Abstract
Fusobacterium species are well described as the causative pathogen in Lemierre’s syndrome, a suppurative thrombophlebitis of the jugular vein. However, they are less recognized for a unique variant of Lemierre’s syndrome presenting with invasive intraabdominal infection and associated portal vein thrombosis. We describe a case of Fusobacterium nucleatum with hepatic abscess and septic pylephlebitis.
Keywords: Fusobacterium, Portal vein thrombosis, Septic pylephlebitis, 16S rRNA sequencing
Introduction
Fusobacterium species are gram-negative anaerobic bacilli which are mainly known for their invasive manifestation of Lemierre’s syndrome usually occurring in otherwise healthy young adults and children. The syndrome was initially recognized in 1936, when Lemierre reported 20 cases of the disease with 69.7% of the cases associated with Fusobacterium necrophorum bacteremia [1]. Although infection with other organisms are also possible, F. necrophorum is usually the primary suspect in Lemierre’s syndrome, accounting for 81.7% of the 109 cases analyzed by Chirinos et al. [2]. In this disease, the organism initially infects the oropharynx and then invades the vasculature of the infected area leading to the classic presentation of septic thrombophlebitis of the internal jugular vein and subsequent septic emboli. Fusobacterium species can also cause a similar variation of intra-abdominal infection in which the organism causes disseminated intraabdominal or pelvic infections with associated portal vein septic thrombophlebitis [3], [4], [5]. We describe a case of Fusobacterium nucleatum hepatic abscess with associated portal vein thrombosis known as septic pylephlebitis.
Case summary
A 59-year-old female with past medical history of multiple sclerosis in remission and not requiring any immunosuppressive medications initially presented to a local emergency room with a 6 week history of fatigue, weight loss, and worsening abdominal pain. The pain was described as intermittent, dull in nature and mainly localizing to the right upper quadrant. Otherwise, no nausea, vomiting, or diarrhea were reported. An initial abdominal computerized tomography (CT)-scan with contrast revealed multiple hepatic lesions with partial thrombosis of the right portal vein, which was radiologically suspicious for metastatic malignant disease. Based on the constellation of symptoms, the patient underwent an outpatient evaluation for malignancy. A position emission tomography scan showed multiple hepatic lesions with associated hypermetabolic activity and re-demonstration of the right portal vein thrombosis, but no other primary foci of malignancy was identified. An esophagogastroduodenoscopy and a colonoscopy were obtained which revealed sigmoid colon diverticulosis, but otherwise unrevealing. Tumor markers were also negative. A liver biopsy performed by interventional radiology showed nonspecific inflammatory process without evidence of malignant cells.
The patient then developed fevers up to 39.4 °C, chills, and soaking night sweats. She was subsequently admitted to an outside hospital for further workup. Upon admission, she had a temperature of 37.1 °C, blood pressure of 98/54 mmHg, and heart rate of 86 beats per minute. On physical examination, she was non-toxic appearing but had localized tenderness to palpation over the right upper quadrant. Her white blood cell count was 25 K/μL (reference range 3.8–10.8 K/μL) with 89% neutrophils, hemoglobin was 10.2 g/dL (reference range 12–15.5 g/dL), and platelet count was 311 K/μL (reference range 150–450 K/μL). Her liver chemistries were significant for alkaline phosphatase of 264 U/L, aspartate aminotransferase of 47 U/L, alanine aminotransferase of 41 U/L, and total bilirubin of 1.6 mg/dL with 0.8 mg/dL direct bilirubin. An abdominal magnetic resonance imaging (MRI) re-demonstrated multiple liver lesions and portal vein thrombosis. Blood cultures were obtained and she was empirically started on intravenous ampicillin-sulbactam and intramuscular enoxaparin. On day two of admission, she underwent a repeat CT-guided liver biopsy, which demonstrated necrosis with acute inflammatory and fibrous reactive changes consistent with abscess. To the best of our knowledge, cultures were either not obtained or did not grow any organisms from the liver biopsy. On day three of admission, Fusobacterium sp. was identified in one of two blood culture sets, and no further speciation or susceptibilities were performed. The patient received one week of intravenous ampicillin-sulbactam and one week of ertapenem prior to being transferred to our facility for further management of the liver abscesses.
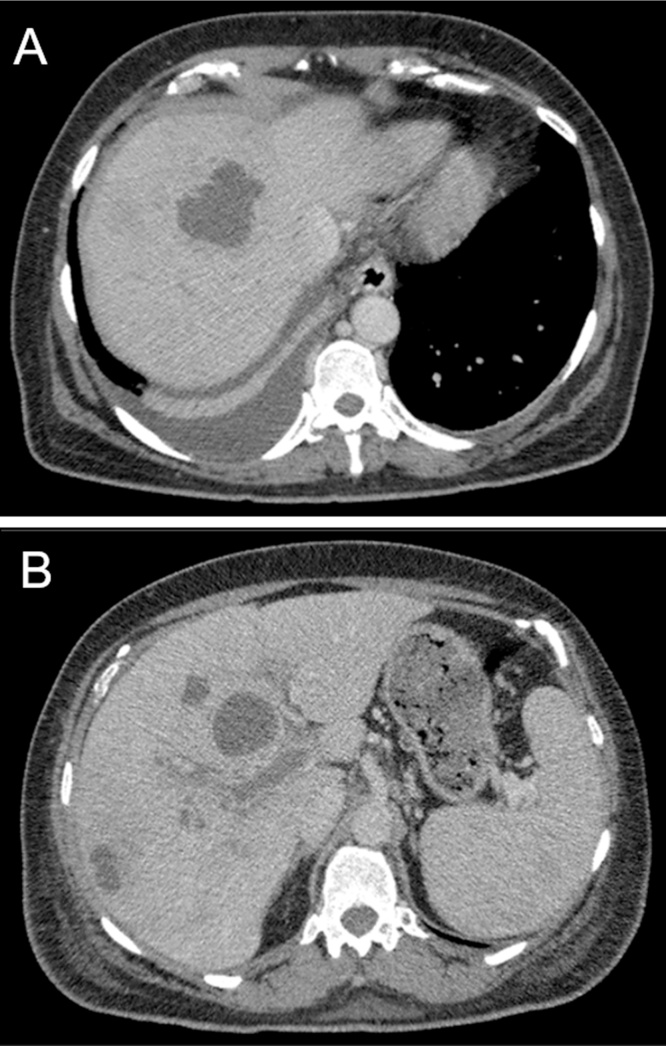
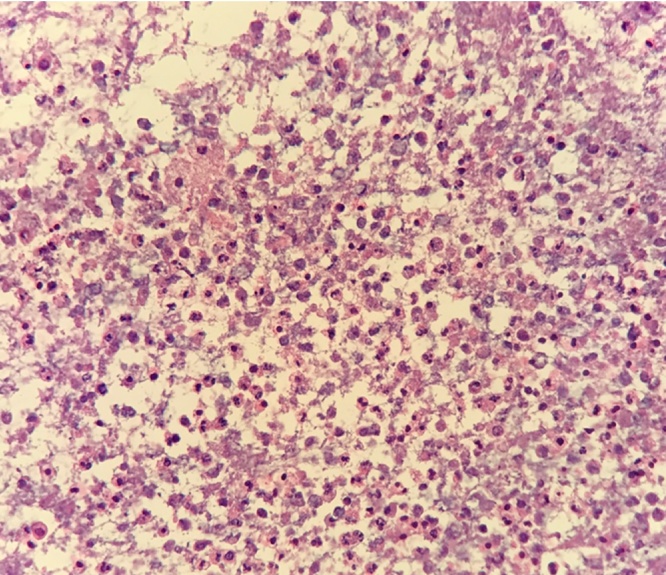
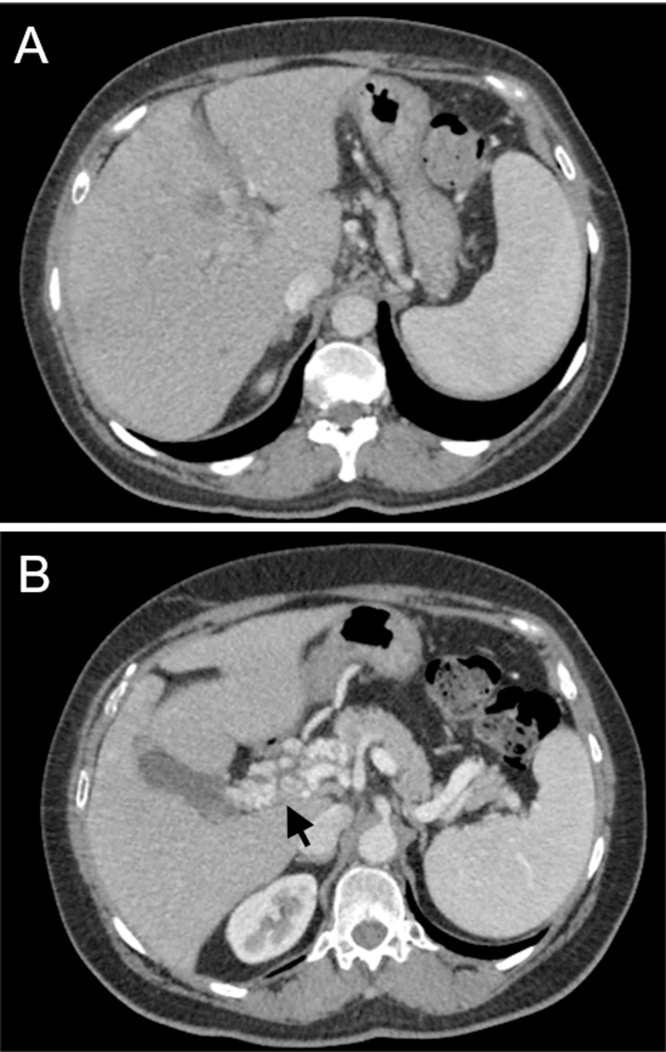
Upon transfer, her white blood cell count had trended down to 9.35 K/μL. Repeat CT abdomen showed multiple persistent low density hepatic lesions, the largest measuring 4.9 × 3.3 × 5.9 cm as well as extension of the portal venous thrombosis (Fig. 1A–B). She subsequently underwent CT-guided drainage of the largest abscess with aspiration of 60 mL of purulent fluid. Histopathology demonstrated acute inflammatory cells and acellular debris consistent with abscess (Fig. 2). Aerobic cultures grew coagulase-negative Staphylococcus. No organisms were isolated from thioglycollate broth, although anaerobic cultures were not specifically obtained. Therefore a sample of the abscess material was sent to a reference laboratory (University of Washington) for broad-range bacterial 16S rRNA gene PCR and sequence analysis which confirmed presence of Fusobacterium nucleatum. The patient was treated with two months course of intravenous ceftriaxone and metronidazole in addition to intramuscular anticoagulation. Given her excellent clinical response, she was then transitioned to oral ampicillin-clavulanic acid to complete an additional two months course of antibacterial. At four months follow-up, repeat CT imaging showed near-complete resolution of the hepatic abscess with interval cavernous transformation of portal vein, a complication of long standing thrombosis (Fig. 3A–B). Based on her resolution of symptoms and favorable serum biomarkers, antibacterial were discontinued and she had no signs of relapse of disease at outpatient follow-up.
Fig. 1.
Transverse CT images through the liver in the portal venous phase demonstrate (A–B) multiple hypoattenuating hepatic lesions (B) a low-attenuation filling defect within the right portal vein (arrow).
Fig. 2.
On examination of the cell block section, the liver abscess drainage consists of acellular debris and acute inflammatory cells (hematoxylin and eosin stain, original magnification ×400).
Fig. 3.
Transverse CT images through the liver demonstrates (A) decreased conspicuity of the hepatic abscesses (B) interval cavernous transformation of the portal vein (arrow).
Discussion
Fusobacterium species are gram-negative anaerobic bacilli which are normally a constituent of the oropharynx, gastrointestinal tract, and female genital flora. Overall, Fusobacterium species are a rare cause of bacteremia accounting for <1% of all bacteremia and <10% of anaerobic bacteremia cases in adults [6]. Fusobacterium bacteremia generally affects males more than females [7], [8], [9] with the primary infection source typically being in the respiratory tract, abdomen, or pelvis [7], [10], [11]. Polymicrobial bacteremia is also common [7], [8], [9], [10], [11]. Although the majority of cases of Fusobacterium bacteremia are thought to be community-acquired, there have also been reports of nosocomial infections [7], [12]. In contrast to Lemierre’s syndrome, in which F. necrophorum is the predominant species affecting young healthy individuals, the majority of intraabdominal and pelvic infections are caused by F. nucleatum affecting an older population with chronic medical conditions and/or malignancies [8], [9], [12], possibly due to tumor invasion of mucosal surfaces. Disseminated Fusobacterium infections involving the brain, liver, heart, and joints are very rare but have been reported [12]. With respect to hepatic abscesses associated with Fusobacterium species, oropharyngeal disease or intestinal sources of infections including diverticulitis have been postulated as the potential initial portal of entry [9].
Although our patient was noted to have diverticulosis on colonoscopy, no evidence of intestinal or oropharyngeal infections were identified. To the best of our knowledge, there have only been a handful of cases of Fusobacterium hepatic abscesses with associated septic pylephlebitis in the literature [3], [4], [5]. All three prior cases were also associated with F. nucleatum with the presentation of gastrointestinal and constitutional symptoms. One case was treated with six weeks of intravenous penicillin without anticoagulation [3], one case was treated with intravenous cefotaxime and metronidazole for three weeks followed by two weeks of oral metronidazole in conjunction with preventive anticoagulation over the initial three weeks [4], and one case was treated with two weeks of clindamycin without any anticoagulation [5]. All patients showed clinical recovery. However, repeat imaging in the two patients in which no anticoagulation was used showed persistent portal vein thrombosis and even atrophy of the liver in one case [3], [5]. Overall, combinations of beta-lactams and/or metronidazole based on susceptibility results, with treatment duration of weeks to months depending on clinical and imaging findings, seem to be the most acceptable approach. Anticoagulation therapy along with antibacterial may also be considered as it may increase the chance of portal venous recanalization in some cases [5], although the definitive role of anticoagulation remains unclear. Finally, although mortality from most Fusobacterium sp. bacteremia is considered to be unusual, the mortality rate with F. nucleatum bacteremia can be as high as 10–30% [7], [11]. As such, prompt recognition of this entity is crucial.
In conclusion, Fusobacterium species bacteremia is a rare entity, but cases may be under-reported due to the limitations of isolation of the organism from anaerobic cultures and tissue samples. Patients with Fusobacterium species bacteremia may warrant further investigation for more invasive disease based on the clinical presentation. As head and neck symptoms would prompt investigation for Lemierre’s syndrome, abdominal symptoms and abnormal liver chemistries should prompt investigation for hepatic abscess and portal vein thrombosis in order to initiate appropriate treatment without delay. An investigation for underlying colonic mucosal injury or malignant processes should also be considered. However, the optimal treatment and duration of therapy, as well as the role of anticoagulation in these cases are still undefined.
References
- 1.Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(March (5874)):701–703. [Google Scholar]
- 2.Chirinos J.A., Lichtstein D.M., Garcia J., Tamariz L.J. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine. 2002;81(November (6)):458–465. doi: 10.1097/00005792-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bultink I.E., Dorigo-Zetsma J.W., Koopman M.G., Kuijper E.J. Fusobacterium nucleatum septicemia and portal vein thrombosis. Clin Infect Dis. 1999;28:1325–1326. doi: 10.1086/517785. [DOI] [PubMed] [Google Scholar]
- 4.Etienne M., Gueit I., Abboud P., Pons J.L., Jacquot S., Caron F. Fusobacterium nucleatum hepatic abscess with pylephlebitis associated with idiopathic CD4+ T lymphocytopenia. Clin Infect Dis. 2001;32(January (2)):326–328. doi: 10.1086/318468. [DOI] [PubMed] [Google Scholar]
- 5.Verna E.C., Larghi A., Faddoul S.G., Stein J.A., Worman H.J. Portal vein thrombosis associated with Fusobacterium nucleatum septicemia in a patient with ulcerative colitis. J Clin Gastroenterol. 2004;1(August (7)):11–12. doi: 10.1097/00004836-200408000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Dorsher C.W., Rosenblatt J.E., Wilson W.R., Ilstrup D.M. Anaerobic bacteremia: decreasing rate over a 15-year period. Rev Infect Dis. 1991;13(July (1)):633–636. doi: 10.1093/clinids/13.4.633. [DOI] [PubMed] [Google Scholar]
- 7.Bourgault A.M., Lamothe F., Dolcé P., Saint-Jean L., Saint-Antoine P. Fusobacterium bacteremia: clinical experience with 40 cases. Clin Infect Dis. 1997;1:S181–S183. doi: 10.1086/516181. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen L.H., Prag J. Lemierre’s syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: a prospective epidemiological and clinical survey. Eur J Clin Microbiol Infect Dis. 2008;1(September (9)):779–789. doi: 10.1007/s10096-008-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huggan P.J., Murdoch D.R. Fusobacterial infections: clinical spectrum and incidence of invasive disease. J Infect. 2008;57(October (4)):283–289. doi: 10.1016/j.jinf.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Henry S., DeMaria A., McCabe W.R. Bacteremia due to Fusobacterium species. Am J Med. 1983;75(August (2)):225–231. doi: 10.1016/0002-9343(83)91196-8. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg E.A., Venkat-Ramani T., Hewit M., Bonilla H.F. Epidemiology and clinical outcomes of patients with Fusobacterium bacteraemia. Epidemiol Infect. 2013;141(February (02)):325–329. doi: 10.1017/S0950268812000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afra K., Laupland K., Leal J., Lloyd T., Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;5(June (1)):264. doi: 10.1186/1471-2334-13-264. [DOI] [PMC free article] [PubMed] [Google Scholar]