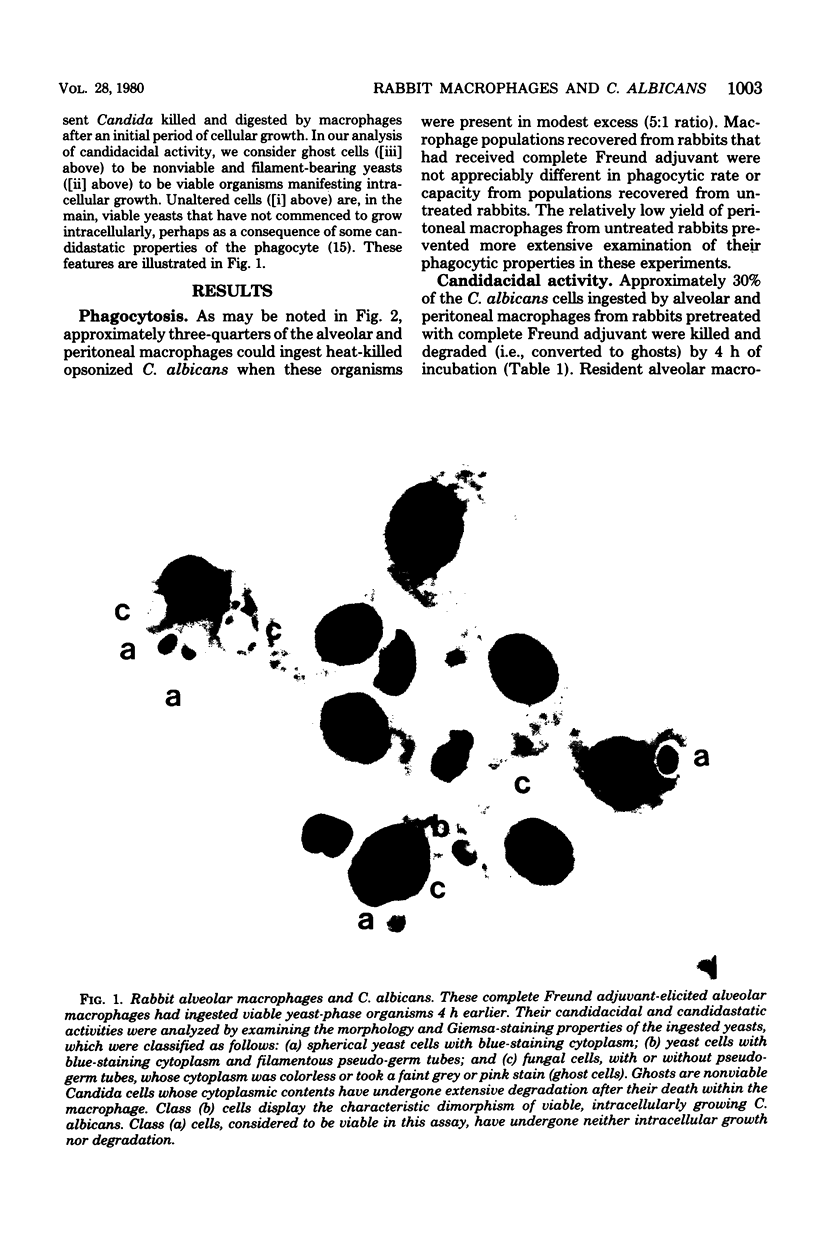
Abstract
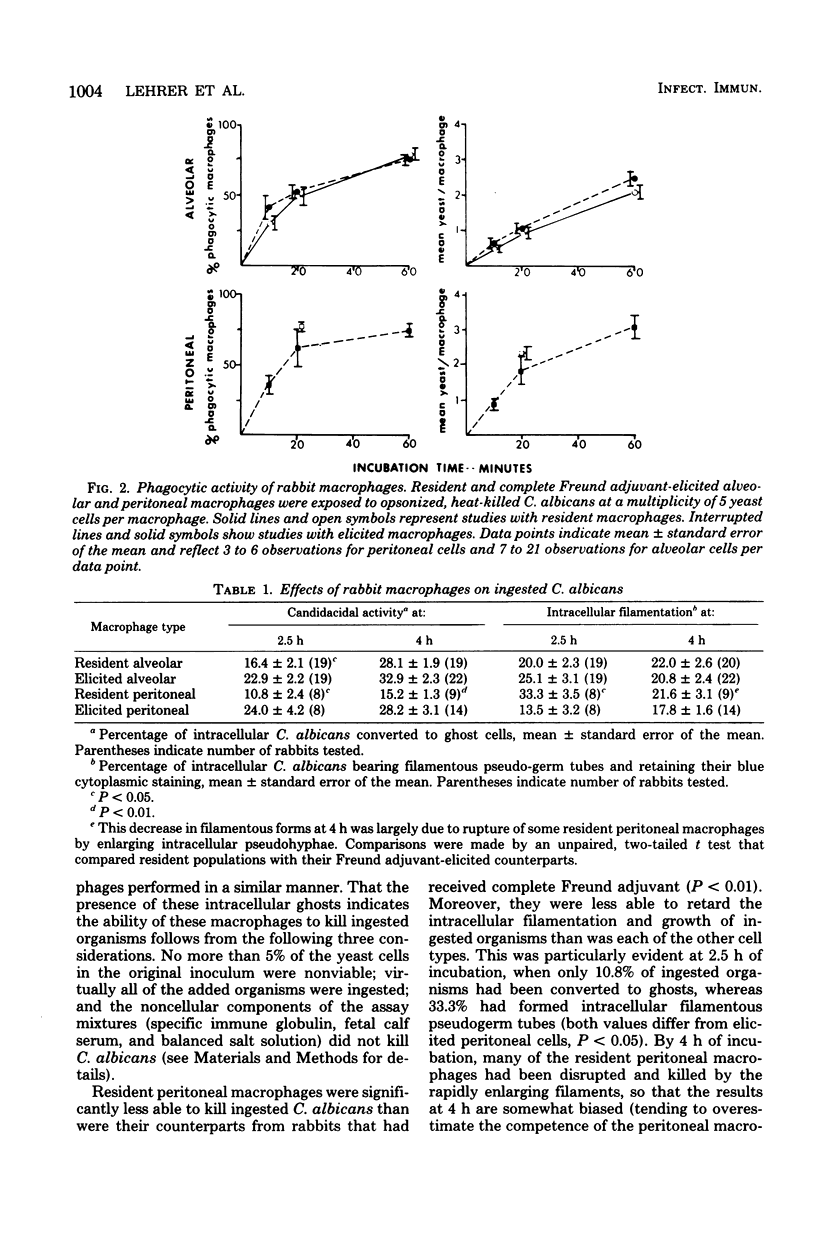
We tested the ability of rabbit macrophages to kill Candida albicans in vitro. Resident (unstimulated) alveolar macrophages killed 28.1 +/- 1.9% of ingested organisms in 4 h, whereas resident peritoneal macrophages killed only 15.2 +/- 1.3% (mean +/- standard error of the mean, P < 0.01). Peritoneal macrophages obtained from rabbits treated 3 weeks earlier with complete Freund adjuvant showed enhanced candidacidal activity relative to normally resident peritoneal cells (28.2 +/- 3.1%, P < 0.01). Candidacidal activity by alveolar macrophages recovered from such treated animals was slightly enhanced relative to untreated alveolar macrophages (32.9 +/- 2.3%). Candidacidal activity by peritoneal and alveolar macrophages was not decreased by several agents (cyanide, azide, sulfadiazine, and phenylbutazone) that inhibit the ability of human blood monocytes to kill C. albicans. In contrast, candidacidal activity by alveolar macrophages was greatly diminished by iodoacetate, an ineffective inhibitor of this function in human monocytes. We conclude that rabbit macrophages kill C. albicans by a fungicidal mechanism distinct from the peroxidase-H2O2 mechanism of human granulocytes and monocytes, and that the fungicidal properties of peritoneal and alveolar macrophage populations are enhanced after nonspecific stimulation with complete Freund adjuvant.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Mikami Y., Yokoyama K. Phagocytosis of Candida albicans by rabbit alveolar macrophages and guinea pig neutrophils. Sabouraudia. 1977 Jul;15(2):171–177. [PubMed] [Google Scholar]
- Biggar W. D., Sturgess J. M. Peroxidase activity of alveolar macrophages. Lab Invest. 1976 Jan;34(1):31–42. [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J. Bactericidal Activity of Human Macrophages: Analysis of Factors Influencing the Killing of Listeria monocytogenes. Infect Immun. 1970 Aug;2(2):156–161. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R. E., Lehrer R. I. Fungicidal properties of a chymotrypsin-like cationic protein from human neutrophils: adsorption to Candida parapsilosis. Infect Immun. 1977 Aug;17(2):382–388. doi: 10.1128/iai.17.2.382-388.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Lehrer R. I., Stiehm E. R., Fischer T. J., Young L. S. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978 Jul;89(1):91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- Faulkner C. S., 2nd, Esterly J. R. Ultrastructural changes in the alveolar epithelium in response to Freund's adjuvant. Am J Pathol. 1971 Sep;64(3):559–566. [PMC free article] [PubMed] [Google Scholar]
- Gee J. B., Vassallo C. L., Bell P., Kaskin J., Basford R. E., Field J. B. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970 Jun;49(6):1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Keist R., Ivatt R. J. Functional and biochemical parameters of activation related to macrophage cytostatic effects on tumor cells. Int J Cancer. 1974 Nov 15;14(5):675–683. doi: 10.1002/ijc.2910140515. [DOI] [PubMed] [Google Scholar]
- King G. W., Bain G., LoBuglio A. F. The effect of tuberculosis and neoplasia on human monocyte staphylocidal activity. Cell Immunol. 1975 Apr;16(2):389–395. doi: 10.1016/0008-8749(75)90127-6. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- Miller T. E. Metabolic event involved in the bactericidal activity of normal mouse macrophages. Infect Immun. 1971 Mar;3(3):390–397. doi: 10.1128/iai.3.3.390-397.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nake T., Nakano M., Saito K. Metabolic study of intracellular killing of bacteria by mouse macrophages. Jpn J Microbiol. 1967 Sep;11(3):189–201. doi: 10.1111/j.1348-0421.1967.tb00336.x. [DOI] [PubMed] [Google Scholar]
- Ouchi E., Selvaraj R. J., Sbarra A. J. The biochemical activities of rabbit alveolar macrophages during phagocytosis. Exp Cell Res. 1965 Dec;40(3):456–468. doi: 10.1016/0014-4827(65)90226-0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Uesaka I. The role of macrophages in Candida albicans infection in vitro. Jpn J Microbiol. 1974 Jan;18(1):29–35. doi: 10.1111/j.1348-0421.1974.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Lehrer R. I. A simple microscopic method for identifying and quantitating phagocytic cells in vitro. J Immunol Methods. 1977;18(3-4):377–379. doi: 10.1016/0022-1759(77)90191-0. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Function of h(2)o(2), myeloperoxidase, and hexose monophosphate shunt enzymes in phagocytizing cells from different species. Infect Immun. 1970 Apr;1(4):338–344. doi: 10.1128/iai.1.4.338-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Selvaraj R. J., Sbarra A. J. Peroxidase mediated antimicrobial activities of alveolar macrophage granules. Science. 1973 Aug 31;181(4102):849–850. doi: 10.1126/science.181.4102.849. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Growth inhibition of Candida albicans by rabbit alveolar macrophages. Infect Immun. 1977 Mar;15(3):910–915. doi: 10.1128/iai.15.3.910-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Inhibition of specific amino acid uptake in Candida albicans by lysosomal extracts from rabbit alveolar macrophages. Infect Immun. 1978 Aug;21(2):506–513. doi: 10.1128/iai.21.2.506-513.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Cramer R., Marzi T., Soranzo M. R., Zabucchi G., Rossi F. Peroxidase activity of alveolar and peritoneal macrophages. J Reticuloendothel Soc. 1973 May;13(5):399–409. [PubMed] [Google Scholar]
- Sorrell T. C., Lehrer R. I., Cline M. J. Mechanism of nonspecific macrophage-mediated cytotoxicity: evidence for lack of dependence upon oxygen. J Immunol. 1978 Feb;120(2):347–352. [PubMed] [Google Scholar]
- Stanley V. C., Hurley R. The growth of Candida species in cultures of mouse peritoneal macrophages. J Pathol. 1969 Feb;97(2):357–366. doi: 10.1002/path.1710970222. [DOI] [PubMed] [Google Scholar]
- TASCHDJIAN C. L., BURCHALL J. J., KOZINN P. J. Rapid identification of Candida albicans by filamentation on serum and serum substitutes. AMA J Dis Child. 1960 Feb;99:212–215. doi: 10.1001/archpedi.1960.02070030214011. [DOI] [PubMed] [Google Scholar]
- Thalinger K. K., Mandell G. L. Bactericidal activity of macrophages in an anaerobic environment. J Reticuloendothel Soc. 1971 May;9(5):393–396. [PubMed] [Google Scholar]
- Volkman A. Disparity in origin of mononuclear phagocyte populations. J Reticuloendothel Soc. 1976 Apr;19(4):249–268. [PubMed] [Google Scholar]