Highlights
-
•
We report an ovarian cancer patient with a prolonged response to immunotherapy.
-
•
Comprehensive genomic profiling may detect patients who benefit from immunotherapy.
-
•
Mutational burden thresholds for ovarian cancer may be lower than other cancers.
1. Introduction
Among gynecologic malignancies, ovarian cancer is the second most common and the leading cause of mortality. Studies have demonstrated that immunologic response can impact prognosis, and with the rapid development of cancer immunotherapy, clinicians must determine how to identify patients most likely to benefit. Here, we report the case of a patient with platinum-resistant ovarian cancer who had a durable response to avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody, with comprehensive genomic profiling (CGP) obtained before and after treatment. We highlight the role of immune checkpoint inhibition in ovarian cancer, associated toxicities, and genetic factors that may have contributed to her response.
2. Case report
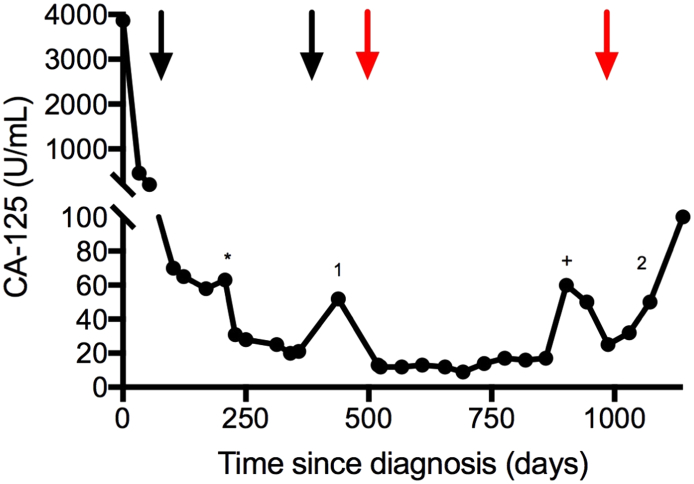
A 71-year-old female presented with abdominal pain, bloating, and dyspnea. A computed tomography (CT) scan showed large volume ascites, peritoneal carcinomatosis, bilateral pleural effusions, and a pulmonary embolism. Cytologic examination of the paracentesis specimen revealed adenocarcinoma of Muellerian origin. Given the patient's advanced disease and medical comorbidities, she was started on neoadjuvant weekly carboplatin (AUC 2 mg/mL/min) and paclitaxel (60 mg/m2). Cancer antigen 125 (CA-125) declined from 3866 U/mL to 58 U/mL after five cycles (Fig. 1). Subsequent complete surgical cytoreduction with exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, and omentectomy resulted in no gross residual disease and microscopic residual high-grade serous carcinoma in the right ovary and omentum.
Fig. 1.
Trend in CA-125 (U/mL) over treatment course. Black arrows indicate the start/end of carboplatin/paclitaxel chemotherapy; red arrows indicate the start/end of avelumab treatment. The first and second recurrences are denoted by 1 and 2, respectively. Cytoreductive surgery is indicated by the (*) and diagnosis of pneumonitis concurrent with transient CA-125 elevation is indicated by the (+). CA-125; cancer antigen 125. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Four additional cycles of chemotherapy resulted in a continued decline in CA-125 to 21 U/mL and no evidence of disease on CT scan and physical exam. Genetic counseling recommended germline testing, which was negative for inherited mutations.
Three months later, a platinum-resistant ovarian cancer recurrence was recognized by a rising CA-125 to 52 U/mL and a new 1.1 cm pleural nodule on CT scan. Subsequently, the patient began immune checkpoint inhibitor (ICPi) therapy with the anti-PD-L1 antibody avelumab (10 mg/kg IV, q2 weeks) on a phase 1 clinical trial (Disis et al., 2015). Her CA-125 declined, ranging from 9 to 16 U/mL (Fig. 1), and multiple sub-centimeter nodules remained stable on CT scan, a partial response by RECIST 1.1 criteria. The patient initially tolerated avelumab therapy well, reporting only minimal fatigue.
After 10 months of treatment, she presented with shortness of breath and was diagnosed with a pleural effusion. Thoracentesis was negative for malignancy and CT scan showed a new left lower lobe ground glass opacity. Pulmonary consultation was obtained per study protocol and pulmonary function testing showed moderately decreased diffusion capacity and prednisone was started for pneumonitis with resolution of dyspnea and resolution of radiographic findings. Follow-up CT scan six months later demonstrated increasing ground glass opacities. Pulmonology recommended bronchoscopy, which was negative for infection but revealed lymphocytic alveolitis with 64% lymphocytes in the lavage fluid, consistent with recurrent avelumab-induced pneumonitis, prompting discontinuation of avelumab after 16.5 months of therapy.
To identify additional therapeutic options, a formalin-fixed, paraffin-embedded cell block prepared from the diagnostic ascites fluid was sent for CGP (FoundationOne®) (Frampton et al., 2013), revealing a tumor mutational burden of 10.2 mutations/megabase (Mb) and 10 clinically relevant genomic alterations (CRGA), including a somatic, loss of function BRCA2 mutation (Table 1).
Table 1.
Somatic tumor mutations from peritoneal fluid (initial diagnosis) and from pelvic lymph node (recurrent disease) detected by next-generation sequencing assay.
| Specimen | NGS panel | Mutations detected |
|---|---|---|
| Peritoneal fluid (prior to chemotherapy and anti-PD-L1 therapy) | FoundationOne® (315 genes) |
BRCA2, c.5079delT, p.R1694fs*12 EGFR, c.940G > A, p.D314N TET2, c.3768_3769insACGGC, p.L1258fs*10 TP53, c.394A > C, p.K132Q MAP2K4 splice site c.115_115 + 62del63 NOTCH1 splice site c.5638 + 2T > G FGFR4 amplification TERC amplification PRKCl amplification RB1 loss exons 19–26 |
| Pelvic lymph node biopsy (after chemotherapy and anti-PD-L1 therapy) | NCI-MATCH (167 genes) |
BRCA2, c.5079delT, p.R1694fs*12 TET2, c.3768_3769insACGGC, p.L1258fs*10 TP53, c.394A > C, p.K132Q |
Differences in the testing results can be attributed to the relative sizes of the gene panels, test sensitivities, and the types of alterations each assay is designed to detect. Abbreviations: NGS, next-generation sequencing; PD-L1, programmed death-ligand 1; NCI-MATCH, National Cancer Institute Molecular Analysis for Therapy Choice.
During the subsequent eight weeks, the patient's CA-125 began to rise and she developed bulky retroperitoneal lymphadenopathy, which was biopsied for NCI-MATCH tumor testing. This assay, which identifies fewer genes, identified 3 CRGA, all concordant with the CGP results, and confirmed the deleterious BRCA2 mutation (Table 1). Based on disease progression and BRCA2 mutation, treatment with the PARP inhibitor rucaparib (600 mg twice daily) was initiated and the patient has had a partial response.
3. Discussion
Over the past two decades, growing evidence has established that critical interactions occur between the immune system and ovarian cancer. The presence of tumor infiltrating lymphocytes has a significant positive impact on both progression-free and overall survival (Zhang et al., 2003). Furthermore, The Cancer Genome Atlas (TCGA) defined four distinct clusters of ovarian cancer based on gene expression, demonstrating that the immunoreactive subgroup has the best survival (Cancer Genome Atlas Research Network et al., 2011). However, significant obstacles remain in harnessing the immune system to treat ovarian cancer.
Many cancers escape elimination by the immune system via binding of programmed death 1 (PD-1), a T-cell coinhibitory protein, to its ligand PD-L1, which is expressed on the surface of many tumors (Topalian et al., 2015). The interaction between PD-1 and PD-L1 is an active area of clinical investigation. One study of 207 patients with non-small cell lung cancer (NSCLC), melanoma, colorectal, renal cell, ovarian, or pancreatic cancer treated with the anti-PD-L1 antibody BMS-936559 reported objective response rates (ORR) of 6–17% with a median duration of therapy of 12 weeks (Brahmer et al., 2012). A companion study of 296 patients who received the PD-1 inhibitor nivolumab (BMS-936558), reported durable ORR of 18–28% among patients with melanoma, renal-cell cancer, or NSCLC (Topalian et al., 2012). In both studies, treatment was well tolerated with grade 3–4 adverse events reported in 9–14% of patients. Biomarkers that potentially identify ICPi responders include immunohistochemical stains for PD-1/PD-L1 protein expression and tumor mutational burden (TMB). PD-L1 positive tumors demonstrated a 36% (9/25) ORR to nivolumab, compared to 0% (0/17) for negative staining.
Avelumab, a fully human anti-PD-L1 IgG1 monoclonal antibody inhibits the immunosuppressive effect of antigen-specific T-cell activation by blocking PD-1/PD-L1 binding. In a cohort of 75 pretreated patients with recurrent or refractory ovarian cancer, avelumab demonstrated clinically activity with a 33.3% progression-free survival (PFS) rate at 24 weeks and a median PFS of 11.9 weeks (Disis et al., 2015). The PD-1 inhibitor nivolumab has also shown activity in platinum-resistant ovarian cancer in a recent phase II trial reporting a 15% ORR and a 45% disease control rate among 20 patients (Hamanishi et al., 2015).
This patient with platinum-resistant ovarian cancer experienced a durable response to single-agent avelumab therapy for 16.5 months, much longer than the 3–4 month responses typically observed with single-agent chemotherapy (Pujade-Lauraine et al., 2014). Interestingly, CGP of DNA extracted from cells in peritoneal fluid collected at initial diagnosis showed a TMB of 10.2 mutations/Mb in addition to 10 clinically relevant genomic alterations (Table 1). In comparison, a recent study assessing 38 ovarian carcinomas by CGP reported an average of 2.9 genomic alterations (range 0–8) and 1.4 clinically actionable mutations (range 0–5) per patient (Ross et al., 2013).
To better understand the potential relationship between this specimen's genomic profile and ICPi response, we compared this case to CGP results (FoundationOne®), including TMB, from 915 predominantly advanced ovarian epithelial carcinomas (Fig. 2) (Elvin et al., 2017). In this series, TMB ranged from zero to 309.9 mutations/Mb, with a median of 3.6 mutations/Mb and a mean of 5.3 mutations/Mb. The upper decile was 8.8 mutations/Mb and illustrates the relatively high TMB of this patient's tumor (10.2 mutations/Mb). The TMB scores observed for ovarian carcinomas are significantly lower than other carcinomas, such as NSCLC and melanoma, for which higher rates of response to ICPi therapy are observed and thresholds for TMB-high scores are close to 20 mutations/Mb. This suggests that the high number of somatic mutations observed in this patient's carcinoma may have contributed to the durable response to avelumab. Lower TMB thresholds for ovarian carcinoma may be needed to identify patients who respond similarly.
Fig. 2.
(A) Peritoneal fluid cell block (H&E, 200 ×) shows abundant single high grade carcinoma cells and small clusters. (B) The distribution of TMB across 915 ovarian epithelial carcinomas - 90th percentile = 8.8 muts/Mb; 75th percentile = 5.7 muts/Mb; 50th percentile = 3.6 muts/Mb; 25th percentile = 1.8 muts/Mb. Patient sample with TMB of 10.2 mutations/Mb ranked as 92th percentile. TMB, tumor mutational burden; muts, mutations; Mb, megabase.
In ovarian cancer, the concept of a “mutator phenotype” has been observed with respect to chemotherapy response. An analysis of the 316 cases from TCGA using whole-exome deep-sequencing demonstrated that BRCA2-mutated cases were enriched with hypermutated samples compared to BRCA wild-type cases (Yang et al., 2011). Additionally, BRCA2-mutated cases were associated with higher chemotherapy response rates and longer PFS. ADAMTS-mutated cases of ovarian cancer are associated with higher genome-wide mutation rates and higher chemotherapy response rates, longer platinum-free duration, and better PFS and overall survival (Liu et al., 2015). There is growing evidence that ovarian cancers with a higher somatic mutation burden also respond better to cytotoxic chemotherapy.
Ultimately, our patient developed pneumonitis, which responded to steroid treatment and she was allowed to continue avelumab after pulmonology consultation since she was benefiting from therapy per study protocol. However, six months later she developed recurrent pneumonitis, confirmed on bronchoscopy, and pulmonology recommended stopping therapy. After discontinuation of avelumab, she developed bulky retroperitoneal lymphadenopathy and elevated CA-125. Pulmonary disorders such as pneumonitis are uncommon but have been reported with ICPi (Topalian et al., 2012). More common side effects of PD-L1 inhibitors are diarrhea, fatigue, arthralgia, rash, nausea, pruritus, headache, and infusion-reactions (Brahmer et al., 2012). Among ovarian cancer patients specifically, endocrine disorders, including hypothyroidism and autoimmune thyroiditis, may occur in 40% of patients (Hamanishi et al., 2015).
This case highlights an impressive and prolonged response to anti-PD-L1 antibody in an ovarian cancer patient with a relatively high number of somatic tumor mutations, suggesting that, similar to melanoma and other carcinomas, TMB may be a biomarker that identifies patients who will respond to ICPi. In contrast to other carcinomas, which generally demonstrate higher median TMB and ICPi response thresholds, an ovarian-specific threshold may be considerably lower. Broader evaluation of samples from ovarian ICPi clinical trials with correlation to TMB scores would be useful to validate this hypothesis and set an appropriate clinical threshold. This case also reinforces the potential clinical value of CGP for ovarian tumor samples. In addition to germline mutation testing for all ovarian cancer patients, further analysis by tumor-based sequencing may reveal somatic alterations in known biomarkers, such as BRCA, and identify those patients more likely to respond to immunotherapy.
Conflicts of interest
Christopher B. Morse reports no conflicts of interest. Julia A. Elvin and Laurie M. Gay report employment by Foundation Medicine, Inc. John B. Liao reports research funding from Merck through his institution.
Informed consent
Written informed consent was obtained from the patient for the publication of this case report.
Acknowledgement
John B. Liao is supported by the Department of Defense, Ovarian Cancer Research Program, Ovarian Cancer Academy: Early-Career Investigator Award, W81XWH-14-1-0161.
References
- Brahmer J.R. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma supplement. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis M.L. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with previously treated, recurrent or refractory ovarian cancer: a phase Ib, open-label expansion trial. J. Clin. Oncol. 2015;33:5509. [Google Scholar]
- Elvin J.A. Poster presented at: Annual Meeting of the United States and Canadian Academy of Pathology, San Antonio, TX, March 4–10, 2017. 2017. Profiling of tumor mutational burden (TMB), microsatellite instability (MSI), and PD1/PD-L1 immunohistochemistry (IHC) in gynecological tumors. [Google Scholar]
- Frampton G.M. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanishi J. Safety and antitumor activity of Anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- Liu Y. Somatic mutations of ADAMTS genes with chemotherapy sensitivity and survival in high-grade serous ovarian carcinoma. JAMA Oncol. 2015;77030:486–494. doi: 10.1001/jamaoncol.2015.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Lauraine E. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- Ross J.S. Comprehensive genomic profiling of epithelial ovarian cancer by next generation sequencing-based diagnostic assay reveals new routes to targeted therapies. Gynecol. Oncol. 2013;130:554–559. doi: 10.1016/j.ygyno.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Topalian S.L. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]