Abstract
Background and Purpose
The goal of carotid artery stenting (CAS) is to decrease the risk of stroke or other adverse events from carotid artery disease. Choosing a treatment strategy requires patient-specific information regarding peri-procedural risk of adverse neurologic events. The aim of this study was to predict individual patient risk following CAS in patients at higher-risk for carotid endarterectomy (CEA).
Methods
Subjects enrolled in the Stenting and Angioplasty with Protection in Patients at High-Risk for Endarterectomy (SAPPHIRE) Worldwide study underwent CAS with distal protection. Only patients with at least one anatomic or co-morbid factor associated with elevated surgical risk were included. Pre-procedural factors were used to develop a model and integer-based risk score predicting stroke or death within 30 days. The model was calibrated and internally validated using bootstrap resampling.
Results
Ten-thousand, one hundred eighty six patients were included in the analysis. The overall rate of stroke or death was 3.6% at 30 days after CAS. Independent predictors of adverse outcomes were increased age (p=0.006), history of stroke (p<0.001), history of transient ischemic attack presentation (p=0.001), recent (<4 weeks) myocardial infarction (p=0.006), dialysis treatment (p=0.007), need for cardiac surgery in addition to carotid revascularization (p=0.005), a right-sided carotid stenosis (p=0.006), a longer carotid plaque (p=0.012), the presence of a type II or III aortic arch (p=0.035), and a tortuous carotid arterial system(p=0.004).The optimism-adjusted C-statistic was 0.691.
Conclusions
Commonly collected clinical and anatomic variables can identify patients at high and low risk for stroke or death after CAS.
Keywords: carotid arteries, stents, stenting, risk factors
Introduction
Treatment options for patients with significant carotid atherosclerosis include surgical carotid endarterectomy (CEA), endovascular carotid artery stenting (CAS), and medical therapy. There has been significant effort to define the optimal treatment choices for specific patients, though this remains a controversial issue.1 Current guidelines from independent international organizations advocate different approaches to this decision.2 The 14 organization multi-society guidelines which included the American Heart Association, American College of Cardiology, and Society for Vascular Surgery advocate decision-making for CAS based on an individual's likelihood of complications and expected benefit. The guidelines also advocate a threshold of 6% as the upper limit of the acceptable rate of peri-procedural stroke or death from CAS in symptomatic patients.3 Previous guidelines have advocated a 6% threshold for carotid revascularization in symptomatic patients and a 3% threshold in asymptomatic patients based on complication rates from surgical studies.4,5 These thresholds are based on anticipated complication rates of routine patients undergoing CEA, rather than on expected risks or benefits of CAS compared with CEA or medical therapy. Recent reports from a large, randomized trial and meta-analysis question the role of CAS, compared with CEA, in asymptomaticpatients.6,7
In clinical practice there is consensus that there are groups of patients at increased risk for complications with CEA due to unfavorable anatomical features and/or medical comorbidities.8-16 Separate international guidelines define “higher risk” patients slightly differently and use different approaches to discuss the appropriate mode or timing of carotid revascularization in these populations.2
Because understanding peri-procedural risk is crucial in decision-making, previous groups have sought to generate risk models or scores to predict adverse events for individual patients undergoing CAS.17-19 None of the previously published risk scores, however, are specifically applicable to higher-surgical risk patients. Previous risk scores also were not developed in cohorts where outcomes were evaluated using independent clinical endpoint committees.
The Stenting and Angioplasty with Protection in Patients at High-Risk for Endarterectomy (SAPPHIRE) randomized trial is the only randomized clinical trial to specifically enroll higher-surgical risk patients for the comparison of CEA and CAS using modern techniques with embolic protection.20 However, with only 334 patients, there was not sufficient data to determine what features were strongly associated with peri-procedural risk.
The goal of this study is to develop and internally validate a model and bedside tool to predict death or stroke within 30 days of CAS in higher surgical-risk patients using easily collected variables that can be assessed in routine clinical practice. The study population is drawn from the SAPPHIRE Worldwide study, a single arm prospective study of higher-risk patients undergoing CAS with embolic protection. The prediction model generated here can be used to support decision making.
Methods
Study Population and Measurements
The SAPPHIRE Worldwide study has been described.21 Patients were enrolled from 364 centers across the United States and Canada. Patients were required to have either ≥50% carotid stenosis (determined by ultrasound or angiogram) if symptomatic (TIA or stroke within 180 days) or ≥80% carotid stenosis if asymptomatic. Patients were required to have at least one factor that made them higher risk for CEA as determined by the enrolling physician. High-risk criteria include: age ≥75 years, class III or IV New York Heart Association heart failure or left ventricular ejection fraction <30%, open heart surgery within 6 weeks, recent myocardial infarction within 4 weeks, unstable angina (Canadian Cardiovascular Society class III/IV), coexistent cardiac and carotid disease requiring cardiac surgery and carotid revascularization, severe pulmonary disease, an abnormal cardiac stress test, contralateral carotid occlusion, post radiation therapy to the neck, recurrent stenosis at the site of prior CEA, high cervical internal carotid artery (ICA) lesion or low common carotid artery (CCA) lesion below the clavicle, and severe tandem lesions. Written informed consent was obtained using forms approved by Institutional Review Boards or Medical Ethics Committees at each center.
CAS procedures were performed using the ANGIOGUARD XP/RX Emboli Capture Guidewire distal protection device and the PRECISE OTW/RX Nitinol stent systems (Cordis; Warren, NJ). Patients were required to have arterial diameters consistent with safe device deployment. The target lesion and stent landing zone must be between 4 mm and 9 mm. The internal ICA at the ANGIOGUARD landing site must be between 3 mm and 7.5 mm. It was recommended that patients be treated with aspirin (81-325 mg daily) at least 72 hours before the procedure and thereafter. Either clopidogrel (300 mg load, 75 mg daily) or ticlopidine (250 mg twice daily) were recommended at least 24 to 48 hours before the procedure and continued atleast 2 weeks after the procedure. Heparin was used during the procedure to attain activated clotting times (ACT) greater than 300 seconds before crossing the lesion.
All physicians performing procedures were stratified according to experience in carotid stenting generally and according to experience with the study device. Operators participated in a training program tailored to previous procedural volume and experience with study devices.22
Patients were evaluated at baseline, hospital discharge, and 30 days post-procedure. The baseline evaluation included a carotid ultrasound and/or angiogram. The National Institutes of Health stroke scale and the Modified Rankin stroke scale were performed by certified providers, but not necessarily neurologists. Adverse events were assessed up to 30-days post procedure. An independent Clinical Events Committee (CEC) at the Harvard Clinical Research Institute, Boston, MA adjudicated all major adverse events including stroke. Remote data monitoring of all endpoints was conducted in all patients, while onsite monitoring by review of medical records was conducted in approximately 15% of patients. The SAPPHIRE Worldwide study is sponsored by Cordis (Warren, NJ). The authors, who had full access to the data, performed the analysis and did not receive funding from Cordis for the analysis.
Candidate Predictors
Stroke was defined as a non-convulsive, focal neurologic deficit of abrupt onset persisting for more than 24 hours, with the deficit corresponding to a vascular territory.
We identified a list of variables to be considered in the multivariable model based on clinical relevance. These included socio-demographic information (age, sex, and race/ethnicity), medical history (hypertension, diabetes mellitus, dyslipidemia, hemodialysis, severe pulmonary disease), cardiovascular and neurovascular history (prior coronary artery disease, priormyocardial infarction, prior stroke, prior TIA, prior coronary artery bypass grafting, prior carotid endarterectomy, prior peripheral angioplasty or stenting, prior heart failure, whether the carotid lesion was symptomatic), and factors associated with increased risk for CEA (low left ventricular ejection fraction or New York Heart Association Class III or IV heart failure, recent or planned heart surgery within 6 weeks, myocardial infarction within four weeks, recent unstable angina, severe pulmonary disease, a significantly abnormal cardiac stress test, age ≥75 years, contralateral carotid artery occlusion, contralateral laryngeal nerve palsy, history of neck radiation, tandem carotid lesions, previous CEA recurrent stenosis, or ICA lesion or a low CCA lesion below the clavicle. We also considered anatomic and angiographic factors including the type of aortic arch (I, II, or III), the presence of significant aortic arch calcification, significant CCA or ICA tortuosity, lesion calcification, lesion length, the presence of lesion ulceration, the presence of thrombus, and the presence of a significantly eccentric lesion.
Statistical Analysis
We first examined the univariate associations of a composite endpoint of stroke or death at 30 days with all candidate variables. Next, multivariable logistic regression was performed using candidates with univariate p<0.2. We performed backwards elimination of candidates until only variables with p<0.05 remained. Age and lesion length were entered as linear functions based on their monotonic relationships with the endpoint.
Internal validation and calibration were performed using bootstrapping (resampling with replacement) techniques.23 We generated 1,000 bootstrap samples with repetition of the variable selection procedure and the final model coefficients were adjusted based on a linear calibration slope.24 We assessed discrimination as measured by the C-statistic, and calibration based oncomparing observed and predicted event rates across deciles of predicted risk over bootstrapped samples. We also adjusted the reported model discrimination based on bootstrap methods to adjust for model optimism and over-fitting. The adjusted C-statistic was calculated: adjusted performance = apparent performance in the original sample – average(bootstrap model performance in bootstrap sample – bootstrapped model performance in original sample).25
The beta-coefficients from the model were used to generate point scores for an integer-based tool.26
Given previous literature relating operator experience to outcomes with CAS,27,28 we evaluated whether the addition of operator experience (coded as a binary variable of > 25 procedures as the primary operator and more than 10 using the study devices or >25 procedures as the primary or secondary operator and >13 as the primary operator), improved the final model based on likelihood ratio testing and based on the calculation of the integrated discrimination improvement (IDI) index.29
Analyses were performed using STATA 11.2 (Statacorp, College Station, TX).
Results
The study population included 10,186 patients who underwent CAS with distal protection between October 30, 2006 and September 30, 2010. Mean age was 72.3 ±9.7 years, and 38.9% of patients were women. History of stroke was present in 22.5% of patients and history of TIA in 22.7%. Symptomatic carotid lesions were present in of 29.8% of patients (Table 1). The most common high-surgical risk feature for CEA was age ≥ 75 years. The frequency of other highsurgical-risk features are in Table 2. Successful use of the study embolic protection device occurred in 96.4% of patients.
Table 1.
Demographics/Baseline Characteristics. Total number of patients=10,186. SD represents standard deviation; TIA, transient ischemic attack.
| Characteristic | Mean (± SD) or Percentage |
|---|---|
| Age (years) | 72.3 ±9.7 |
| Male | 61.1% |
| Symptomatic | 29.8% |
| Caucasian | 91.6% |
| Hypertension | 82.3% |
| Diabetes | 32.7% |
| Prior myocardial infarction | 19.3% |
| Prior carotid endarterectomy | 27.8% |
| History stroke | 22.5% |
| History TIA | 22.7% |
| History chronic kidney disease (Cr >2.5) | 5.1% |
| Dialysis | 1.4% |
Table 2.
High risk characteristics for carotid endarterectomy. MI indicates myocardial infarction; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; ICA, internal carotid artery; CCA, common carotid artery.
| Characteristic | Percentage of Total Subjects(n=10,186) |
|---|---|
| Physiologic High Risk Characteristic | |
| CHF (Class III or IV) or LVEF ≤ 30% | 11.0% |
| Heart surgery in 6 weeks | 0.9% |
| MI within 4 weeks | 1.7% |
| Unstable angina | 4.2% |
| Severe pulmonary disease | 12.2% |
| Abnormal stress test | 10.6% |
| Age>75 years | 39.8% |
| Severe simultaneous cardiac disease requiring surgery and carotid disease | 3.8% |
| Anatomic High Risk Characteristic | |
| Contralateral occlusion | 13.0% |
| Contralateral laryngeal palsy | 0.5% |
| Post neck radiation | 7.1% |
| Tandem lesions | 2.5% |
| High ICA or CCA lesions below clavicle | 10.4% |
| Previous CEA recurrent stenosis | 23.1% |
Death occurred in 123 patients (1.2%) and stroke in 301 (3.0%) within 30 days of CAS. A total of 366 patients had either stroke or death within 30 days. Two hundred forty five strokes were ipsilateral (79.5% of strokes) and 276 were ischemic (91.7%). Lacunar strokes occurred in 33 subjects (11.9% of ischemic strokes). There were 25 hemorrhagic strokes.
The final multivariable model with calibration-slope adjusted coefficients is presented in Table 3. There are 10 significant predictors in the final model. The raw C-statistic was 0.709 and the optimism-adjusted C-statistic is 0.691. Over 100 bootstrapped samples, the predicted probability of death or stroke within 30 days was well-calibrated with the observed rates of death or stroke (Figure 1, Hosmer-Lemeshow p=0.62).
Table 3.
Final Logistic Regression Model for Death or Stroke at 30 days after carotid artery stenting. The optimism-adjusted C-statistic is 0.691. The associated Hosmer-Lemeshow p-value is 0.62. OR indicates odds ratio.
| Variable | Adjusted-Beta | Adjusted-OR | 95% CI for Adjusted-OR | p-value |
|---|---|---|---|---|
| Age (per 10 years) | 0.417 | 1.520 | 1.32-1.81 | <0.001 |
| Stroke | 0.731 | 2.080 | 1.55-2.75 | <0.001 |
| Transient ischemic attack | 0.534 | 1.710 | 1.24-2.22 | 0.001 |
| Myocardial infarcation within 4weeks | 1.025 | 2.790 | 1.34-5.82 | 0.006 |
| Dialysis | 0.986 | 2.680 | 1.34-6.01 | 0.007 |
| Need for concomitant cardiac surgery plus carotid revascularization | 0.772 | 2.160 | 1.27-3.77 | 0.005 |
| Left-sided lesion | -0.385 | 0.680 | 0.51-0.89 | 0.006 |
| Lesion length (per 10 mm) | 0.183 | 1.200 | 1.03-1.33 | 0.012 |
| Type II or Type III aortic arch | 0.291 | 1.240 | 1.02-1.49 | 0.035 |
| Two 90-degree bends | 0.463 | 1.590 | 1.17-2.21 | 0.004 |
| Constant | -7.350 |
Figure 1.
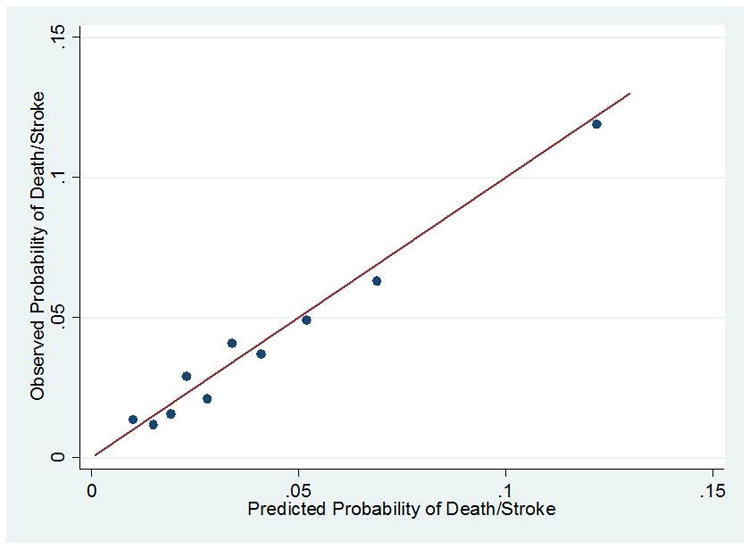
Observed versus predicted probability of death or stroke within 30 days with the full model over 100 bootstrapped samples. Individuals are grouped into 10 deciles based on their predicted probability of death or stroke in 30 days. Displayed line is y=x.
An integer-based tool intended for bedside use is in Figure 2. Individuals with ≤8 pointshave a predicted risk of death or stroke of less than 3% at 30 days. The C-statistic of the integer-based score is 0.683. Individuals with more than 12 points have a predicted rate of death or stroke higher than 6%. We also demonstrate that the rate of observed events was similar to the rate of events predicted based on the bedside tool (Figure 3).
Figure 2.
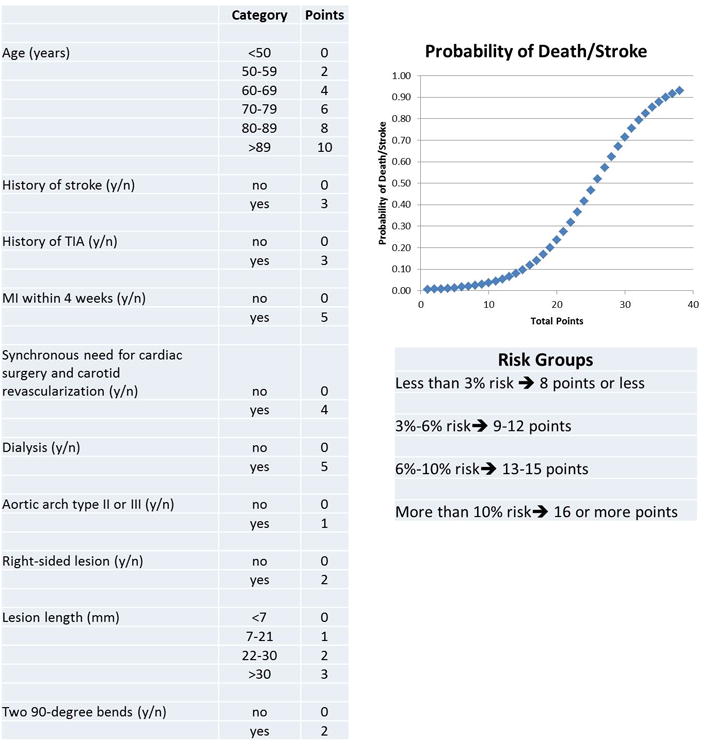
Bedside prediction tool for death or stroke at 30 days after carotid artery stenting. The total point score gives the predicted probability for death or stroke at 30 days according to the following equation:
indicates transient ischemic attack; MI, myocardial infarction.
Figure 3.
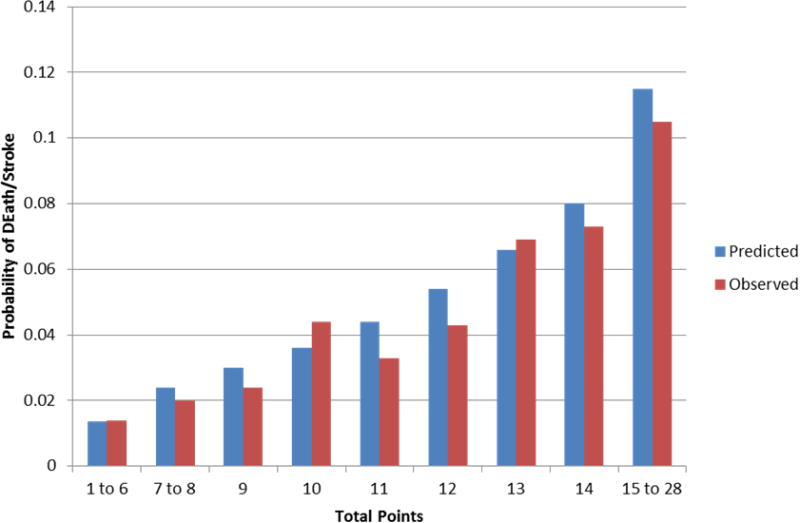
Observed versus predicted probability of death or stroke at 30 days using the bedside tool over 100 bootstrapped samples.
The addition of two different binary variables for operator experience did not significantly improve the final model based on likelihood ratio testing or based on calculation of the IDI (0.00027, p=0.45 or 0.00030, p=0.49).
Discussion
In a large study of patients at higher-risk for CEA, commonly collected variables were able to identify patients at high and low risk for stroke or death after CAS. We generated a risk model and simple risk score to predict stoke or death within 30 days using these variables. We found that elevated age, history of stroke, history of TIA, recent myocardial infarction, the need for both cardiac surgery and carotid revascularization, dialysis treatment, the presence of a type II or III aortic arch, a right sided carotid stenosis, a longer carotid plaque, and a severely tortuous carotid arterial system were all important risk factors for the development of stroke or death within 30 days of CAS. These findings are consistent with previous observations regarding the risk factors for adverse events associated with CAS.30 31-34 Our findings also reinforce that in addition to co-morbid conditions, anatomic and lesion-specific considerations simultaneously confer risk and should be factored into the decision about carotid revascularization with CAS.
Our study differs from previous studies that have considered CAS risk prediction,17-19 and represents an improvement in a number of ways. First, we were able to use data from many centers across the spectrum of clinical practice. Our study used routine, impartial clinical endpoint adjudication and the administration of standard stroke assessment tools not available in previous studies that generated risk models. Most importantly, however, our study is restricted to higher-surgical risk patients who were not well-represented in the original surgical studies that led to the adverse event rate thresholds that are present in the multi-society guidelines.35-38 Non-randomized studies have demonstrated higher rates of adverse events with CEA in patients with multiple risk factors.10,11,39 In routine clinical practice, clinicians are currently referring patients with more severe co-morbid conditions to CAS and rather than to CEA more frequently. 40 Thus, even without adequate tools to precisely predict the risk of adverse events after CAS forhigh surgical-risk patients, clinicians are more likely to refer the most severely ill patients to CAS rather than to CEA. The risk prediction model presented here will now allow clinicians to assess CAS risk in a more quantitative manner than previously possible.
Our analyses should be interpreted in the context of important limitations. Although we retained a large number of clinically relevant variables in our model, the discriminative ability of the model was modest. Whereas our reported discrimination compares favorably to the discrimination of previously reported models, there may be unobserved social, biological, or procedural factors associated with stroke or death after CAS that are not accounted for in our models. We also are limited by the fact that not every patient was subject to evaluation by a neurologist to ascertain the endpoint of stroke. While certified study coordinators administered standard assessment tools, ascertainment of post-procedure complications may be lower than if neurologists performed routine assessments. We are also limited by self-reporting of angiographic data elements without core lab data ascertainment. External validity of the model can also be questioned given that the model was developed in patients who underwent CAS using specific study devices. Finally, while the goal was to present a clinically useful prediction model, our analysis only reflects risk for CAS. We did not consider the relative treatment effect compared with other therapies, including CEA or medical therapy without revascularization.
While our risk score quantifies the risk for individuals contemplating CAS, a particular score for a given individual does not imply that CAS is appropriate therapy. There remain large controversies, specifically with regard to the role of medical therapy alone, in this population. The role of medical therapy alone is particularly important to study in elderly patients, in those with significant comorbidities, and in asymptomatic patients.
Summary/Conclusions
We developed and validated a predictive model and integer-based tool to predict the occurrence of death or stroke within 30 days of CAS. The prospective use of individualized assessments may support rational decision making for the treatment of carotid atherosclerosis and may aide communication between clinicians and patients before CAS. In the future, prospective testing should be performed to ascertain whether this model improves patient outcomes and understanding.
Acknowledgments
Sources of Funding: Dr. Wimmer is supported by NIH T32-HL007604.
Footnotes
Disclosures: Dr. Wimmer reports no disclosures. Dr. Yeh is an investigator for the Harvard Clinical Research Institute. Dr. Cutlip receives institutional research support from Medtronic. Dr. Mauri receives institutional research support from Boston Scientific, Medtronic, Abbot, Cordis, Sanofi, Bristol Myers Squibb, Eli Lilly, and Daiichi Sankyo and has consulted for Medtronic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Neil J. Wimmer, Division of Cardiovascular Medicine, Brigham and Women's Hospital, Boston, MA.
Robert W. Yeh, Division of Cardiology, Massachusetts General Hospital, Boston, MA.
Donald E. Cutlip, Division of Cardiology, Beth Israel Deaconess Medical Center, Boston, MA.
Laura Mauri, Division of Cardiovascular Medicine, Brigham and Women's Hospital, Boston, MAHarvard Medical School.
References
- 1.Kinlay S. Fire in the hole: Carotid stenting versus endarterectomy. Circulation. 2011;123:2522–2525. doi: 10.1161/CIRCULATIONAHA.111.034314. [DOI] [PubMed] [Google Scholar]
- 2.Paraskevas KI, Mikhailidis DP, Veith FJ. Comparison of the five 2011 guidelines for the treatment of carotid stenosis. J Vasc Surg. 2012;55:1504–1508. doi: 10.1016/j.jvs.2012.01.084. [DOI] [PubMed] [Google Scholar]
- 3.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 asa/accf/aha/aann/aans/acr/asnr/cns/saip/scai/sir/snis/svm/svs guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american stroke association, american association of neuroscience nurses, american association of neurological surgeons, american college of radiology, american society of neuroradiology, congress of neurological surgeons, society of atherosclerosis imaging and prevention, society for cardiovascular angiography and interventions, society of interventional radiology, society of neurointerventional surgery, society for vascular medicine, and society for vascular surgery. Circulation. 2011;124:e54–130. doi: 10.1161/CIR.0b013e31820d8c98. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. Primary prevention of ischemic stroke: A guideline from the american heart association/american stroke association stroke council: Cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary working group; cardiovascular nursing council; clinical cardiology council; nutrition, physical activity, and metabolism council; and the quality of care and outcomes research interdisciplinary working group. Circulation. 2006;113:e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the american heart association/american stroke association council on stroke: Co-sponsored by the council on cardiovascular radiology and intervention: The american academy of neurology affirms the value of this guideline. Circulation. 2006;113:e409–449. [PubMed] [Google Scholar]
- 6.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murad MH, Shahrour A, Shah ND, Montori VM, Ricotta JJ. A systematic review and meta-analysis of randomized trials of carotid endarterectomy vs stenting. J Vasc Surg. 2011;53:792–797. doi: 10.1016/j.jvs.2010.10.101. [DOI] [PubMed] [Google Scholar]
- 8.Gansera B, Angelis I, Weingartner J, Neumaier-Prauser P, Spiliopoulos K, Kemkes BM. Simultaneous carotid endarterectomy and cardiac surgery--additional risk factor or safety procedure? Thorac Cardiovasc Surg. 2003;51:22–27. doi: 10.1055/s-2003-37282. [DOI] [PubMed] [Google Scholar]
- 9.Coyle KA, Smith RB, 3rd, Gray BC, Salam AA, Dodson TF, Chaikof EL, et al. Treatment of recurrent cerebrovascular disease. Review of a 10-year experience. Ann Surg. 1995;221:517–521. doi: 10.1097/00000658-199505000-00009. discussion 521-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein LB, Samsa GP, Matchar DB, Oddone EZ. Multicenter review of preoperative risk factors for endarterectomy for asymptomatic carotid artery stenosis. Stroke. 1998;29:750–753. doi: 10.1161/01.str.29.4.750. [DOI] [PubMed] [Google Scholar]
- 11.Wong JH, Findlay JM, Suarez-Almazor ME. Regional performance of carotid endarterectomy.Appropriateness, outcomes, and risk factors for complications. Stroke. 1997;28:891–898. doi: 10.1161/01.str.28.5.891. [DOI] [PubMed] [Google Scholar]
- 12.Das MB, Hertzer NR, Ratliff NB, O'Hara PJ, Beven EG. Recurrent carotid stenosis.A five-year series of 65 reoperations. Ann Surg. 1985;202:28–35. doi: 10.1097/00000658-198507000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamdan AD, Pomposelli FB, Jr, Gibbons GW, Campbell DR, LoGerfo FW. Renal insufficiency and altered postoperative risk in carotid endarterectomy. J Vasc Surg. 1999;29:1006–1011. doi: 10.1016/s0741-5214(99)70241-7. [DOI] [PubMed] [Google Scholar]
- 14.Leseche G, Castier Y, Chataigner O, Francis F, Besnard M, Thabut G, et al. Carotid artery revascularization through a radiated field. J Vasc Surg. 2003;38:244–250. doi: 10.1016/s0741-5214(03)00320-3. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: A risk-modelling study. European carotid surgery trialists' collaborative group. Lancet. 1999;353:2105–2110. doi: 10.1016/s0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]
- 16.Vassilidze TV, Cernaianu AC, Gaprindashvili T, Gallucci JG, Cilley JH, Jr, DelRossi AJ. Simultaneous coronary artery bypass and carotid endarterectomy. Determinants of outcome. Tex Heart Inst J. 1994;21:119–124. [PMC free article] [PubMed] [Google Scholar]
- 17.Setacci C, Chisci E, Setacci F, Iacoponi F, de Donato G, Rossi A. Siena carotid artery stenting score: A risk modelling study for individual patients. Stroke. 2010;41:1259–1265. doi: 10.1161/STROKEAHA.110.578583. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald S, Lee R, Williams R, Stansby G. Towards safer carotid artery stenting: A scoring system for anatomic suitability. Stroke. 2009;40:1698–1703. doi: 10.1161/STROKEAHA.109.547117. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann R, Niessner A, Kypta A, Steinwender C, Kammler J, Kerschner K, et al. Risk score for peri-interventional complications of carotid artery stenting. Stroke. 2006;37:2557–2561. doi: 10.1161/01.STR.0000240688.81918.32. [DOI] [PubMed] [Google Scholar]
- 20.Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579. doi: 10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 21.Massop D, Dave R, Metzger C, Bachinsky W, Solis M, Shah R, et al. Stenting and angioplasty with protection in patients at high-risk for endarterectomy: Sapphire worldwide registry first 2,001 patients. Catheter Cardiovasc Interv. 2009;73:129–136. doi: 10.1002/ccd.21844. [DOI] [PubMed] [Google Scholar]
- 22.Katzen BT, Criado FJ, Ramee SR, Massop DW, Hopkins LN, Donohoe D, et al. Carotid artery stenting with emboli protection surveillance study: Thirty-day results of the cases-pms study. Catheter Cardiovasc Interv. 2007;70:316–323. doi: 10.1002/ccd.21222. [DOI] [PubMed] [Google Scholar]
- 23.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 24.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 25.Steyerberg EW. Clinical prediction models : A practical approach to development, validation, and updating. New York, NY: Springer; 2009. [Google Scholar]
- 26.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The framingham study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 27.Nallamothu BK, Gurm HS, Ting HH, Goodney PP, Rogers MA, Curtis JP, et al. Operator experience and carotid stenting outcomes in medicare beneficiaries. Jama. 2011;306:1338–1343. doi: 10.1001/jama.2011.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenfield K, Babb JD, Cates CU, Cowley MJ, Feldman T, Gallagher A, et al. Clinical competence statement on carotid stenting: Training and credentialing for carotid stenting--multispecialty consensus recommendations: A report of the scai/svmb/svs writing committee to develop a clinical competence statement on carotid interventions. J Am Coll Cardiol. 2005;45:165–174. doi: 10.1016/j.jacc.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D'Agostino RB, Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: Net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31:101–113. doi: 10.1002/sim.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White CJ. Carotid artery stent placement. JACC Cardiovasc Interv. 2010;3:467–474. doi: 10.1016/j.jcin.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Hobson RW, 2nd, Howard VJ, Roubin GS, Brott TG, Ferguson RD, Popma JJ, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the crest lead-in phase. J Vasc Surg. 2004;40:1106–1111. doi: 10.1016/j.jvs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 32.White CJ, Iyer SS, Hopkins LN, Katzen BT, Russell ME. Carotid stenting with distal protection in high surgical risk patients: The beach trial 30 day results. Catheter CardiovascInterv. 2006;67:503–512. doi: 10.1002/ccd.20689. [DOI] [PubMed] [Google Scholar]
- 33.Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The capture registry: Predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc Interv. 2007;70:1025–1033. doi: 10.1002/ccd.21359. [DOI] [PubMed] [Google Scholar]
- 34.Roubin GS, Iyer S, Halkin A, Vitek J, Brennan C. Realizing the potential of carotid artery stenting: Proposed paradigms for patient selection and procedural technique. Circulation. 2006;113:2021–2030. doi: 10.1161/CIRCULATIONAHA.105.595512. [DOI] [PubMed] [Google Scholar]
- 35.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North american symptomatic carotid endarterectomy trial collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 36.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the mrceuropean carotid surgery trial (ecst) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 37.Endarterectomy for asymptomatic carotid artery stenosis. Executive committee for the asymptomatic carotid atherosclerosis study. Jama. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 38.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 39.van Lammeren GW, Catanzariti LM, Peelen LM, de Vries JP, de Kleijn DP, Moll FL, et al. Clinical prediction rule to estimate the absolute 3-year risk of major cardiovascular events after carotid endarterectomy. Stroke. 2012;43:1273–1278. doi: 10.1161/STROKEAHA.111.647958. [DOI] [PubMed] [Google Scholar]
- 40.Longmore RB, Yeh RW, Kennedy KF, Anderson HV, White CJ, Longmore LS, et al. Clinical referral patterns for carotid artery stenting versus carotid endarterectomy: Results from the carotid artery revascularization and endarterectomy registry. Circ Cardiovasc Interv. 2011;4:88–94. doi: 10.1161/CIRCINTERVENTIONS.110.958843. [DOI] [PubMed] [Google Scholar]


