SUMMARY
The contributions of the innate immune system to the development of pancreatic cancer are still ill-defined. Inflammatory macrophages can initiate metaplasia of pancreatic acinar cells to a duct-like phenotype (ADM), which then give rise to pancreatic intraepithelial neoplasia (PanIN) when oncogenic KRas is present. However, it remains unclear when and how this inflammatory macrophage population is replaced by tumor-promoting macrophages. We here demonstrate presence of interleukin-13 (IL-13), which can convert inflammatory into Ym1+ alternatively-activated macrophages, at ADM/PanIN lesions. We further show that Ym1+ macrophages release factors such as IL-1ra and CCL2 to drive pancreatic fibrogenesis and tumorigenesis. Treatment of mice expressing oncogenic KRas under an acinar cell-specific promoter with a neutralizing antibody for IL-13 significantly-decreased the accumulation of alternatively-activated macrophages at these lesions, resulting in decreased fibrosis and lesion growth.
Graphical abstract

INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) is tightly associated with pancreatitis (pancreatic inflammation) and desmoplasia (Greer and Whitcomb, 2009; Kolodecik et al., 2013; Raimondi et al., 2010). Although oncogenic KRas (present in over 95% of PDA) by itself can induce a chronic micro-inflammation to initiate the formation of low-grade pancreatic intraepithelial neoplasia (PanIN) (Liou et al., 2015b), in order for these precancerous lesions to progress to PDA additional inflammatory stimuli are needed (Daniluk et al., 2012; Huang et al., 2014). In KRasG12D-expressing mice chronic pancreatic inflammation can be achieved by a high fat diet (Dawson et al., 2013) or caerulein-induced release of digestive enzymes (Guerra et al., 2007). The presence of chronic pancreatitis then dramatically accelerates the development of PDA by abrogating oncogenic KRas-induced senescence in precancerous lesions (Guerra et al., 2011). While genetic animal models clearly demonstrated that in presence of oncogenic KRas chronic pancreatitis can drive the development of PDA (Guerra et al., 2011; Guerra et al., 2007), the interplay between different immune cells and pancreatic cells is not sufficiently understood. Especially the contributions of differently polarized macrophage subtypes to the development of PanIN and their progression to PDA have not been well characterized.
Inflammatory macrophages can have an initiating function for the development of precancerous lesions since they induce the transdifferentiation of pancreatic acinar cells to a progenitor-like phenotype (Liou et al., 2013; Liou and Storz, 2015). This acinar-to-ductal metaplasia (ADM) process is initiated by macrophage-secreted inflammatory cytokines such as TNF and RANTES, and microenvironment remodeling facilitated by macrophage released matrix-metalloproteinases (MMPs) such as MMP-9 (Liou et al., 2015b; Liou et al., 2013). However, ADM is a reversible process and only after acquisition of an oncogenic KRas mutation do these progenitor cells progress to a pre-neoplastic cell type that forms PanIN (Stanger and Hebrok, 2013).
Studies investigating the roles of macrophage subpopulations in chronic pancreatitis indicate pancreatic stellate cells (PSC) can be a source of IL-4 and IL-13, leading to the presence of alternatively-activated macrophages which then promote pancreatic fibrosis (Xue et al., 2015). The increasing occurrence of fibrosis during progression from ADM to PanIN and PDA suggests that a similar switch in macrophages populations towards an alternatively-activated phenotype is needed for the development of pancreatic cancer.
We here show that IL-13, a factor that can initiate a polarization switch from an inflammatory towards an alternatively-activated macrophage phenotype, is expressed in oncogenic KRas-caused low grade ADM/PanIN lesion cells. We further show that resulting alternatively-activated macrophages secrete molecules such as CCL-2 and IL-1ra that promote the growth of PanIN and fibrosis. In vivo, treatment with a neutralizing antibody for IL-13 significantly decreased the accumulation of alternatively-activated macrophages at PanIN, as well as fibrosis and lesion growth, in mice with pancreas-specific expression of KRas.
RESULTS
ADM and PanIN show different predominance of macrophage polarization types
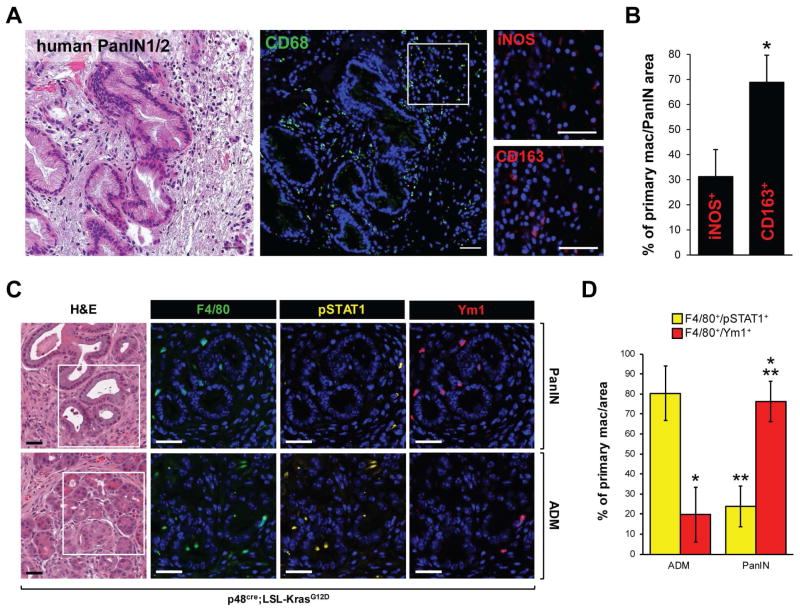
In human and mouse pancreas, acinar cell regions that undergo metaplasia to a duct-like phenotype (ADM) are characterized by increased presence of inflammatory macrophages (Liou et al., 2015b; Liou et al., 2013). These macrophages contribute to ADM by secreting inflammatory cytokines such as TNF, as well as factors that degrade extracellular matrix such as MMP-9 (Liou et al., 2015b; Liou et al., 2013). Typical markers for inflammatory macrophages are iNOS or phosphorylated STAT1 (Murray and Wynn, 2011). In presence of an oncogenic KRas mutation, cells that have undergone ADM can progress to PanIN1/2 lesions. Human PanIN1/2 lesions show a high presence of macrophages (Fig. 1A; CD68 staining; green). However, a more detailed analysis of macrophage polarization types populating the areas of human PanIN1/2 indicated presence of inflammatory (nitric oxide synthase 2 (iNOS) positive, M1-polarized) and alternatively-activated (CD163 positive, M2-polarized) macrophages at a ratio of approximately 1:2 (Figs. 1A, 1B). To investigate this shift in macrophage populations in more detail, we next analyzed regions of ADM and PanIN lesions in pancreata of p48cre; KrasG12D mice for presence of inflammatory or alternatively-activated macrophages. Similar as observed with human samples, we found an approximately 3-fold increase in alternatively-activated (F4/80/Ym1-positive, M2-polarized) macrophages in PanIN regions (Fig. 1C; top row), whereas the predominant subtype in ADM regions were inflammatory (F4/80/pSTAT1-positive, M1-polarized) macrophages (Fig. 1C; bottom row). A quantification of macrophage polarization subtypes indeed indicated a switch in macrophage populations when pancreatic abnormal structures progress from ADM to the PanIN stage (Fig. 1D).
Figure 1. ADM and PanIN show different predominance of macrophage polarization types.
A: Patient samples that contain PanIN1/2 lesions were stained with H&E (left panel), or analyzed by immunofluorescence for presence of macrophages (anti-CD68, middle panel). The inset marks the zoom-in area for which presence of different macrophage subtypes were determined by using iNOS or CD163 as markers. DAPI stains nuclei. Scale bar: 50 μm. B: Quantification of iNOS+ or CD163+ macrophages localized at human PanIN1/2 areas (n=10). The asterisk indicates statistical significance (p < 0.05) of the increase in CD163+ as compared to iNOS+ cells. C: H&E staining of pancreatic tissue of 14 week old KC mice showing typical areas of PanIN1 and ADM. Immunofluorescence pictures show analysis of presence of macrophage subtypes by labeling with F4/80 in combination with pSTAT1 or YM1. Scale bar: 50 μm. D: Quantification of F4/80/pSTAT1+ or F4/80/Ym1+ macrophages in ADM (n=8) and PanIN areas (n=9). * indicates statistical significance (p < 0.05) as compared to F4/80/pSTAT1+ macrophages in ADM regions; ** indicates statistical significance (p < 0.05) as compared to F4/80/Ym1+ macrophages in ADM regions.
Presence of IL-13 at PanIN and in pancreatic cancer cells
We next tested if inflammatory macrophages during ADM-PanIN transition can get re-polarized to become alternatively-activated (tumor-associated) macrophages. In vitro, peritoneal F4/80/iNOS-positive (inflammatory) mouse macrophages can be re-polarized to a F4/80/Ym1-positive (alternatively-activated) phenotype in presence of IL-13 or IL-4 (Suppl. Fig. S1A). Therefore, we determined if cells in pancreatic lesions express IL-4 or IL-13 to possibly initiate shifting of macrophage populations. We did not observe IL-4 expression in pancreata of normal or p48cre; KrasG12D (KC) mice (Suppl. Fig. S1B). However, expression of IL-13 was detected in regions of ADM and PanIN1 of KC mice (Fig. 2A). A quantification of IL-13 expression in regions of ADM and PanIN1/2 indicated an approximately 2.5 fold increase in PanIN (Fig. 2B), correlating with the occurrence of alternatively-activated macrophages at these structures (Fig. 1D). To test if PanIN cells express IL-13 mRNA, we microdissected normal acinar regions and PanIN1A/B from KC mice (Fig. 2C), or performed in situ hybridization (ISH) on tissue (Fig. 2D). While PanIN cells showed IL-13 expression, adjacent fibrotic and normal acinar cell areas were negative. In experimentally-induced pancreatitis, stellate cells and Th-cells previously have been shown to be capable of expressing IL-13 (Xue et al., 2015). However, ISH for IL-13 in combination with IHC for desmin (stellate cells) indicated that these cells do not produce IL-13 in our animal model (Fig. 2E). Moreover, we hardly detected CD4+ cells (Th-cells) in ADM/PanIN regions (Suppl. Fig. S1C, left side), and only found a few cell clusters in the periphery of the pancreas that were CD4+. Analyses of these cells indicated that they do not express significant amounts of IL-13 mRNA (Suppl. Fig. S1C, right side).
Figure 2. Expression of IL-13 is increased in PanIN and pancreatic cancer.
A, H: Pancreatic tissue of 18 week old KC or control mice (A) or pancreatic cancer patient samples (H) were analyzed by immunohistochemistry for expression of IL-13. Scale bar: 50 μm. B: Quantification of samples (from A) for IL-13 expression. * indicates statistical significance (p < 0.05) as compared to normal acinar cells; ** indicates statistical significance (p < 0.05) as compared to ADM regions. C: PanIN1A/B (n=50) or adjacent “normal” acinar cells (n=50) were microdissected from KC mice and analyzed by q-PCR for IL-13 mRNA expression. D: Indicated pancreatic tissues from KC mice were analyzed for IL-13 mRNA expression (brown dots) using RNAscope in situ hybridization (ISH). E, F: PanIN areas from KC mice were analyzed for IL-13 mRNA expression (brown dots) using RNAscope ISH and DCLK1 or Desmin protein expression (pink staining) using IHC. G–I: Pancreatic tissue of 14 week old KC or control mice (G) or pancreatic cancer patient samples (H, I) were analyzed by immunofluorescence labeling (G, I) for IL-13 and acetyl-α-tubulin. Nuclei were visualized by DAPI staining. Samples in H were stained by IHC for IL-13. In I, the rectangle window represents an area for which single channels are shown. Scale bars in G–I: 50 μm.
The patchy staining pattern of IL-13 (Fig. 2A) indicated that it is mainly expressed in Tuft cells, a stem cell-like cell type that is present in early PanIN lesions and was linked to progression of pancreatic cancer (Bailey et al., 2014; Delgiorno et al., 2014). Expression of DCLK1 and acetylated α-tubulin are bona fide markers for Tuft cells (Suppl. Fig. S1D). In order to test if Tuft cells produce IL-13 we performed an ISH for IL-13 in combination with IHC for DCLK1 (Fig. 2F), as well as immunofluorescence co-staining for IL-13 and acetyl-α-tubulin (Fig. 2G and Suppl. Fig. S1E). Additional analysis of FACS-sorted DCLK1+ cells confirmed the presence of IL-13 mRNA (Suppl. Fig. S1F). As proof of principle to the data obtained with the KC mouse model, we detected increased IL-13 expression in pancreatic cancerous lesions from patient samples (Fig. 2H). And similar as in mice, IL-13 expression correlated with presence of the Tuft cell marker acetylated α-tubulin (Fig. 2I).
Neutralization of IL-13 decreases the presence of Ym1+ macrophages at ADM/PanIN lesions and results in an overall decrease in abnormal pancreatic structures
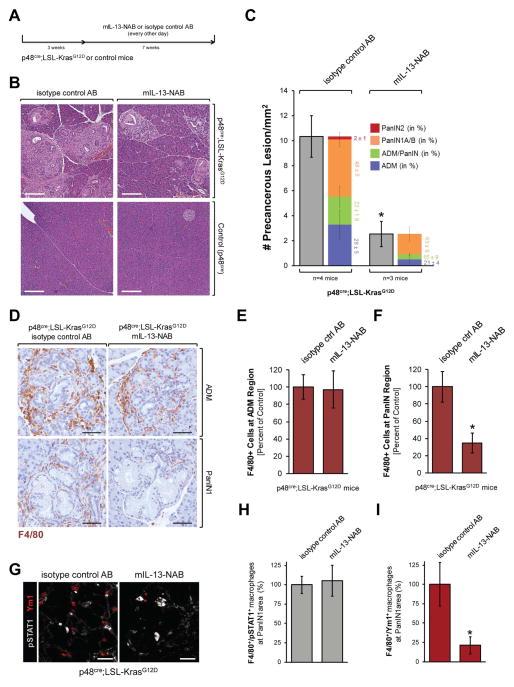
IL-13 can amplify the presence of M2 tumor-associated macrophages (Kratochvill et al., 2015). In the pancreas of our mouse model we found the IL-13 receptor IL-13-Rα1 mainly expressed in acinar cell regions (Suppl. Fig. S2A), in which mainly inflammatory macrophages are present (Fig. 1D). A co-immunofluorescence analysis of pancreatic tissue indicated that inflammatory macrophages indeed express IL-13-Rα1, while Ym1-positive macrophages do not express this receptor (Suppl. Fig. S2B). Isolation of F4/80-positive cells from pancreata of KC mice by FACS and subsequent treatment with IL-13 indicated that IL-13 indeed can enrich Ym1-positive populations (Suppl. Fig. S2C). To test if this can contribute to progression of pre-neoplastic lesions, we treated KC or control mice with mouse IL-13 neutralizing antibody (mIL-13 NAB) or isotype control antibody for a time period of 7 weeks (Fig. 3A). Neutralization of IL-13 led to a drastic (almost 80%) decrease in abnormal pancreatic structures (Figs. 3B, 3C). This went along with a 30% decrease of total macrophages in the pancreas from 100% −/+ 3.14% in KC + IgG control to 69.1% −/+ 6.15% in KC + IL-13-NAB (data not shown). This reflected a decrease in macrophages in PanIN areas (from 100% −/+ 17.8% in control to 34.7% −/+ 11.4% in IL-13-NAB treated mice), while total macrophage populations at ADM regions are similar under both conditions (100% −/+ 14.3% in control + IgG control and 97.1% −/+ 21.7% IL-13-NAB) (Figs. 3D, 3E and 3F). Further analyses of PanIN1 areas indicated that the decrease in F4/80 positive cells is due to an approximately 80% decrease in alternatively-activated (F4/80/Ym1-positive) macrophages, while the relative presence of inflammatory (F4/80/pSTAT1-positive) macrophages stayed unchanged (Figs. 3G, 3H and 3I). However, it should be noted that the depletion of IL-13 at this stage of tumorigenesis also affects presence of neutrophils (Ly6B.2-positive cells) at the abnormal areas of the pancreas. Other cell populations such as T-cells (CD3+), Th-cells (CD4+) were sparsely present and remained unaffected by neutralization of IL-13, while mature dendritic cells (CD83+) were not present (Suppl. Figs. S3A and S3B).
Figure 3. Neutralization of IL-13 decreases the presence of Ym1+ macrophages at PanIN lesions and results in an overall decrease in abnormal pancreatic structures.
A: Treatment scheme. KC or control mice at an age of 3 weeks were treated with indicated antibodies for 7 weeks. At 10 weeks pancreas tissues were harvested. B: Representative H&E staining of pancreas tissue samples from KC mice treated as indicated in A. Scale bar: 200 μm. C: Quantification of total abnormal structures including ADM and PanIN (grey) and grading of different pancreatic lesions. The asterisk indicates statistical significance (p<0.05) as compared to isotype control antibody-treated KC mice. D–F: IHC for F4/80+ macrophages and quantification of their presence in regions of ADM and PanIN1 in KC mice either treated with mIL-13-NAB or isotype control AB. Scale bar: 100 μm. G–I: Further analyses of pSTAT1+ and Ym1+ macrophages at PanIN1 regions of KC mice either treated with mIL-13-NAB or isotype control AB using immunofluorescent labeling and quantification. Scale bar: 50 μm.
Neutralization of IL-13 decreases fibrosis and proliferation of PanIN lesion cells
The neutralization of IL-13 correlated with a decrease in fibrosis as measured by several means. Masson’s trichrome staining indicated a decrease in collagen (Fig. 4A), and additional immunohistochemical staining for smooth muscle actin (SMA) and desmin indicated a decrease in stellate cells at pre-neoplastic lesions (Figs. 4B, 4C).
Figure 4. Neutralization of IL-13 decreases fibrosis in pancreatic lesions and the proliferation of PanIN lesion cells.
A–C: Indicated pancreatic tissues were analyzed for presence of collagen using trichrome staining (A), smooth muscle actin (SMA) (B) or desmin (C). D–G: Indicated pancreatic tissues were analyzed for Ki-67 (D) and quantified for Ki-67+ cells per PanIN1 (E); or analyzed for expression of phospho-T202/Y204-ERK1/2 as proliferation marker (F, G). Scale bars: 50 μm.
An analysis of Ki-67-positive cells in PanIN1 lesions suggested that the neutralization of IL-13 also negatively-affects proliferation of PanIN cells (Figs. 4D, 4E). Active ERK1/2 as detected by determining phosphorylation at T202/Y204 (labeled pERK1/2) is a typical marker for proliferating PanINs. Neutralization of IL-13 led to an over 90% reduction of pERK1/2 positive cells (Figs. 4F, 4G), indicating that factors secreted by IL-13-responsive macrophages may impact PanIN cell proliferation.
Anti-inflammatory molecules from Ym1+ macrophages induce the growth of PanIN lesions
Of 40 different cytokines or chemokines analyzed, supernatants of peritoneal Ym1+ macrophages mainly contained tissue inhibitor of metalloproteinase-1 (TIMP-1), interleukin-1 receptor antagonist (IL-1ra) and CCL2 at detectable levels (Suppl. Fig. S4A). An ISH combined with IHC for Ym1 in pancreatic PanIN1 regions of KC mice indicated that CCL2 is mainly expressed by Ym1+ cells (Fig. 5A), IL-1ra is expressed by both Ym1+ and PanIN cells (Fig. 5B), whereas TIMP-1 is expressed by a multitude of cells (Suppl. Fig. S4B). We therefore focused on CCL2 and IL-1ra in our further analyses. The receptors for both factors (IL-1R for IL-1ra and CCR2 for CCL2) are expressed in pancreatic lesion structures, and their expression remained unchanged when IL-13 was neutralized (Suppl. Figs. S4C, S4D). Although Ym1+ cells are the main producers of CCL2 mRNA (Fig. 5A), CCL2 protein is mainly detected at PanIN lesion cells (Fig. 5D), which also express CCR2 (Suppl. Fig. S4C). This as well as the dramatic decrease of CCL2 protein when IL-13 is neutralized (Figs. 5D, 5F) suggests that, while Ym1+ cells produce this factor, PanIN cells are main responders. In contrast to CCL2, depletion of Ym1+ macrophages by neutralization of IL-13 led only to a moderate decrease of IL-1ra mRNA (Fig. 5C), possibly due to its additional expression in PanIN cells (Fig. 5B). This translated to a moderate decrease of IL-1ra protein at pancreatic lesions (Figs. 5F, 5G)
Figure 5. CCL2 and IL-1ra are expressed by Ym1+ cells and induce the growth of PanIN lesions.
A, B: PanIN areas from KC mice were analyzed for CCL2 (A) or IL-1ra (B) mRNA expression (brown dots) using RNAscope ISH and Ym1 expression (pink) using IHC. The arrows indicate CCL2 or IL-1ra mRNA C: Quantification of CCL2 or IL-1ra mRNA expression from pancreata from KC or control mice treated as indicated. The asterisk indicates statistical significance (p<0.05) as compared to isotype control antibody group. D–G: Detection (D, F) and quantification (E, G) of CCL2 and IL-1ra protein in pancreas tissues of KC mice treated with indicated antibodies. Scale bars: 50 μm. The asterisk indicates statistical significance (p<0.05) as compared to the isotype control antibody group. H: Ductal structures of SM3 cells seeded on Matrigel and treated with control vehicle or indicated factors (50 ng/ml) at day 2. Scale bar: 50 μm. I: Ductal area covered by n=60 structures (sorted from smallest to largest) from three independent experiments for each condition at day 2. The asterisk indicates statistical significance (p<0.0001; One-way ANOVA test). J: SM3 cells were treated as indicated for 48 hours and analyzed by immunoblotting for pERK1/2, ERK1/2 and Akt. The fold increase was determined by quantification (Image J software) of three independent experiments.
To test if CCL2 or IL-1ra can affect the growth of ductal structures we used SM3 cells which were isolated from pre-neoplastic ductal structures. Grown on top of Matrigel, these cells form duct-like structures comparable to PanIN lesions; and treatment with IL-1ra as well as CCL2 increased their growth (Fig. 5H), in average covering double the ductal area (Fig. 5I). A combination of both did not lead to synergistic growth, indicating that both factors activate similar signaling pathways to mediate proliferation. One of them is through induction of ERK1/2 activity as measured by probing for its activating phosphorylations (Fig. 5J). Effects observed with IL-1ra were blocked with IL-1β, indicating that IL-1ra indeed acts on the IL-1 receptor (Suppl. Figs. S5A, S5B). Moreover, IL-1ra did not affect cell-cell adhesions as documented by intact localization of ZO-1 and E-cadherin (Suppl. Fig. S5C).
Overall, this suggested that alternatively-activated (Ym1+) macrophages not only affect collagen deposition and fibrosis in abnormal regions of the pancreas of KC mice, but also growth of duct-like structures. However, factors secreted by alternatively-activated macrophages show a high grade of redundancy in mediating growth of pre-neoplastic lesions and this explains the somewhat dramatic decreases of abnormal structures after treatment of mice with IL-13 NAB.
DISCUSSION
Chronic pancreatic inflammation contributes to the development of pancreatic cancer (Guerra et al., 2011; Guerra et al., 2007). Inflammatory macrophages in the pancreas induce ADM (Liou et al., 2013) and enhance formation of KRas-initiated precancerous lesions (Liou et al., 2015b). However, it is unknown at which point a switch in macrophage populations occurs to drive additional pro-tumorigenic events such as increased fibrogenesis. Our data now indicate that IL13, an anti-inflammatory cytokine that can re-polarize inflammatory macrophages to an alternatively-activated phenotype, is expressed in PanIN lesions.
Macrophages show high plasticity and can change their phenotype and physiology dependent on the microenvironment. Initial inflammatory macrophage populations attracted to the pancreas to induce ADM either in pancreatitis or oncogenic KRas settings are most likely of peritoneal origin (Gea-Sorli and Closa, 2009) since in vivo they can be in direct contact with ascitic fluid (Dugernier et al., 2000). While these inflammatory macrophages play an important role in the initiation of pancreatic precancerous lesions (Guerra et al., 2011; Liou et al., 2015b; Liou et al., 2013), in order to facilitate fibrogenesis and further progression they need to be replaced with alternatively-activated macrophages. This is because inflammatory processes and inflammatory macrophages can initiate apoptotic or necrotic responses of precancerous cells. Alternatively-activated macrophages are immunosuppressive (Huber et al., 2010; Momi et al., 2012), restrain the inflammatory response (Hao et al., 2012), facilitate angiogenesis (Lin et al., 2006) and accelerate lymphatic metastasis (Kurahara et al., 2011). Therefore, alternatively-activated macrophages are generally associated with poor prognosis (Kurahara et al., 2011).
Another long-standing question is if inflammatory macrophages during ADM-PanIN transition are re-polarized to become tumor-associated macrophages, or if these alternatively-activated macrophages are a different population recruited to the pancreas. In vitro, a conversion from inflammatory to alternatively-activated macrophages can be achieved after treatment with IL-4 or IL-13 (Stout et al., 2005). For chronic pancreatitis such a polarization shift can be initiated by IL-4/IL-13, secreted by Th and pancreatic stellate cells (Xue et al., 2015). However, it was unclear how such a shift occurs in developing pancreatic cancer (Gea-Sorli and Closa, 2009), and our data indicate that PanIN cells and Tuft cells within PanIN lesions are major producers of IL-13 (Fig. 2). The differences between both models may be due to the occurrence of cell death and acinar cell regeneration during pancreatitis, which may trigger different signaling in stellate cells.
We identified inflammatory macrophages in the pancreas as recipients for IL-13 since these cells express the receptor IL-13-Rα1 (Suppl. Fig. S2B). Moreover, IL-13 can mediate a transformation of primary peritoneal inflammatory macrophages to alternatively-activated Ym1+ macrophages in vitro (Suppl. Fig. S1A). Similarly, treatment of macrophages isolated from the pancreas of KC mice with IL-13 led to an increase in Ym+ populations (Suppl. Fig. S2C). In vivo, a similar switch in populations is detected in proximity to PanIN lesions (Fig. 1).
The desmoplastic reaction of PDA is characterized by increases in deposition of collagens and fibronectin, with collagen I as the predominant component. Our data using an IL-13 neutralization strategy in vivo, indicate that the prevention of the presence of Ym1+ macrophages at PanIN leads to a decrease in fibrosis and stellate cells, as determined with staining for SMA, desmin or collagen (Trichrome staining) (Figs. 4A–C). Another outcome of presence of Ym1+ macrophages at PanIN lesion is that they produce factors such as CCL2 and IL-1ra, which increase PanIN cell proliferation (Fig. 5 and Suppl. Figs. S4, S5). CCL2-producing macrophages have been identified as pro-cancerous (Hermano et al., 2014) and CCL2 has been shown to be critical for immunosuppression to promote metastasis (Kudo-Saito et al., 2013). Our finding that CCL2 also can regulate lesion growth is in line with data showing that inhibition of its receptor (CCR2) in orthotopic models of murine pancreatic cancer leads to decreased tumor growth (Sanford et al., 2013). IL-1ra also has anti-inflammatory functions since it antagonizes IL-1β which is produced within the pancreas during inflammation, and then reaches levels that are cytotoxic (Fink et al., 1997). In addition, IL-1β contributes to anti-proliferative and pro-apoptotic activities of inflammatory macrophages toward pancreatic cancer cells (Monti et al., 2003). In our model system IL-1ra may have dual functions. First it may attenuate inflammatory processes similar as it was shown for acute or chronic pancreatitis (Shen et al., 2012; Vasseur et al., 2014); and second it affects lesion growth. The latter may be due to activation of ERK1/2 signaling (Fig. 5J), but also to a block of oncogene-induced senescence, since senescent cells produce IL-1, and IL-1ra acts as an antagonist for this cytokine.
CCL2 serum levels in pancreatic cancer patients have been shown to positively correlate with tumor macrophage infiltration (Monti et al., 2003). Therefore, understanding of the crosstalk between different macrophage subtypes, pancreatic cells and the pancreatic microenvironment may allow the development of early detection markers. It also may lead to new possibilities for intervention. For example, targeting macrophages with CD40 agonists destructed the tumor stroma and reestablished the tumor immune surveillance in PDA (Beatty et al., 2011). Neutralizing antibodies for IL-13 as an anti-fibrotic strategy have been successfully tested in animal models for other diseases (Corren et al., 2011; Murray et al., 2014; Ramalingam et al., 2016), and our data now suggests that decreased fibrosis and PanIN progression can be achieved with IL-13 neutralizing antibodies (Figs. 3 and 4).
EXPERIMENTAL PROCEDURES
Antibodies and reagents
Antibodies used for Western blotting, immunohistochemistry, immunofluorescence and fluorescent cell sorting are described in detail in Supplemental Table 1. The neutralizing antibody for mouse IL-13 (mabg-mil13) was from InvivoGen (San Diego, CA), and its matching isotype antibody (mouse IgG1) was from R&D Systems. Recombinant murine EGF, murine CCL2, human IL-1ra, murine IL-1β, murine IL-4, murine IL-10 and murine IL-13 were purchased from PeproTech (Rocky Hill, NJ). Hoechst 33342 was from Invitrogen. DAPI was from Sigma-Aldrich, and Matrigel was from BD Biosciences.
Primary duct-like cells (SM3)
SM3 primary duct-like cells were isolated from pancreata of 6 week old Pdx1cre/+; KrasG12D/+ mice as previously described (Agbunag et al., 2006) and propagated in laminin (BD Biosciences). See Supplemental Experimental Procedures for maintenance of SM3 cells. To determine formation and growth of ductal structures, SM3 single cells were seeded on top of Matrigel (200 μl/well of a 24 well plate) and stimulated with indicated recombinant proteins (50 ng/ml) or vehicle control. Area of ductal structures formed was determined using Image J software.
Mouse lines and treatment
Ptf1a/p48cre/+ and LSL-KrasG12D/+ mouse strains and genotyping of mice have been described previously (Liou et al., 2015a; Liou et al., 2015b). To neutralize IL-13 in vivo, 3 week old mice (n=4 per treatment group) were intraperitoneally (IP) injected with mIL-13 neutralizing antibody (or isotype control antibody) at a dose of 1 mg/kg every other day for 7 weeks (Fig. 3A). The sex of the animal subjects was random, since we do not observe gender-specific differences using the KC animal model. All animal experiments were approved by the Mayo Clinic IACUC committee and were performed in accordance with relevant institutional and national guidelines and regulations.
Human pancreatic tissue samples
Patient tissues were obtained from archival materials in accordance with institutional guidelines and prior institutional review board (IRB) approval. The experiments using patient material are proof of principle experiments to confirm our mouse data.
Western Blotting
Cells were washed twice with ice-cold PBS (140 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2), lysed with lysis buffer A (50 mM Tris-HCl, pH 7.4, 1% Triton X-100, 150 mM NaCl, 5 mM EDTA, pH 7.4) containing 1X protease inhibitor cocktail (PIC; Sigma-Aldrich), and incubated on ice for 30 min. Samples were subjected to SDS-PAGE and then transferred to nitrocellulose membrane. Proteins of interest were detected using indicated specific primary antibodies and HRP-conjugated secondary antibodies.
Immunohistochemistry (DAB and IF)
Slides were deparaffinized, dewaxed and rehydrated as previously described (Liou et al., 2016). Antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0). Tissue samples were then treated with 3% H2O2 (5 min), washed with 0.5% Tween 20/PBS, and blocked with Protein Block Serum-Free Solution (DAKO; 5 min, RT). For DAB IHC, primary antibodies (dilutions listed in Supplemental Table 1) were diluted in Antibody Diluent Background Reducing Solution (DAKO) and visualized using EnVision Plus Anti-Rabbit Labelled Polymer Kit (DAKO), or biotin-streptavidin (Biocare Medical, Concord, CA) 2-step conjugation when primary goat antibodies were used. H&E staining was performed as described (Liou et al., 2015a). Masson Trichrome Stain Kit (Sigma-Aldrich) was used for trichrome staining. Images were captured using ScanScope XT scanner and ImageScope software (Aperio, Vista, CA). For fluorescent IHC, slides were incubated with primary antibodies (dilutions listed in Supplemental Table 1) in Antibody Diluent Background Reducing solution (DAKO) at 4°C, overnight. After 3 washes with 0.05% Tween-20/PBS, Alexa Fluor 488, 594, or 633 labeled secondary antibodies (Invitrogen) at a 1:500 dilution were added, and samples incubated at RT for 1 hr. For nuclear staining, 125 μg/ml DAPI was added to samples (RT, 5 min) after incubation with secondary antibodies. After several washes with 0.05 % Tween-20/PBS, LabVision PermaFluor (Thermo Scientific) was used as mounting medium. Images were captured using a ScanScope FL scanner and ImageScope software (Aperio).
RNAscope in situ hybridization (ISH) and IHC
In-situ hybridization (ISH) was performed using RNAscope® Assay 2.5 HD Reagent Kit-Brown (Advanced Cell Diagnostics [ACD], Hayward, CA) according to the manufacturer’s protocol, with some modifications (for details see Supplemental Experimental Procedures). ACD mouse target probes were IL1-ra (NM_001159562.1, region 203–1270, Il-13 (NM_008355.3, region 20–632), CCL2 (NM_011333.3, region 136–754) or TIMP-1 (NM_001044384.1, region 2–772), and mRNA signal was detected with DAB. To continue with IHC, ISH tissue samples were blocked with Protein Block (Dako, Carpinteria, CA) for 30 min at RT, and then incubated overnight at 4°C with indicated antibodies diluted in Antibody Diluent (Dako). After three washes with TBS, the slides were incubated with Rabbit on Rodent AP-Polymer (Biocare Medical, Concord, CA) for 30 min at RT, rinsed with TBS 3 times, treated with Warp Red Chromogen Kit (Biocare Medical), counterstained with hematoxylin, dehydrated and mounted. Images were captured using ScanScope XT scanner and ImageScope software (Aperio, Vista, CA).
Quantification and Statistical Analysis
All cell biological and biochemical experiments have been performed at least 3 times. Each repeat experiment was performed with a pancreas from a different individual mouse. For animal experiments, if not stated otherwise in the figure legends, n = 3 pancreatic samples have been used for quantification analyses. 5–6 fields per sample were subject to quantification using a 10x magnification. IHC data was quantified by manual counting of positive cells. Co-expression of proteins in cells was judged by analyses of IF for each protein on the same slide. Data are presented as mean ± SD. P values (if not stated otherwise in the figure legends) were acquired with the unpaired student’s t-test with Welch’s correction using Graph Pad software (GraphPad Inc., La Jolla, CA). p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the Chartrand Foundation, a pilot grant from the Mayo Clinic SPORE in Pancreatic Cancer (CA102701-12DRP3) and NIH CA200572 to PS, NIH P01 CA117969-07 to NB, and grants from the Hirshberg Foundation for Pancreatic Cancer Research, American Cancer Society (RSG-12-083-01-TBG), and NIH P01 CA163200 and NIH P01 DK098108 to DWD. We thank Veethika Pandey (statistical analyses), Brian Necela (isolation of primary macrophages), and Laura Lewis-Tuffin (FACS sorting) for help with the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: PS, GYL, LB, HD. Performed the experiments: GYL, LB, AF, HD, BHE. Analyzed the data: GYL, LB, AF, LZ, PS. Contributed reagents/materials/analysis tools: NB, DWD. Wrote the paper: PS, GYL, LB, AF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agbunag C, Lee KE, Buontempo S, Bar-Sagi D. Pancreatic duct epithelial cell isolation and cultivation in two-dimensional and three-dimensional culture systems. Methods Enzymol. 2006;407:703–710. doi: 10.1016/S0076-6879(05)07055-2. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, Cruz-Monserrate Z, Wang H, Ji B, Logsdon CD. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–1528. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, Hertzer K, Moro A, Donald G, Chang HH, Go VL, Pandol SJ, Lugea A, Gukovskaya AS, Li G, et al. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 2013;6:1064–1073. doi: 10.1158/1940-6207.CAPR-13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B, Wright CV, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146:233–244. e235. doi: 10.1053/j.gastro.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugernier T, Laterre PF, Reynaert MS. Ascites fluid in severe acute pancreatitis: from pathophysiology to therapy. Acta Gastroenterol Belg. 2000;63:264–268. [PubMed] [Google Scholar]
- Fink G, Yang J, Carter G, Norman J. Acute pancreatitis-induced enzyme release and necrosis are attenuated by IL-1 antagonism through an indirect mechanism. J Surg Res. 1997;67:94–97. doi: 10.1006/jsre.1996.4935. [DOI] [PubMed] [Google Scholar]
- Gea-Sorli S, Closa D. In vitro, but not in vivo, reversibility of peritoneal macrophages activation during experimental acute pancreatitis. BMC Immunol. 2009;10:42. doi: 10.1186/1471-2172-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermano E, Meirovitz A, Meir K, Nussbaum G, Appelbaum L, Peretz T, Elkin M. Macrophage polarization in pancreatic carcinoma: role of heparanase enzyme. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Daniluk J, Liu Y, Chu J, Li Z, Ji B, Logsdon CD. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2014;33:532–535. doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–3320. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- Kolodecik T, Shugrue C, Ashat M, Thrower EC. Risk factors for pancreatic cancer: underlying mechanisms and potential targets. Front Physiol. 2013;4:415. doi: 10.3389/fphys.2013.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, McEvoy J, Roussel MF, Dyer MA, Qualls JE, Murray PJ. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12:1902–1914. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo-Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin Exp Metastasis. 2013;30:393–405. doi: 10.1007/s10585-012-9545-6. [DOI] [PubMed] [Google Scholar]
- Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- Liou GY, Doppler H, Braun UB, Panayiotou R, Scotti Buzhardt M, Radisky DC, Crawford HC, Fields AP, Murray NR, Wang QJ, et al. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun. 2015a;6:6200. doi: 10.1038/ncomms7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Doppler H, DelGiorno KE, Zhang L, Leitges M, Crawford HC, Murphy MP, Storz P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep. 2016;14:2325–2336. doi: 10.1016/j.celrep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, Storz P. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov. 2015b;5:52–63. doi: 10.1158/2159-8290.CD-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Doppler H, Necela B, Krishna M, Crawford HC, Raimondo M, Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. J Cell Biol. 2013;202:563–577. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Storz P. Inflammatory macrophages in pancreatic acinar cell metaplasia and initiation of pancreatic cancer. Oncoscience. 2015;2:247–251. doi: 10.18632/oncoscience.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momi N, Kaur S, Krishn SR, Batra SK. Discovering the route from inflammation to pancreatic cancer. Minerva Gastroenterol Dietol. 2012;58:283–297. [PMC free article] [PubMed] [Google Scholar]
- Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–7461. [PubMed] [Google Scholar]
- Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, Flaherty KR, Lee J, Bell M, Knight DA, Martinez FJ, et al. Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. Am J Respir Cell Mol Biol. 2014;50:985–994. doi: 10.1165/rcmb.2013-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Ramalingam TR, Gieseck RL, Acciani TH, KMH, Cheever AW, Mentink-Kane MM, Vannella KM, Wynn TA. Enhanced protection from fibrosis and inflammation in the combined absence of IL-13 and IFN-gamma. J Pathol. 2016;239:344–354. doi: 10.1002/path.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Gao J, Zhang J, Xiang D, Wang X, Qian L, Yang L, Zhu S, Wu M, Yu Y, Han W. Recombinant human interleukin-1 receptor antagonist (rhIL-1Ra) attenuates caerulein-induced chronic pancreatitis in mice. Biomed Pharmacother. 2012;66:83–88. doi: 10.1016/j.biopha.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Hebrok M. Control of cell identity in pancreas development and regeneration. Gastroenterology. 2013;144:1170–1179. doi: 10.1053/j.gastro.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Vasseur P, Devaure I, Sellier J, Delwail A, Chagneau-Derrode C, Charier F, Tougeron D, Tasu JP, Rabeony H, Lecron JC, Silvain C. High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology. 2014;14:465–469. doi: 10.1016/j.pan.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.