroimaging studies of methamphetamine dependence has suggested that early enlarged striatal volume might be followed by more neurodegenerative changes with a longer period of exposure (Chang et al., 2005; Jernigan et al., 2005). Accordingly, smaller striatal volumes have been associated with larger cumulative methamphetamine doses and impaired cognitive function (Chang et al., 2005). Tentative cellular mechanisms of enlarged striatal volume in the early phase of dependence have been proposed to include neuroinflammatory reactions or reactive gliosis as possible compensatory responses to methamphetamine use (Chang et al., 2005, 2007; Jernigan et al., 2005). In some respects, our findings suggest the neurodegenerative changes of the ventral striatum in response to chronic methamphetamine use. For example, although our findings do not address the exact direction nor the localization of methamphetamine use-related striatal volume changes, significant aging effects in the ventricular expansion adjacent to the bilateral ventral striatum indirectly suggest accelerating atrophic changes in these regions. However, we did not find the significant relationships between methamphetamine use patterns and ventricular expansion. This may be partly because the ranges of data related to methamphetamine use patterns were likely to be wide (average daily dose of methamphetamine use, sd = 0.55; duration of regular methamphetamine use, sd = 48.5) or the current data could not reflect exact cumulative effects of methamphetamine exposure. Future studies with a larger sample should be necessary to confirm the dose-dependent relationships between methamphetamine use and focal expansion of the lateral ventricles.
Given preclinical findings suggesting methamphetamine-specific neurodegenerative effects on axonal arbors of dopamine neurons with sparing of cell bodies (Ricaurte et al., 1980), dopamine circuits from the ventral tegmental area through the ventral striatum finally extending to the medial prefrontal cortical areas have also been identified as regions associated with damage related to methamphetamine use (Berman et al., 2008; Salo and Fassbender, 2012). Long-term changes in the fronto-striatal regions, which play an important role in selective attention and top-down control of drug-seeking behaviors, have been reported in methamphetamine-exposed animals (Ricaurte et al., 1982; Villemagne et al., 1998). Preferential involvement in the medial frontal lobe has also been observed in methamphetamine-dependent human brain (Thompson et al., 2004; Kim et al., 2006; Daumann et al., 2011). We found that significant lateral ventricular expansion was localized in an area adjacent to the right medial frontal lobe. As this region functions en bloc with the striatum and midbrain dopamine areas, less tissue resource in the prefrontal lobe might confer increased risk for prolonged and uncontrolled drug use (Goldstein and Volkow, 2011). Findings of the present study of the lateral ventricles may have clinical relevance in terms of supporting the prefrontal-striatal regional deficits associated with methamphetamine dependence.
Thalamic volumes may be relatively preserved in methamphetamine dependence, although there is a paucity of data (Chang et al., 2005; Jernigan et al., 2005). However, dopaminergic signaling from the striatum (Scheel-Kruger, 1986) may render the thalamus vulnerable to the neurotoxic effects of methamphetamine. Likewise, functional imaging findings indicate that methamphetamine use is associated with lower metabolism not only of the striatum but also of the thalamus (Volkow et al., 2001). Therefore, ventricular expansion due to thalamic contraction in the methamphetamine cohort may be interpreted in the context of methamphetamine effects on the dopamine system. Further, given that the thalamus is a major hub region conveying information between cortical and subcortical regions (Byne et al., 2009) and is frequently involved as a mediator of schizophrenia-like psychotic symptoms (Gaser et al., 2004; Welch et al., 2011a,b; Janssen et al., 2012), the current findings of thalamic regional involvement in methamphetamine dependence may have clinical implications in terms of the frequently comorbid psychotic symptoms observed in individuals exposed to chronic methamphetamine use.
Localized gray matter volume deficits related to chronic methamphetamine use have been reported in cortical regions including the prefrontal, cingulate, insula and superior temporal gyri as well as subcortical regions including the caudate and hippocampus (Kim et al., 2006; Nakama et al., 2011; Schwartz et al., 2010; Thompson et al., 2004; Morales et al., 2012). The current findings that methamphetamine-related focal lateral ventricular enlargement was distributed mainly in the right lateral ventricle are in general consistent with previous structural neuroimaging studies suggesting right gray matter deficits accompanying lateral ventricular expansion (Kim et al., 2006; Thompson et al., 2004). Although the precise cellular mechanisms underlying these laterality effects is still unknown, volume expansion predominantly in the right ventricle might also be attributed to lateralized atrophic changes in the right prefrontal regions induced by chronic methamphetamine exposure (Thompson et al., 2004; Kim et al., 2006). However, since these lateralization effects were not prominent in other studies on methamphetamine dependence (Schwartz et al., 2010; Morales et al., 2012), further studies are required to confirm the potential lateralization effects of chronic methamphetamine use.
There are several methodological considerations and limitations in interpreting the results. Although the present study aimed to detect chronic methamphetamine-use related focal changes in the lateral ventricular system, it should be noted that the effect size of lateral ventricular volume differences between groups was small to medium (right lateral ventricle, Cohen’s d = 0.39; left lateral ventricle, Cohen’s d = 0.18). Furthermore, additional covariation of alcohol and smoking history was likely to decrease statistical significances in volume differences although there was a similar focal regional pattern of lateral ventricular expansion. Future studies with a larger sample size will be required to confirm the methamphetamine-use related lateral ventricular volume enlargement that may indicate diffuse cerebral atrophy.
The prevalence of HIV infection in injecting drug abusers has been known to be very low in South Korea (Min et al., 2013). However, the lack of clinical information of HIV infection, which could potentially affect cerebral atrophic changes, would be a limitation of the present study.
It is notable that dehydration state, which is frequently observed in acute methamphetamine intoxication state (Kosten et al., 2012), might be related to brain atrophic changes and ventricular enlargements (Dickson et al., 2005; Duning et al., 2005; Kempton et al., 2009, 2011a,b; Streitburger et al., 2012). Although the current methamphetamine-dependent subjects had a period of abstinence of at least 4 weeks, a subclinical and prolonged dehydration state related to chronic methamphetamine use may be partly responsible for lateral ventricular enlargement in this sample.
Current analysis excluded the temporal horns of the lateral ventricles as in other studies using the morphometric approach because of the low resolution of its connection to the main body of the lateral ventricle (Styner et al., 2005; Jackson et al., 2011; Vidal et al., 2008). Our findings should thus be interpreted within the focused regions that were included in the analysis. For instance, medial temporal lobe involvement associated with methamphetamine dependence (Thompson et al., 2004) could not be readily determined based on this study.
Although the lateral ventricles can be very easily segmented and the automated segmentation method has been validated (Kempton et al., 2011a,b), it should be noted that segmentation using manual tracings is regarded as the gold standard for region-of-interest volumetry. In addition, although lateral ventricular enlargement might be interpreted as the consequence of reflecting cortical and subcortical deficits (Frisoni et al., 2010; Nestor et al., 2008; Kempton et al., 2010; Wright et al., 2000; Pfefferbaum et al., 2001; Thompson et al., 2004; Wobrock et al., 2009), future studies using imaging methodologies optimized for determining each structural change will be necessary to define the neurotoxic effects of methamphetamine on individual cortical and subcortical structures. In a related limitation, our ventricular findings suggesting that methamphetamine-induced brain parenchymal deficits might be highly localized rather than diffuse will also require replication in future studies using direct measurements of relevant structural changes.
A further limitation of the study is that cross-sectional design limits conclusions as to whether the focal expansion of the lateral ventricles reflects constitutional vulnerability, results from damage, or compensatory responses to chronic methamphetamine use (Berman et al., 2008).
Three-dimensional surface-based morphometric analysis of the lateral ventricles, as implemented in the current study, has methodological advantages in terms of a greater sensitivity to identify focal changes related to diseases (Styner et al., 2005; Apostolova et al., 2012; Jackson et al., 2011). In this study, we found that chronic methamphetamine use might be related to focal lateral ventricular expansion primarily located in areas adjacent to prefrontal-ventral striatal-thalamic circuits. These findings have clinical implications suggesting the structural vulnerability of certain brain regions subserving top-down control of compulsive drug administration to methamphetamine-induced neurotoxic influences that poses further problematic and prolonged drug-use behaviors.
Supplementary Material
Acknowledgments
Role of funding source
This study was supported by a grant of the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A101219). The funding source of the study had no role in the study design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2013.05.009.
Footnotes
Supplementary material can be found by accessing the online version of this paper. See Appendix A for more details.
Contributors
HSJ and SL contributed equally to this work. PFR and IKL designed the study and wrote the protocol. Data collection and image analyses were performed by HSJ, SL, SY, JJJ, HBC, PFR, and IKL. HSJ, SL, and SY wrote the first draft of the manuscript. All authors contributed to the data interpretation and manuscript composition. All authors have also approved the manuscript.
Conflict of interest
IKL has received research support from Lundbeck, AstraZeneca, GSK, and Boryung Pharmaceutical. PFR has been a consultant for Ridge Diagnostics and Kyowa Hakko. Other authors report no financial relationships with commercial interests.
References
- Apostolova L, Alves G, Hwang KS, Babakchanian S, Bronnick KS, Larsen IP, Thompson PM, Chou YY, Tysnes OB, Vefring HK, Beyer MK. Hippocampal and ventricular changes in Parkinson’s disease mild cognitive impairment. Neurobiol Aging. 2012;33:2113–2124. doi: 10.1016/j.neurobiolaging.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S, O’Neill L, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein F. Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge Univ. Press; Cambridge: 1991. [Google Scholar]
- Brechbuhler C, Gerig G, Kubler O. Parameterization of closed surfaces for 3-D shape description. Comput Vision Image Underst. 1995;61:154–170. [Google Scholar]
- Buckley PF, Dean D, Bookstein FL, Friedman L, Kwon D, Lewin JS, Kamath J, Lys C. Three-dimensional magnetic resonance-based morphometrics and ventricular dysmorphology in schizophrenia. Biol Psychiatry. 1999;45:62–67. doi: 10.1016/s0006-3223(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21:14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl. 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann J, Koester P, Becker B, Wagner D, Imperati D, Gouzoulis-Mayfrank E, Tittgemeyer M. Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometric. Neuroimage. 2011;54:794–801. doi: 10.1016/j.neuroimage.2010.08.065. [DOI] [PubMed] [Google Scholar]
- Dickson JM, Weavers HM, Mitchell N, Winter EM, Wilkinson ID, Van Beek EJ, Wild JM, Griffiths PD. The effects of dehydration on brain volume - preliminary results. Int J Sports Med. 2005;26:481–485. doi: 10.1055/s-2004-821318. [DOI] [PubMed] [Google Scholar]
- Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–550. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am J Psychiatry. 2004;161:154–156. doi: 10.1176/appi.ajp.161.1.154. [DOI] [PubMed] [Google Scholar]
- Gerig G, Styner M, Jones D, Weinberger D, Lieberman J. Shape analysis of brain ventricles using spharm. In: Staib L, Rangarajan A, editors. Mathematical Methods in Biomedical Image Analysis. IEEE Computer Society; Washington DC: 2001. pp. 171–178. [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Irwin W, Dabbs K, Lin JJ, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP. Ventricular enlargement in new-onset pediatric epilepsies. Epilepsia. 2011;52:2225–2232. doi: 10.1111/j.1528-1167.2011.03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J, Aleman-Gomez Y, Reig S, Schnack HG, Parellada M, Graell M, Moreno C, Moreno D, Mateos-Perez JM, Udias KM, Arango C, Desco M. Regional specificity of thalamic volume deficits in male adolescents with early-onset psychosis. Br J Psychiatry. 2012;200:30–36. doi: 10.1192/bjp.bp.111.093732. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Ettinger U, Foster R, Williams SC, Calvert GA, Hampshire A, Zelaya FO, O’Gorman RL, McMorris T, Owen AM, Smith MS. Dehydration affects brain structure and function in healthy adolescents. Hum Brain Mapp. 2011a;32:71–79. doi: 10.1002/hbm.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Ettinger U, Schmechtig A, Winter EM, Smith L, McMorris T, Wilkinson ID, Williams SC, Smith MS. Effects of acute dehydration on brain morphology in healthy humans. Hum Brain Mapp. 2009;30:291–298. doi: 10.1002/hbm.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Metaanalysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Stahl D, Williams SC, DeLisi LE. Progressive lateral ventricular enlargement in schizophrenia: a meta-analysis of longitudinal MRI studies. Schizophr Res. 2010;120:54–62. doi: 10.1016/j.schres.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Underwood TS, Brunton S, Stylios F, Schmechtig A, Ettinger U, Smith MS, Lovestone S, Crum WR, Frangou S, Williams SC, Simmons A. A comprehensive testing protocol for MRI neuroanatomical segmentation techniques: evaluation of a novel lateral ventricle segmentation method. Neuroimage. 2011b;58:1051–1059. doi: 10.1016/j.neuroimage.2011.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Cho GJ, Hong SK, Chang KH, Chung JS, Choi YH, Song YG, Huh A, Yeom JS, Lee KS, Choi JY. Epidemiology and clinical features of HIV infection/aids in Korea. Yonsei Med J. 2003;44:363–370. doi: 10.3349/ymj.2003.44.3.363. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim L, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Newton TF, De La Garza R, Haile IICN. Cocaine and Methamphetamine Dependence: Advances in Treatment. American Psychiatric Publishing; Washington, DC: 2012. [Google Scholar]
- Kremen WS, Panizzon MS, Neale MC, Fennema-Notestine C, Prom-Wormley E, Eyler LT, Stevens A, Franz CE, Lyons MJ, Grant MD, Jak AJ, Jernigan TL, Xian H, Fischl B, Thermenos HW, Seidman LJ, Tsuang MT, Dale AM. Heritability of brain ventricle volume: converging evidence from inconsistent results. Neurobiol Aging. 2012;33:1–8. doi: 10.1016/j.neurobiolaging.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JA, Yoon Y, Lee HJ, Choi J, Kwon M, Kim K, Lee CU, Kim DJ, Yun H. Prevalence and associated clinical characteristics of hepatitis b, c, and HIV infections among injecting drug users in Korea. J Med Virol. 2013;85:575–582. doi: 10.1002/jmv.23523. [DOI] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106:1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R. Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain. 2008;131:2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiol Aging. 2004;25:175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Preul C, Hubsch T, Lindner D, Tittgemeyer M. Assessment of ventricular reconfiguration after third ventriculostomy: what does shape analysis provide in addition to volumetry? AJNR Am J Neuroradiol. 2006;27:689–693. [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Salo R, Fassbender C. Structural, functional and spectroscopic MRI studies of methamphetamine addiction. Curr Top Behav Neurosci. 2012;11:321–364. doi: 10.1007/7854_2011_172. [DOI] [PubMed] [Google Scholar]
- Scheel-Kruger J. Dopamine–GABA interactions: evidence that GABA transmits, modulates and mediates dopaminergic functions in the basal ganglia and the limbic system. Acta Neurol Scand Suppl. 1986;107:1–54. [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitburger DP, Moller HE, Tittgemeyer M, Hund-Georgiadis M, Schroeter ML, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS ONE. 2012;7:e44195. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner M, Gerig G, Lieberman JA, Jones D, Weinberger D. Statistical shape analysis of neuroanatomical structures based on medial models. Med Image Anal. 2003;7:207–220. doi: 10.1016/s1361-8415(02)00110-x. [DOI] [PubMed] [Google Scholar]
- Styner M, Lieberman IA, McClure RK, Weinberger DR, Lones DW, Gerig G. Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proc Natl Acad Sci USA. 2005;102:4872–4877. doi: 10.1073/pnas.0501117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS/WHO Joint United Nations Programme on HIV/AIDS (UNAIDS) World epidemic of HIV/AIDS report 2012 [Google Scholar]
- Vidal CN, Nicolson R, Boire JY, Barra V, DeVito TJ, Hayashi KM, Geaga LA, Drost DJ, Williamson PC, Rajakumar N, Toga AW, Thompson PM. Three-dimensional mapping of the lateral ventricles in autism. Psychiatry Res. 2008;163:106–115. doi: 10.1016/j.pscychresns.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35, 428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower sub-cortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Ward B. Simultaneous Inference for FMRI Data. AFNI 3dDeconvolve Documentation. Medical College of Wisconsin 2000 [Google Scholar]
- Welch KA, McIntosh AM, Job DE, Whalley HC, Moorhead TW, Hall J, Owens DG, Lawrie SM, Johnstone EC. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011a;37:1066–1076. doi: 10.1093/schbul/sbq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KA, Stanfield AC, McIntosh AM, Whalley HC, Job DE, Moorhead TW, Owens DG, Lawrie SM, Johnstone EC. Impact of cannabis use on thalamic volume in people at familial high risk of schizophrenia. Br J Psychiatry. 2011b;199:386–390. doi: 10.1192/bjp.bp.110.090175. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, Frommann N, Wolwer W, Gaebel W. Effects of abstinence on brain morphology in alcoholism: a MRI study. Eur Arch Psychiatry Clin Neurosci. 2009;259:143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Wu CW, Ping YH, Yen JC, Chang CY, Wang SF, Yeh CL, Chi CW, Lee HC. Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol. 2007;220:243–251. doi: 10.1016/j.taap.2007.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


