Abstract
Background and Purpose
Low-grade and anaplastic oligodendrogliomas are often difficult to differentiate on the basis of conventional MR imaging characteristics. Dynamic contrast-enhanced (DCE) MRI can assess tumor microvasculature and has demonstrated utility for predicting glioma grade and prognosis in primary brain tumors. The aim of our study was to evaluate the performance of plasma volume (Vp) and volume transfer coefficient (Ktrans) derived from DCE MRI in differentiating between grade II and grade III oligodendrogliomas.
Materials and Methods
Twenty-four consecutive patients with pathologically confirmed oligodendroglioma (World Health Organization [WHO] grade II, n=14 and grade III, n=10) were retrospectively assessed. Pretreatment DCE MRI was performed and regions of interest were manually drawn around the entire tumor volume to calculate Vp and Ktrans. The Mann-Whitney U test and receiver operating characteristic analysis were performed to compare pharmacokinetic parameters between the 2 groups.
Results
The Vpmean values for grade III oligodendrogliomas were significantly higher (p=0.03) than those for grade II oligodendrogliomas. The Ktransmean values were higher in grade III lesions, but the difference between the 2 groups was not statistically significant (p>0.05). Based on receiver operating characteristic (ROC) analysis, the Vpmean (area under curve (AUC)=0.757, standard deviation=0.1) cut off value that provided the best combination of high sensitivity and specificity to distinguish between grade II and III oligodendrogliomas was 2.35 (p<0.03).
Conclusion
The results of our study suggest the DCE MRI parameter Vpmean can noninvasively differentiate between grade II and grade III oligodendrogliomas.
INTRODUCTION
Oligodendroglial tumors constitute approximately 5%–18% of all brain gliomas.1 They are mainly seen in adult patients with a peak of occurrence between the fourth and sixth decade of life although low-grade oligodendroglial tumors can appear in younger patients.2 These tumors are classified as grade II (low-grade) and III (anaplastic) according to the WHO classification system. The treatment and prognosis of low-grade and anaplastic oligodendrogliomas are different. Initial treatment of anaplastic oligodendrogliomas usually involves tumor resection, adjuvant radiation and often chemotherapy.3 In contrast, patients with low-grade oligodendrogliomas typically undergo less treatment and survive longer than patients with anaplastic tumors.4 Accurate determination of tumor grade in patients with oligodendrogliomas is therefore clinically relevant. The current standard for glioma grading is histopathologic analysis. This can only be accomplished after invasive neurosurgery and its inherent morbidity and mortality risks to the patients.5, 6 Additionally, this method is subject to sampling error and can be limited by tumor location in inaccessible brain regions, which can potentially result in inaccurate grading. A noninvasive method of confidently predicting tumor grade would help address some of these issues. Previous studies that analyzed the predictive capabilities of conventional MRI and dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion imaging for oligodendroglioma grading demonstrated mixed results 7–12 with neither method proving reliable enough for noninvasive tumor grading.
Dynamic contrast-enhanced (DCE)-MRI is an advanced imaging technique that has recently demonstrated promise for predicting glioma grade 13, 14, differentiating different primary brain tumor histologies 15 and predicting prognosis in brain tumor patients.16 Unlike conventional brain imaging, DCE-MRI can quantitatively assess tumor microvasculature by measurement of a range of parameters that reflect specific physiologic characteristics such as plasma volume (Vp), volume transfer coefficient (Ktrans) and extravascular extracellular distribution volume (Ve). DCE-MRI also offers several advantages over DSC perfusion imaging such as not being limited by susceptibility effects and allowing for more absolute quantitative measurements of pharmacokinetic parameters. The aim of our study was to evaluate the ability of DCE-MRI to differentiate between low-grade and anaplastic oligodendrogliomas. We hypothesized that Vp derived from the entire tumor volume would be an accurate predictor of grade since it provides an estimate of tumor microvascular density which is known to be increased in high-grade tumors.17, 18
MATERIALS AND METHODS
Patients
This retrospective study was performed in accordance with the Health Insurance Portability and Accountability Act and after local Institutional Review Board approval, including waiver of informed consent. Records of potentially eligible patients with oligodendroglioma from January 2011 through January 2015 were reviewed and included in the study based on the following criteria: 1) Histopathologically-confirmed oligodendroglioma diagnosis according to the WHO classification system; 2) pretreatment DCE-MRI perfusion scans. A total of 24 consecutive patients (13 female and 11 male) with a mean age of 45.2 (range, 39–82) years were included in the study. There were 14 patients with grade II (low-grade) oligodendrogliomas and 10 patients with grade III (anaplastic) oligodendrogliomas based on WHO criteria as determined by a neuropathologist with over 25 years of experience.
DCE MRI Acquisition and Analysis
Patients were scanned on 1.5T or 3T scanners (Signa Excite, HDx and Discovery 750, GE Healthcare, Milwaukee, WI) using an 8-channel head coil. Standard T1-weighted, T2-weighted, diffusion-weighted, fluid-attenuated inversion recovery, susceptibility-weighted and contrast T1-weighted images were acquired in multiple planes. Gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) was power-injected via an intravenous catheter (18–21 gauge) at doses standardized by patient body weight (0.2 mL/kg body weight, maximum 20 mL) at 2–3 mL/s. T1-weighted DCE perfusion data was acquired using an axial 3D echo-spoiled gradient-echo sequence (TR, 4–5 ms; TE, 1–2 ms; section thickness, 5 mm; flip angle, 25°; FOV, 24 cm; matrix, 256 × 128). Ten phases were acquired pre-injection followed by another 30 phases during the dynamic injection of intravenous contrast. This was followed by a 40-mL saline flush. The time between phases (temporal resolution) was 5–6 seconds. Matching contrast T1-weighted (TR/TE, 600/8 ms; thickness, 5 mm; matrix, 256 × 224) spin-echo images were obtained. Ten to twelve slices were obtained for the DCE color maps and matching T1 post contrast images to cover the volumes of the lesions. The native T1 was not measured and a fixed baseline value of 1000 ms was utilized.
An off-line workstation with available commercial imaging analysis software (NordicICE; Nordic Neuro Lab, Bergen, Norway) was used to process all raw perfusion data. Perfusion data preprocessing consisted of noise correction, motion artifact rectification and semi-automatic selection of arterial input function (AIF) from the middle cerebral artery. Curves displaying an optimal relationship between AIF and concentration-time curve were carefully chosen. The 2-compartment pharmacokinetic model proposed by Tofts [19] was applied to calculate the pharmacokinetic parameters plasma volume (Vp) and volume transfer coefficient (Ktrans). Regions of interest encompassing the entire enhancing and non-enhancing tumor volume were drawn on each transaxial slice, generating a volume of interest (VOI). Large vessels and cystic/necrotic regions were excluded from the VOI in order not to bias measurements. The volumes of interest (VOIs) were then transferred onto the matching Vp and Ktrans perfusion maps and the average of the mean values for all VOIs from each transaxial slice were used to calculate the mean Vp (Vpmean) and Ktrans (Ktransmean) of the entire lesion. A board-certified attending neuroradiologist with 10 years of neuroimaging experience approved all regions of interest. The data processing was performed with all investigators blinded to histopathologic tumor grade and patient demographics.
Statistical Analysis
A Mann-Whitney U test was used to assess the differences between the DCE-MRI pharmacokinetic parameters (Vp and Ktrans). A p value <0.05 was considered statistically significant. ROC curve analysis was performed using Statistical Package for the Social Sciences Statistics (Version 22; IBM, Armonk, New York) to determine which cut off values provided the best combination of sensitivity and specificity for differentiating between low-grade and anaplastic oligodendrogliomas.
RESULTS
Pharmacokinetic Parameters
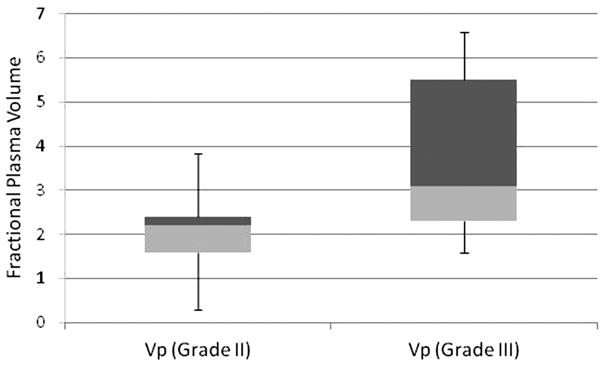
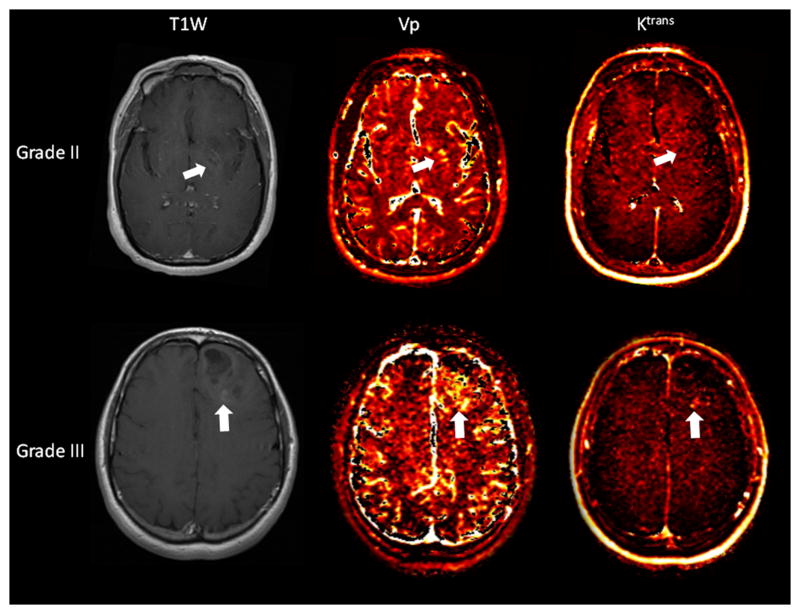
The Vpmean values for WHO grade III oligodendrogliomas were significantly higher (p=0.03) than those for grade II oligodendrogliomas (Figure 1, Table 1). Vpmean for grade III oligodendrogliomas was 3.78 (SD=1.83) and Vpmean for grade II oligodendrogliomas was 2.08 (SD=1.05). The Ktransmean values were also higher in grade III lesions (mean=0.057, SD=0.063) compared to grade II (mean=0.036, SD=0.036), but the difference between the 2 groups was not statistically significant (p>0.05). Representative images of grade II and grade III oligodendrogliomas are provided in Figure 2.
Figure 1.
Box plot illustrating the mean values and standard deviations for Vp of grade II and grade III oligodendrogliomas.
Table 1.
Demographics, Tumor Grade and Pharmacokinetic Parameters of Patients
| Patient | Age | Gender | Grade | Average VP | Average K12 |
|---|---|---|---|---|---|
| 1 | 82 | M | III | 1.59 | 0.222 |
| 2 | 71 | M | III | 5.95 | 0.046 |
| 3 | 43 | M | III | 6.58 | 0.1 |
| 4 | 69 | F | III | 5.71 | 0.032 |
| 5 | 43 | F | III | 3.2 | 0.01 |
| 6 | 63 | M | III | 4.96 | 0.038 |
| 7 | 58 | M | III | 2.38 | 0.0343 |
| 8 | 41 | F | III | 2.28 | 0.007 |
| 9 | 52 | M | III | 2.2 | 0.057 |
| 10 | 40 | M | III | 2.98 | 0.0263 |
| 11 | 42 | M | II | 1.13 | 0.139 |
| 12 | 64 | F | II | 3.84 | 0.0635 |
| 13 | 46 | F | II | 2.34 | 0.0104 |
| 14 | 42 | M | II | 2.21 | 0.013 |
| 15 | 61 | M | II | 0.363 | 0.004 |
| 16 | 48 | F | II | 2.35 | 0.007 |
| 17 | 42 | M | II | 1.92 | 0.019 |
| 18 | 42 | M | II | 1.57 | 0.026 |
| 19 | 51 | F | II | 2.22 | 0.0355 |
| 20 | 53 | M | II | 0.296 | 0.005 |
| 21 | 50 | F | II | 1.67 | 0.0261 |
| 22 | 39 | F | II | 3.45 | 0.044 |
| 23 | 47 | F | II | 2.41 | 0.0406 |
| 24 | 52 | F | II | 3.38 | 0.073 |
Figure 2.
Axial T1-weighted post contrast images with corresponding Vp and Ktrans perfusion color maps of grade II (top row) and grade III (bottom row) oligodendrogliomas. Both lesions demonstrate mild enhancement (arrows), but the grade III lesion demonstrates greater Vp than the grade II lesion. The Ktrans is also higher in the grade III lesion but the difference is less striking.
ROC Analysis
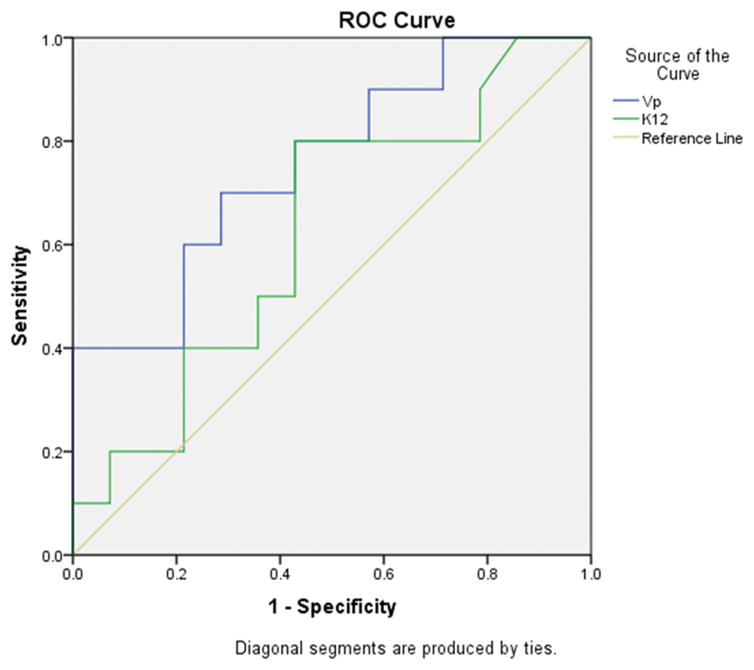
Based on ROC analysis, the cut-off for value Vpmean (AUC=0.757 with standard deviation=0.1) that provided the best combination of high sensitivity (70%) and specificity (70%) to distinguish between grade II and III oligodendrogliomas was 2.35 (p<0.03) (Figure 3).
Figure 3.
Receiver operating characteristic (ROC) curves depicting the true positive rate (specificity) and the false positive rate (sensitivity) of DCE-MRI pharmacokinetic parameters Vp and Ktrans in differentiating grade II versus grade III oligodendrogliomas.
DISCUSSION
In this study, we found that pretherapeutic measurements of Vpmean within oligodendrogliomas were predictive of tumor grade. Grading oligodendrogliomas accurately is important because the grade impacts how the tumors will be treated and the overall prognosis. Surgical morbidity, tumor inaccessibility, sampling errors and inconsistencies in histopathologic grading can lead to unfavorable results for patients. An accurate and reliable imaging predictor of tumor grade would be a useful tool to help optimize patient care. To our knowledge, this is the first study to demonstrate the utility of Vpmean as a noninvasive imaging biomarker for differentiating grade II and grade III oligodendrogliomas.
It has been well documented that rCBV measurements correlate with both conventional angiographic assessment of glioma vascular density and histologic determination of glioma microvascular density and vascular endothelial growth factor (VEGF) expression.19–23 VEGF is a potent angiogenic factor that is known to play an important role in induction of tumor neovascularization.24 High-grade gliomas, including oligodendrogliomas, have been shown to demonstrate increased VEGF expression and microvascular density compared to their lower grade counterparts.17, 18
Multiple studies have assessed the potential of relative cerebral blood volume (rCBV) measurements derived from DSC perfusion for differentiating between low-grade and anaplastic oligodendrogliomas. The results regarding the predictive value of rCBV have been mixed with some reports demonstrating significantly higher rCBV in grade III oligodendrogliomas 25–28 and others reporting that rCBV values were not reliable for making the distinction.10, 11, 29–31 The reasons for the inconsistencies in the literature are probably multi-factorial, but may in part be due to the inherent limitations of DSC technique. For example, rCBV measurements derived from DSC are semi-quantitative and can be influenced by multiple post-processing steps, including the choice of normal contralateral white matter and correction technique to address contrast extravasation.32, 33 Other limitations of DSC perfusion imaging include sensitivity to susceptibility effects from calcification, hemorrhage and bone 34 and potential for biased measurements secondary to T1 effects from extravascular contrast leakage in tumor vessels.35
Instead of DSC perfusion, we utilized DCE-MRI which is a T1-weighted perfusion technique that is less sensitive to susceptibility artifacts and can better quantify absolute cerebral blood volume and the volume transfer coefficient (Ktrans), which measures the degree of contrast leakage from the intravascular to the extravascular compartment. Vp is a pharmacokinetic parameter derived from DCE-MRI and is defined as the blood plasma volume per unit volume of tissue. This parameter has the same physiologic meaning as rCBV obtained from DSC but Vp has been demonstrated to be superior for quantitative evaluation of cerebral blood volume 36 because it is based on the preferred T1-weighted perfusion method. A recent study comparing estimates of blood volume obtained by DCE-MRI and DSC perfusion on a pixel-by-pixel basis within high-grade gliomas found a statistically significant difference between the 2 sets of values and only a weak (although significant) correlation.37 There was also a statistically significant difference and weak correlation between Ktrans and Vp suggesting they provide different information.
Multiple investigators have reported the usefulness of DCE-MRI for glioma grading 13, 14, 38–40 but these studies included a broader (grades II-IV) and more heterogeneous group of low- and high-grade gliomas such as diffuse astrocytomas, oligoastrocytomas, anaplastic astrocytomas and glioblastomas, in addition to oligodendrogliomas. We focused only on differentiating grade II and grade III oligodendrogliomas in an attempt to begin addressing this relative gap in the DCE-MRI literature and because DSC perfusion studies have been inconsistent. An investigation by Jia et al that employed DCE-MRI for differentiating oligodendroglioma grades 41 found that Ktrans and Ve could distinguish between grade II and grade III oligodendrogliomas in a statistically significant manner. Interestingly, our study did not demonstrate that measurements of Ktrans were significantly different between grades. The reasons for this are unclear but may be related to our smaller sample size of 24 lesions (14 grade II, 10 grade III) compared to their 65 lesions (28 grade II and 37 grade III). The Jia et al study focused on differences in tumor microvascular permeability and did not report on Vp, an estimate of microvascular density and blood volume, which we found to be a statistically significant (p<0.03) imaging biomarker for differentiating grade II and grade III oligodendrogliomas despite a small number of patients.
Another difference between this study and numerous prior DCE-MRI and DSC perfusion studies for glioma grading is that we used the entire tumor volume to generate the data as opposed to the more common approach of selecting only the most abnormal appearing ROIs within tumor volumes. Quantitative analysis of the entire tumor is potentially a more reproducible and less subjective method than selecting ROIs, which vary in number and size from study to study and observer to observer. Several DCE-MRI glioma-grading studies that generated data from whole tumor volumes demonstrated that pharmacokinetic parameters could successfully differentiate grade II and grade III gliomas. 13, 14
Aside from its retrospective nature, our study had several additional limitations. Some of the oligodendrogliomas we analyzed had poorly-defined margins and delineation of the tumor from normal adjacent parenchyma or edema was occasionally difficult. Even though this could potentially result in diminished reproducibility, we believe the quantitative data extracted from the inclusion of the entire tumor volume would more than compensate for the relatively minor errors or differences in exactly defining the tumor edges. Another limitation is that our data may have been influenced by the relatively small number of lesions analyzed, particularly as it pertains to Ktrans, which has been shown to be a reliable perfusion parameter for glioma grading in multiple prior studies. We also did not assess extravascular extracellular distribution volume (Ve), which has been demonstrated in the literature to be useful for brain tumor analysis. Unpublished data at our institution has demonstrated that Ve is not as helpful as other DCE parameters. One possible explanation is that our total DCE acquisition time of 3 minutes and 20 seconds to 4 minutes may not be sufficiently long enough to reach the washout phase. It has been suggested that longer acquisition times may be necessary for this parameter to be accurately assessed.42, 43 The assumption of a fixed T1 value is another potential limitation as some studies have shown that Ktrans estimates can vary based on native T1 values, whereas other reports have concluded this is a minor limitation and that fixed baseline T1 values may actually contribute to more consistent results while protecting dynamic data from incorrect scale factors or patient movement during DCE acquisition.13, 44, 45 Finally, we were not able to correlate pharmacokinetic parameters with molecular differences between oligodendrogliomas because of (1) unavailable data and (2) the overwhelming majority of the remaining lesions demonstrated 1p/19q chromosomal co-deletions which did not allow for meaningful subgroup comparison.
CONCLUSIONS
The results of our study suggest that pretherapeutic quantitative analysis of the DCE-MRI parameter Vpmean can noninvasively differentiate between grade II and grade III oligodendrogliomas. Larger and prospective studies are needed to validate our findings.
Acknowledgments
Grant: NIH cancer center (P30 CA004748)
Grant support: Julio Arevalo-Perez was supported by a grant from the Spanish foundation “Fundación Alfonso Martín Escudero”.
Abbreviations
- WHO
World Health Organization
- AUC
area under curve
- DCE
dynamic contrast-enhanced
- VP
plasma volume
- Ktrans
contrast transfer coefficient
- AIF
arterial input function
- ROC
receiver operating characteristic
- VEGF
vascular endothelial growth factor
References
- 1.Chawla S, Krejza J, Vossough A, et al. Differentiation between oligodendroglioma genotypes using dynamic susceptibility contrast perfusion-weighted imaging and proton MR spectroscopy. AJNR Am J Neuroradiol. 2013;34:1542–9. doi: 10.3174/ajnr.A3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromberg JE, van den Bent MJ. Oligodendrogliomas: molecular biology and treatment. Oncologist. 2009;14:155–63. doi: 10.1634/theoncologist.2008-0248. [DOI] [PubMed] [Google Scholar]
- 3.Le Rhun E, Taillibert S, Chamberlain MC. Anaplastic glioma: current treatment and management. Expert Rev Neurother. 2015;15:601–20. doi: 10.1586/14737175.2015.1042455. [DOI] [PubMed] [Google Scholar]
- 4.Forst DA, Nahed BV, Loeffler JS, Batchelor TT. Low-grade gliomas. Oncologist. 2014;19:403–13. doi: 10.1634/theoncologist.2013-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field M, Witham TF, Flickinger JC, Kondziolka D, Lunsford LD. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg. 2001;94:545–51. doi: 10.3171/jns.2001.94.4.0545. [DOI] [PubMed] [Google Scholar]
- 6.Kreth FW, Muacevic A, Medele R, Bise K, Meyer T, Reulen HJ. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours--a prospective study. Acta Neurochir. 2001;143:539–45. doi: 10.1007/s007010170058. discussion 45–6. [DOI] [PubMed] [Google Scholar]
- 7.Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. Am J Neuroradiol. 2004;25:746–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg LE, Fuller GN, Hashmi M, Leeds NE, Schomer DF. The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg Neurol. 1998;49:436–40. doi: 10.1016/s0090-3019(97)00360-1. [DOI] [PubMed] [Google Scholar]
- 9.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology. 1999;211:791–8. doi: 10.1148/radiology.211.3.r99jn46791. [DOI] [PubMed] [Google Scholar]
- 10.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected] AJNR Am J Neuroradiol. 2004;25:214–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, See SJ, Ng WH, et al. Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery. 2005;56:919–26. discussion -26. [PubMed] [Google Scholar]
- 12.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–98. [PMC free article] [PubMed] [Google Scholar]
- 13.Jung SC, Yeom JA, Kim JH, et al. Glioma: Application of histogram analysis of pharmacokinetic parameters from T1-weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR Am J Neuroradiol. 2014;35:1103–10. doi: 10.3174/ajnr.A3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arevalo-Perez J, Peck KK, Young RJ, Holodny AI, Karimi S, Lyo JK. Dynamic Contrast-Enhanced Perfusion MRI and Diffusion-Weighted Imaging in Grading of Gliomas. J Neuroimaging. 2015 doi: 10.1111/jon.12239. [DOI] [PMC free article] [PubMed]
- 15.Kickingereder P, Sahm F, Wiestler B, et al. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am J Neuroradiol. 2014;35:1503–8. doi: 10.3174/ajnr.A3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen TB, Cron GO, Mercier JF, et al. Preoperative prognostic value of dynamic contrast-enhanced MRI-derived contrast transfer coefficient and plasma volume in patients with cerebral gliomas. AJNR Am J Neuroradiol. 2015;36:63–9. doi: 10.3174/ajnr.A4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan AS, Leung SY, Wong MP, et al. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. The Am J Surg Pathol. 1998;22:816–26. doi: 10.1097/00000478-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry IH, O’Donovan DG, Brenchley PE, Reid H, Roberts IS. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology. 2001;39:409–15. doi: 10.1046/j.1365-2559.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 19.Cha S, Johnson G, Wadghiri YZ, et al. Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn Reson Med. 2003;49:848–55. doi: 10.1002/mrm.10446. [DOI] [PubMed] [Google Scholar]
- 20.Maia AC, Jr, Malheiros SM, da Rocha AJ, et al. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol. 2005;26:777–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 22.Aronen HJ, Pardo FS, Kennedy DN, et al. High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin Cancer Res. 2000;6:2189–200. [PubMed] [Google Scholar]
- 23.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol. 1998;171:1479–86. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]
- 24.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Human pathology. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor GS, Gocke TA, Chawla S, et al. Magnetic resonance perfusion-weighted imaging defines angiogenic subtypes of oligodendroglioma according to 1p19q and EGFR status. J Neurooncol. 2009;92:373–86. doi: 10.1007/s11060-009-9880-x. [DOI] [PubMed] [Google Scholar]
- 26.Fellah S, Caudal D, De Paula AM, et al. Multimodal MR imaging (diffusion, perfusion, and spectroscopy): is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am J Neuroradiol. 2013;34:1326–33. doi: 10.3174/ajnr.A3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitmore RG, Krejza J, Kapoor GS, et al. Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J Neurosurg. 2007;107:600–9. doi: 10.3171/JNS-07/09/0600. [DOI] [PubMed] [Google Scholar]
- 28.Spampinato MV, Smith JK, Kwock L, et al. Cerebral blood volume measurements and proton MR spectroscopy in grading of oligodendroglial tumors. AJR Am J Roentgenol. 2007;188:204–12. doi: 10.2214/AJR.05.1177. [DOI] [PubMed] [Google Scholar]
- 29.Jenkinson MD, Smith TS, Joyce KA, et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology. 2006;48:703–13. doi: 10.1007/s00234-006-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilario A, Ramos A, Perez-Nunez A, et al. The added value of apparent diffusion coefficient to cerebral blood volume in the preoperative grading of diffuse gliomas. AJNR Am J Neuroradiol. 2012;33:701–7. doi: 10.3174/ajnr.A2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalid L, Carone M, Dumrongpisutikul N, et al. Imaging characteristics of oligodendrogliomas that predict grade. AJNR Am J Neuroradiol. 2012;33:852–7. doi: 10.3174/ajnr.A2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellingson BM, Zaw T, Cloughesy TF, et al. Comparison between intensity normalization techniques for dynamic susceptibility contrast (DSC)-MRI estimates of cerebral blood volume (CBV) in human gliomas. JMRI J Magn Reson Imaging. 2012;35:1472–7. doi: 10.1002/jmri.23600. [DOI] [PubMed] [Google Scholar]
- 33.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–67. [PMC free article] [PubMed] [Google Scholar]
- 34.Calamante F, Vonken EJ, van Osch MJ. Contrast agent concentration measurements affecting quantification of bolus-tracking perfusion MRI. Magn Reson Med. 2007;58:544–53. doi: 10.1002/mrm.21362. [DOI] [PubMed] [Google Scholar]
- 35.Boxerman JL, Prah DE, Paulson ES, Machan JT, Bedekar D, Schmainda KM. The Role of preload and leakage correction in gadolinium-based cerebral blood volume estimation determined by comparison with MION as a criterion standard. AJNR Am J Neuroradiol. 2012;33:1081–7. doi: 10.3174/ajnr.A2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hacklander T, Reichenbach JR, Modder U. Comparison of cerebral blood volume measurements using the T1 and T2* methods in normal human brains and brain tumors. J Comput Assist Tomogr. 1997;21:857–66. doi: 10.1097/00004728-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Alcaide-Leon P, Pareto D, Martinez-Saez E, Auger C, Bharatha A, Rovira A. Pixel-by-pixel comparison of volume transfer constant and estimates of cerebral blood volume from dynamic contrast-enhanced and dynamic susceptibility contrast-enhanced MR imaging in high-grade gliomas. AJNR Am J Neuroradiol. 2015;36:871–6. doi: 10.3174/ajnr.A4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awasthi R, Rathore RK, Soni P, et al. Discriminant analysis to classify glioma grading using dynamic contrast-enhanced MRI and immunohistochemical markers. Neuroradiology. 2012;54:205–13. doi: 10.1007/s00234-011-0874-y. [DOI] [PubMed] [Google Scholar]
- 39.Jia Z, Geng D, Xie T, Zhang J, Liu Y. Quantitative analysis of neovascular permeability in glioma by dynamic contrast-enhanced MR imaging. J Clin Neurosci. 2012;19:820–3. doi: 10.1016/j.jocn.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 40.Mills SJ, Soh C, O’Connor JP, et al. Enhancing fraction in glioma and its relationship to the tumoral vascular microenvironment: A dynamic contrast-enhanced MR imaging study. AJNR Am J Neuroradiol. 2010;31:726–31. doi: 10.3174/ajnr.A1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Z, Geng D, Liu Y, Chen X, Zhang J. Low-grade and anaplastic oligodendrogliomas: differences in tumour microvascular permeability evaluated with dynamic contrast-enhanced magnetic resonance imaging. J Clin Neurosci. 2013;20:1110–3. doi: 10.1016/j.jocn.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Alcaide-Leon P, Rovira A. Dynamic contrast-enhanced MR: importance of reaching the washout phase. AJNR Am J Neuroradiol. 2013;34:E58–9. doi: 10.3174/ajnr.A3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathore RK, Gupta RK. Dynamic contrast-enhanced MR: importance of reaching the washout phase. Author reply AJNR Am J Neuroradiol. 2013;34:E60. doi: 10.3174/ajnr.A3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo JY, Reddick WE, Rosen MA, Song HK. Dynamic contrast-enhanced magnetic resonance imaging parameters independent of baseline T10 values. Magn Reson Imaging. 2009;27:1208–15. doi: 10.1016/j.mri.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haacke EM, Filleti CL, Gattu R, et al. New algorithm for quantifying vascular changes in dynamic contrast-enhanced MRI independent of absolute T1 values. Magn Reson Med. 2007;58:463–72. doi: 10.1002/mrm.21358. [DOI] [PubMed] [Google Scholar]