Abstract
Hypercholesterolemia is one of the main risk factors for coronary heart disease and significantly contributes to the high mortality associated with cardiovascular diseases. Statin therapy represents the gold standard in the reduction of low-density lipoprotein cholesterol concentration. Nevertheless, many patients still cannot achieve the recommended target levels, due to either inadequate effectiveness or intolerance of these drugs. Monoclonal antibodies that inhibit proprotein convertase subtilisin/kexin type 9 (PCSK9) have emerged as a promising option in lipid-lowering treatment. After confirmation of their efficacy and safety in clinical trials, evolocumab and alirocumab received approval from the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for introduction into clinical practice. In this review, we present a history of the development and mechanisms of action, as well as the results of the most important studies concerning PCSK9 inhibitors.
Keywords: hypercholesterolemia, proprotein convertase subtilisin/kexin type 9, monoclonal antibodies, lipid targets, statin intolerance
Introduction
For over half a century, hypercholesterolemia has been considered an indisputable risk factor for cardiovascular diseases (CVD), especially coronary heart disease (CHD), which is a major cause of death worldwide [1]. Introduction of statins into clinical practice revolutionized lipid control and significantly contributed to the reduction of mortality [2]. Nevertheless, about 50% of treated patients and even 80% of the population at very high risk do not achieve the recommended cholesterol values [3–5]. This may be partly explained by the ‘rule of 6’, which means that every doubling of statin dose causes only a 6% additional decrease in the low-density lipoprotein cholesterol (LDL-C) concentration [6]. Moreover, the target levels of lipids are still controversial. The results of recent studies supported the concept “the lower the LDL-C, the better”. The IMPROVE-IT trial revealed that combining ezetimibe with simvastatin therapy caused a further decrease of LDL-C level (53 vs. 70 mg/dl) and significantly reduced cardiovascular event rates (32.7% vs. 34.7%) [7]. On the other hand, statin intolerance remains an important problem in routine clinical settings. Its incidence was reported to be as high as 10% to 20% [8, 9]. According to the unified definition recommended by the International Lipid Expert Panel (ILEP), statin intolerance is an inability to tolerate at least 2 different statins (one at the lowest starting average daily dose and the other at any dose) because of their adverse effects. These include muscle symptoms, headache, sleep disorders, alopecia, rash, dyspepsia, nausea, erectile dysfunction, gynecomastia, and/or arthritis. The diagnosis is supported by the improvement of symptoms and/or biomarker changes following dose reduction or drug withdrawal. In each case predisposing conditions, such as hypothyroidism and drug-drug interaction, should be excluded [10]. These limitations of standard lipid-lowering therapy created the need to seek new, more effective, as well as safer drugs.
Genetic background
The level of cholesterol depends on hereditary factors and lifestyle. In some cases, lipid disorders are caused by single genetic mutations of proteins involved in metabolic pathways. Familial hypercholesterolemia (FH) may serve as an example determined by mutations in the genes encoding low-density lipoprotein receptor (LDLR) or apolipoprotein B (APOB). The prevalence of heterozygous FH is estimated at 1 per every 500 individuals in European countries. In Poland, more than 80,000 people may be affected [11]. The contemporary data indicate even higher rates of overall frequency (1 : 200–1 : 300), suggesting > 30 million people with FH worldwide [12]. The vast majority of cases remain underdiagnosed and undertreated. The main clinical symptoms of FH include xanthelasma palpebrarum, senile corneal arcus and xanthomas in the Achilles tendons as well as tendons of hand extensor muscles. In heterozygous FH, total cholesterol (TC) concentration is usually between 290 mg/dl and 500 mg/dl (7.5–12.9 mmol/l), while in homozygotes it rises to the range of 600–1000 mg/dl (15.5–25.8 mmol/l). The affected individuals suffer from atherosclerosis, which develops at an early age and leads to premature myocardial infarctions (MI), strokes, and death [13].
In 2003, Abifadel et al. identified a novel mutation in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene in two French families with autosomal dominant hypercholesterolemia [14]. A short time later, other scientists reported FH cases inherited in a similar way and proved the relationship between polymorphism in the PCSK9 gene and the levels of TC as well as LDL-C in the general population [15, 16]. They noted that phenotype varied in accordance with the type of mutation. Gain-of-function mutations cause hypercholesterolemia and represent the least common form of FH, albeit associated with the highest risk for CHD [17]. Conversely, when the expression of PCSK9 is reduced or its activity is blocked, the concentration of LDL-C decreases [18]. In the ARIC study, nonsense mutations in the PCSK9 gene resulted in a substantial reduction of the LDL-C level as well as the incidence of CHD (by 28% and 88% in the black population, and by 15% and 47% in the white population) [19]. Zhao et al. described a woman of African descent who was a compound heterozygote for two inactivating mutations in the PCSK9 gene. Despite a very low LDL-C plasma concentration (14 mg/dl), she showed no signs of health problems [20]. These discoveries shed a new light on cholesterol homeostasis and became an incentive for research aimed at designing novel lipid-lowering drugs.
Role of PCSK9 in metabolism
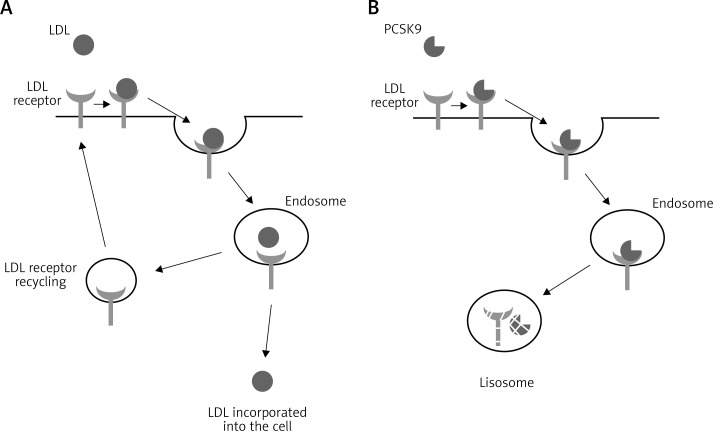
Proprotein convertase subtilisin/kexin type 9 belongs to the family of enzymes which activate or deactivate other proteins by proteolytic cleavage. It consists of 692 amino acids encoded by a gene located in chromosome 1p32.3. Following synthesis in the nucleus, PCSK9 undergoes intramolecular autocatalytic cleavage of its N-terminal prosegment in the endoplasmic reticulum. This process is required for protein maturation and secretion. PCSK9 is mainly expressed in the liver, but also in neuronal tissue, kidney mesenchymal cells, and intestinal epithelia [21]. Its pivotal role in cholesterol metabolism is modulation of LDL clearance by disrupting recycling of LDL receptors (Figure 1). The PCSK9 molecule binds to the epidermal growth factor-like repeat A (EGF-A) domain of LDLR, which leads to its internalization into hepatocytes and subsequently lysosomal degradation [22]. It also acts as an intracellular chaperone and impairs the transport of LDLR premature forms to the cell surface membrane [23]. Moreover, PCSK9 promotes hepatic and intestinal production of the triglyceride-rich APOB lipoproteins [24].
Figure 1.
A – Recycling of the LDL receptor. B – Degradation of the LDL receptor after binding with PCSK9 molecule
PCSK9 in physiological and pathological conditions
The concentration of PCSK9 varies depending on the fasting state (decreased during prolonged fasting), gender (higher in women than in men) and time of the day (nadir between 3 pm and 9 pm, peak at around 4 am) [25, 26]. It declines in adolescence (boys aged 9–16 years, and girls about the age of 16), as well as in postmenopausal women. Pregnancy is associated with an increase in PCSK9 levels. Growth hormone and estrogen play a key role in these phenomena [27]. Lifestyle also affects PCSK9 concentration. A Mediterranean-type diet reduces it significantly [28]. A similar trend was observed in high-fat-fed mice after aerobic treadmill exercise training [27].
Changes in PCSK9 level have also been reported in different diseases. The literature concerning the influence of insulin on the expression of the PCSK9 molecule is inconsistent [27, 29, 30]. However, in diabetics its concentration is increased due to insulin resistance and obesity [27]. The expression of PCSK9 correlates positively with TSH and inversely with thyroid hormones [27, 31].
Patients with chronic kidney disease on hemodialysis therapy have lower PCSK9 values than those on peritoneal dialysis therapy [27]. Nevertheless, no relationship between PCSK9 levels and kidney function was found [32].
The role of PCSK9 in cases of liver dysfunction has been assessed in several animal studies. PCSK9 modulates the infectivity of the hepatitis C virus by down-regulation of its receptors (LDLR and CD81). In rats following partial hepatectomy, an increase in PCSK9 mRNA was observed. The regenerating liver capacity was impaired in PCSK9-knockout mice, but resolved after implementation of a high-cholesterol diet [27].
PCSK9 also influences the immune defense system. Inflammation stimulates its expression followed by an increase of LDL-C [33]. Walley et al. demonstrated that PCSK9 molecules reduce pathogen lipid clearance via the LDLR and proved that the inhibition of this mechanism can mitigate the inflammatory response, as well as improving the septic shock outcome [34, 35].
The PCSK9 level in serum is directly correlated not only with LDL-C and TC values but also with atheroma burden in coronary arteries [36]. This was observed in 400 patients with chest pain, independently of LDL-C or other CVD risk factors [37]. Some PCSK9 gene haplotypes associate with the severity of intracranial atherosclerosis and incidence of ischemic stroke [38]. Furthermore, PCSK9 concentration predicts cardiovascular events [39].
Drugs and PCSK9 level
The influence of different drugs on PCSK9 concentration has been reported by many authors. Careskey et al. demonstrated that treatment with atorvastatin increased circulating PCSK9 levels by 34% and decreased LDL-C by 42% when compared with baseline and placebo [40]. Sahebkar et al. confirmed these findings in a large meta-analysis [41]. The induction of PCSK9 expression involving hepatocyte nuclear factor 1 α (HNF1-α) and sterol response element binding protein 2 (SREBP2) may diminish the beneficial effect of statins [42]. On the other hand, PCSK9 concentration, both at baseline and during treatment, may differentiate individuals who respond versus those who are resistant to such therapy [43]. Some authors claim that fenofibrate reduces PCSK9 values by repressing its promoter activity and stimulating two degrading convertases: PC5/6A and furin [44, 45]. However, the majority of published reports present the opposite result. Sahebkar et al. performed a meta-analysis of 6 studies including a total of 218 subjects, where he showed that fibrate therapy was associated with a significant increase in PCSK9 concentration [46]. Niacin co-administered with statin or fibrate counteracts this phenomenon and improves the efficacy of such therapy [47]. Ezetimibe seems to have a neutral influence on PCSK9 level [48]. The aforementioned data suggest that the combination of a PCSK9 inhibitor and currently available drugs may result in an additive, or even synergistic, lipid-lowering effect [49].
Discovery of PCSK9 inhibitors
The first reports regarding such therapy appeared in 2007, when Graham et al. proposed antisense oligonucleotide inhibitors of PCSK9 (ASO) as an attractive approach to the treatment of hypercholesterolemia. These agents reduced TC concentration by 53% in mice and caused a 2-fold increase in hepatic LDLR protein levels [50]. Lindholm et al. observed similar changes in nonhuman primates [51]. In 2008 Frank-Kamenetsky et al. proved that small interfering RNAs (siRNAs) decreased the values of PCSK9, APOB, and LDL-C without a measurable influence on either high-density lipoprotein cholesterol (HDL-C) or triglycerides (TG) in rodents, as well as cynomolgus monkeys [52]. A year later Duff et al. demonstrated that antibodies targeting PCSK9 can reverse the PCSK9-mediated regulation of cell-surface LDLR and identified them as potential drugs in the therapy of lipid disorders [53]. Chan confirmed these findings with results of 36% reduction in the TC concentration in mice and 80% reduction of LDL-C levels in cynomolgus monkeys by using a neutralizing anti-PCSK9 antibody. Furthermore, he suggested its synergistic effect with statins [54]. Ni et al. and Liang et al. presented similar data with other high-affinity anti-PCSK9 antibodies: 1D05-IgG2 and J16, respectively [55, 56]. As a consequence of these reports, several pharmaceutical companies, such as Regeneron Pharmaceuticals, Amgen, Pfizer, and Merck, engaged in long-term research aimed at designing the best PCSK9 inhibitor [57].
Antibodies targeting PCSK9
Evolocumab (AMG 145)
Evolocumab is a fully human monoclonal IgG2 antibody directed against PCSK9. In phase I studies, it reduced LDL-C levels by up to 64% and up to 81% after one dose of ≥ 21 mg and with repeated doses of ≥ 35 mg once a week, respectively [58].
Several phase II trials, such as MENDEL, RUTHERFORD, LAPLACE-TIMI 57, and GAUSS, have confirmed the efficacy and safety of evolocumab [59–62]. It reduced dose-dependently the LDL-C concentration by 39–66% compared with placebo. At the end of the 12 weeks of the LAPLACE-TIMI 57 study, over 90% of patients at high risk achieved the recommended LDL-C values of < 70 mg/dl with evolocumab at doses of 140 mg every 2 weeks and ≥ 280 mg every 4 weeks [63]. Apart from that, it lowered the level of lipoprotein(a) (Lp(a)), which is a proven important risk factor in the development of CVD [64, 65]. Active treatment in the RUTHERFORD study was associated with a reduction in the levels of Lp(a)by 23–32% and TG by 15–20%, while HDL-C increased by 7% [60]. In the OSLER-1 study, which was composed of patients who had taken part in the 4 aforementioned trials, evolocumab lowered the LDL-C concentration on average by approximately 50% beyond the values achieved with optimal standard therapy. The effect was stable over the course of 52 weeks. Conversely, discontinuation of treatment caused a rapid return to the baseline [66]. In another combined analysis of OSLER-1 and OSLER-2, including the results of phase III studies, with 4465 patients enrolled, evolocumab reduced LDL-C by 61% after 12 weeks of treatment. The rate of cardiovascular events at 1 year was diminished by 53% compared with standard therapy alone (0.95% in the evolocumab group vs. 2.18% in the standard therapy group (HR = 0.47; 95% CI: 0.28–0.78; p = 0.003)) [67].
The PROFICIO Program revealed in a group of over 1300 patients that evolocumab significantly reduced the levels of LDL-C, APOB, non-HDL-C, TG and Lp(a), as well as increasing HDL-C and apolipoprotein A1 (APOA1). Adverse events (AEs) occurred in 56.8% and 49.2% of patients in the evolocumab and placebo groups respectively, but only a minority of them (11.5% and 9.6%) were assigned as treatment-related events. The most common complaints included nasopharyngitis, upper respiratory infection symptoms, headache, diarrhea, myalgia, and back pain. Serious AEs (SAEs) occurred in 2.0% of patients receiving evolocumab and 1.2% of patients in the control group, but none of these cases were considered treatment-related. Reactions at the site of injection affected 4.1% and 3.3% of individuals in the evolocumab and placebo groups, while muscle-related AEs concerned 6% and 3.9% of them, respectively. The rate of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) elevations exceeding the upper limit of normal (ULN) by more than 3 times was similar in both study arms. An increase in creatine kinase (CK) activity more than 5 times the ULN was reported in 1.4% and 0.9% of patients taking evolocumab and placebo, respectively. All changes were asymptomatic and resolved spontaneously [68].
In the TESLA trial, evolocumab caused a significant and dose-related reduction of LDL-C concentration in homozygous FH patients with defective LDL-R activity. There was no effect in receptor negative subjects (< 2% LDL-R activity) [69].
The characteristics of the most important studies concerning evolocumab are presented in Table I [70–78]. In summary, in both the phase II and III trials, evolocumab administered at doses of 140 mg every 2 weeks or 420 mg once a month significantly reduced LDL-C levels by approximately 50–75% compared with placebo and 35–45% compared with ezetimibe [78]. The important advantage of evolocumab from a practical standpoint is the fact that there is no necessity of dose adjustments for age (18–79 years), gender, race/ethnicity, body weight, or statin therapy [78].
Table I.
Clinical trials concerning evolocumab (AMG 145) – PROFICIO Program
| Phase II | |||||
|---|---|---|---|---|---|
| Study | Population | Dosage | LDL-C reduction | ||
| MENDEL (n = 406, evolocumab = 271, ezetimibe = 45, placebo = 90) [59] | Patients with hypercholesterolemia (LDL-C in the range 100–190 mg/dl) without lipid-lowering therapy | Evolocumab 70, 105, or 140 mg every 2 weeks, 280, 350, or 420 mg every 4 weeks, ezetimibe 10 mg only; time of treatment = 12 weeks |
37–53% (vs. placebo) | ||
| RUTHERFORD (n = 168, evolocumab = 112, placebo = 56) [60] | Patients with heterozygous FH and hypercholesterolemia (LDL-C ≥ 100 mg/dl) during statin treatment with or without ezetimibe | Evolocumab 350 or 420 mg every 4 weeks; time of treatment = 12 weeks |
44–56% (vs. placebo) | ||
| LAPLACE-TIMI 57 (n = 631, evolocumab = 474, placebo = 157) [61] | Patients with hypercholesterolemia (LDL-C ≥ 85 mg/dl) during statin treatment with or without ezetimibe | Evolocumab 70, 105, or 140 mg every 2 weeks, 280, 350, or 420 mg every 4 weeks; time of treatment = 12 weeks |
42–66% (vs. placebo) | ||
| GAUSS (n = 160, placebo = 33, evolocumab = 127) [62] | Patients with history of statin intolerance | Evolocumab 280, 350, or 420 mg every 4 weeks, 420 mg + ezetimibe 10 mg daily every 4 weeks, ezetimibe 10 mg only; time of treatment = 12 weeks |
26–47% (vs. placebo) | ||
| YUKAWA (n = 310, evolocumab = 207, placebo = 103) [70] | Patients at high risk for cardiovascular events with hypercholesterolemia (LDL-C ≥ 116 mg/dl) during statin treatment | evolocumab 70 or 140 mg every 2 weeks, 280 or 420 mg every 4 weeks; time of treatment = 12 weeks |
53–69% (vs. placebo) | ||
| Phase III | |||||
| Study | Population | Dosage | LDL-C reduction | Percentage of patients achieving LDL-C level < 70 mg/dl | Additional results |
| MENDEL 2 (n = 614) [71] | Patients with hypercholesterolemia (LDL-C in the range 100–190 mg/dl) and Framingham risk scores ≤ 10% | Evolocumab 140 mg every 2 weeks, 420 mg every 4 weeks, ezetimibe 10 mg daily, placebo; time of treatment = 12 weeks |
55–57% in evolocumab group compared with placebo and 38–40% compared with ezetimibe | 69–72% vs. 0–1% vs. 1–2% (evolocumab vs. placebo vs. ezetimibe) | Significant reduction of APOB, Lp(a), non-HDL-C, TG, VLDL as well as TC/HDL-C and APOB/APOA1; significant increase in HDL-C compared with placebo |
| RUTHERFORD-2 (n = 331) [72] | Patients with heterozygous FH and hypercholesterolemia (LDL-C ≥ 100 mg/dl) despite intense lipid-lowering therapy | Evolocumab 140 mg every 2 weeks, 420 mg every 4 weeks, placebo; time of treatment = 12 weeks |
60–66% in evolocumab group compared with placebo | 63–68% vs. 2% (evolocumab vs. placebo) | Reduction of TG by 19.6% and Lp(a) by 31.6%; increase of HDL-C by 9.2% compared with placebo |
| LAPLACE-2 (n = 1899) [73] | Patients with hypercholesterolemia (LDL-C ≥ 150 mg/dl (no statin at screening), ≥ 100 mg/dl (non-intensive statin), or ≥ 80 mg/dl (intensive statin)). Intensive statin therapy was defined as daily atorvastatin (≥ 40 mg), rosuvastatin (20 mg), simvastatin (80 mg), or any statin plus ezetimibe |
Evolocumab 140 mg every 2 weeks, 420 mg every 4 weeks, ezetimibe 10 mg daily, placebo; time of treatment = 12 weeks |
59–66% in evolocumab group from baseline and 63–75% compared with placebo | 86–94% of patients in moderate-intensity statin therapy with evolocumab, 93–95% of patients in high-intensity statin therapy with evolocumab, 17–20% of patients receiving moderate-intensity statins and 51–62% of those receiving high-intensity statins with ezetimibe | Significant reduction of non-HDL-C (52–59% from baseline, 58–65% vs. placebo), APOB (47–56% from baseline, 51–59% vs. placebo) and Lp(a) (24–39% from baseline, 21–36% vs. placebo) for all statin groups; reduction in TG (6–16% from baseline, 12–30% vs. placebo); increase in HDL-C (5–10% from baseline, 4–10% vs. placebo) |
| GAUSS-2 (n = 307) [74] | Patients with hypercholesterolemia and statin intolerance (LDL-C ≥ 100 mg/dl with diagnosed CHD or risk equivalent, ≥ 130 mg/dl without CHD or risk equivalent and ≥ 2 risk factors, ≥ 160 mg/dl without CHD or risk equivalent and one risk factor, or ≥ 190 mg/dl without CHD or risk equivalent and no risk factors) |
Evolocumab 140 mg every 2 weeks, 420 mg every 4 weeks, ezetimibe 10 mg daily; time of treatment = 12 weeks |
55–56% from baseline, 37–39% compared with ezetimibe | 87.5% vs. 2% (evolocumab vs. ezetimibe) | Reduction of Lp(a) by 22–27% vs. 1.7–5.8% (evolocumab vs. ezetimibe) |
| DESCARTES (n = 901) [75] | Patients with hypercholesterolemia (LDL-C ≥ 75 mg/dl after 4–12 weeks run-in period of lipid-lowering therapy (diet alone, diet plus atorvastatin 10 mg daily, atorvastatin 80 mg daily, or atorvastatin 80 mg plus ezetimibe 10 mg daily)) | Evolocumab 420 mg every 4 weeks, ezetimibe 10 mg daily, placebo; time of treatment = 52 weeks |
49–62% compared with placebo | 82.3% vs. 6.4% (evolocumab vs. placebo) | Significant reduction of APOB (44.2%), non-HDL-C (50.3%), Lp(a) (22.4%) and TG (11.5%); significant increase of HDL-C (5.4%) and ApoA1 (3.0%) |
| TESLA (n = 50) [76] | Patients with homozygous FH on lipid-lowering therapy for at least 4 weeks | Evolocumab 420 mg every 4 weeks, placebo; time of treatment = 12 weeks |
30.9% compared with placebo | ||
| YUKAWA-2 (n = 404) [77] | Patients with hypercholesterolemia (LDL-C ≥ 100 mg/dl) at high risk for cardiovascular events based on Japan Atherosclerosis Society criteria | Evolocumab 140 mg every 2 weeks, 420 mg every 4 weeks, placebo; time of treatment = 12 weeks |
67–76% compared with placebo | 96–98% in evolocumab group, 0–4% in placebo group receiving atorvastatin 5 mg/day, 20% in placebo group receiving atorvastatin 20 mg/day | Reduction of APOB (56–66%), HDL-C (10–17%), Lp(a) (40–53%) |
| Ongoing | |||||
| Study | Purpose | ||||
| GAUSS-3 | to assess the efficacy and safety of evolocumab in subjects with statin intolerance | ||||
| OSLER-2 | to assess the long-term safety, tolerability and efficacy of evolocumab in subjects with hyperlipidemia or mixed dyslipidemia | ||||
| TAUSSIG | to assess the long-term efficacy and safety of evolocumab in subjects with severe FH | ||||
| FOURIER | to evaluate the influence of LDL-C reduction with evolocumab used in addition to other lipid-lowering treatment on the risk of cardiovascular death, MI, hospitalization for unstable angina, stroke, or coronary revascularization in subjects with clinically evident CVD | ||||
| GLAGOV | to evaluate the influence of LDL-C reduction with evolocumab on atherosclerotic plaque regression measured by intravascular ultrasound in subjects with CHD taking lipid-lowering therapy | ||||
| EBBINGHAUS | to evaluate the influence of treatment with evolocumab on neurocognitive functions in high cardiovascular risk subjects | ||||
| FLOREY | to evaluate the effect of evolocumab, atorvastatin, and combination therapy on lipoprotein kinetics (completed) | ||||
APOA1 – apolipoprotein A1, APOB – apolipoprotein B, CHD – coronary heart disease, CVD – cardiovascular disease, FH – familial hypercholesterolemia, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, Lp(a) – lipoprotein (a), MI – myocardial infarctions, TC – total cholesterol, TG – triglycerides.
Alirocumab (SAR236553/REGN727)
Alirocumab is a fully human, monoclonal IgG1 anti-PCSK9 antibody. In 2012, Stein et al. reported the results of the phase I study exploring this agent. Co-administered with atorvastatin, alirocumab reduced LDL-C concentration by 39%, 54%, and 61%, when taken in doses of 50, 100, and 150 mg, respectively [79]. In another study, it reduced LDL-C by 40–72% as well as lowering non-HDL-C, APOB, and Lp(a) [80]. All patients who received alirocumab and 52% of those treated with atorvastatin (80 mg daily) plus placebo attained LDL-C levels of less than 100 mg/dl. More than 90% of patients from the alirocumab study arm achieved LDL-C levels of less than 70 mg/dl, compared with 17% in the control group [81]. In individuals with gain-of-function mutations in the PCSK9 gene, alirocumab reduced LDL-C by 62.5% from the baseline, and by 53.7% when compared with placebo. The effect was sustained after 8 weeks of treatment [82].
Alirocumab not only affects the concentration of plasma lipids, but also improves the atherosclerosis burden and plaque morphology by reducing macrophage-rich and necrotic areas, while increasing the smooth muscle cell and collagen content [83]. The PCSK9 and LDL-C levels reach a nadir at about 3–4 days and 15 days respectively, following the single administration of a 75 mg dose. The pharmacokinetic and pharmacodynamic profiles are similar regardless of the injection site (upper arm, abdomen, thigh) [84].
The efficacy of alirocumab has been confirmed in several phase II and III studies. Their results are presented in Table II [85–89]. The data concerning the influence of PCSK9 inhibitors on cardiovascular disease risk are limited, but some trends are worth noting. In post hoc analysis of the ODYSSEY LONG TERM trial, alirocumab treatment was associated with a significantly lower rate of adjudicated major adverse cardiovascular events (a composite of death from CHD, nonfatal MI, ischemic stroke, and unstable angina requiring hospitalization) (1.7% in the alirocumab group versus 3.3% in the placebo group (HR = 0.52; 95% CI: 0.31–0.90; p = 0.02)) [87].
Table II.
Clinical trials concerning alirocumab (SAR236553/REGN727) – ODYSSEY Program
| Phase II | |||||
|---|---|---|---|---|---|
| Study | Population | Dosage | LDL-C reduction | ||
| NCT01288443 (McKenney et al.) (n = 183, alirocumab = 152, placebo = 31) [80] | Patients with hypercholesterolemia (LDL-C ≥ 100 mg/dl) during treatment with statins | 300 mg every 2 or 4 weeks | 40–72% (from baseline), 35–67% (vs. placebo) | ||
| CT01266876 (Stein et al.) (n = 77, alirocumab = 62, placebo = 15) [85] | Patient with heterozygous FH and hypercholesterolemia (LDL-C ≥ 100 mg/dl) during statin treatment with or without ezetimibe | 150 mg every 2 weeks, 150, 200, or 300 mg every 4 weeks | 29–68% (from baseline), 18–57% (vs. placebo) | ||
| Phase III | |||||
| Study | Population | Dosage | LDL-C reduction | Percentage of patients achieving LDL-C level < 70 mg/dl | Additional results |
| ODYSSEY MONO (n = 103) [86] | Patients with hypercholesterolemia (LDL-C 100–190 mg/dl) and 10-year risk of fatal cardiovascular events 1–5% (SCORE scale) | Alirocumab 75 mg every 2 weeks with dose up-titrated to 150 mg every 2 weeks if LDL-C at week 8 was ≥ 70 mg/dl, ezetimibe 10 mg daily; time of treatment = 24 weeks |
47% in alirocumab group vs. 16% in ezetimibe group | N/A | Significant reduction of APOB, TC and non-DL-C; increase in HDL-C and APOA1; no difference in the levels of Lp(a) and TG compared to ezetimibe |
| ODYSSEY LONG TERM (n = 2341) [87] | Patients at high risk for cardiovascular events with hypercholesterolemia (LDL-C ≥ 70 mg/dl) when receiving treatment with statins at the maximum tolerated dose, with or without other lipid-lowering therapy | Alirocumab 150 mg every 2 weeks, placebo; time of treatment = 78 weeks |
61% in alirocumab group vs. 0.8% in placebo group at week 24, 52.4% vs. 3.6% at week 78 | 79.3% in alirocumab group vs. 8.0% in placebo group at week 24 | Reduction of non-HDL-C (52.3%), APOB (54%), TC (37.5%), Lp(a) (25.6%) and TG (17.3%); increase of HDL-C (4.6%) and ApoA1 (2.9%) compared with placebo |
| ODYSSEY COMBO I (n = 316) [88] | Patients with established CHD or its equivalents and hypercholesterolemia (LDL-C ≥ 70 mg/dl and established CVD or LDL-C ≥ 100 mg/dl with CHD risk equivalents (e.g., diabetes mellitus with other risk factors or chronic kidney disease)) | Alirocumab 75 mg every 2 weeks with dose up-titrated to 150 mg every 2 weeks if LDL-C at week 8 was ≥ 70 mg/dl, placebo; time of treatment = 52 weeks |
48.2% in alirocumab group vs. 2.3% in placebo group at week 24 | 75% in alirocumab group vs. 9% in placebo group | Significant reduction of non-HDL-C, TC, APOB and Lp(a) |
| ODYSSEY FH I, FH II (n = 735) [89] | Patients with heterozygous FH who did not have a history of cardiovascular events, and those who had suffered an MI or ischemic stroke if their LDL-C levels were not at goal according to current guidelines for primary (≥ 100 mg/dl) or secondary (≥ 70 mg/dl) prevention. All patients were receiving stable high-dose statin therapy with or without other lipid-lowering drugs | Alirocumab 75 mg every 2 weeks with dose up-titrated to 150 mg every 2 weeks if at week 8 LDL-C was ≥ 70 mg/dl, placebo; time of treatment = 78 weeks |
57.9% in alirocumab group compared to placebo in FH I and 51.4% in alirocumab group compared to placebo in FH II at week 24; reduction from baseline by 51.8% in alirocumab group in FH I and 52.1% in FH II at week 78 | 59.8% in alirocumab group vs. 0.8% in placebo group in FH I and 68.2% in alirocumab group vs. 1.2% in placebo group in FH II at week 24 | Significant reduction of APOB, non-HDL-C, Lp(a), and TG; increase of HDL-C and APOA1 |
| Ongoing | |||||
| Study | Purpose | ||||
| ODYSSEY OLE | to assess the long-term efficacy and safety of alirocumab when added to lipid-lowering therapy in patients with heterozygous FH | ||||
| ODYSSEY High FH | to assess the efficacy and safety of alirocumab in subjects with heterozygous FH (completed) | ||||
| ODYSSEY CHOICE 1 ODYSSEY CHOICE 2 | to assess the efficacy and safety of alirocumab in subjects with primary hypercholesterolemia when administered every 4 weeks alone or added to current lipid-lowering therapy (completed) | ||||
| ODYSSEY-ALTERNATIVE | to assess the efficacy and safety of alirocumab in patients with primary hypercholesterolemia and moderate, high, or very high cardiovascular risk, who are intolerant to statins | ||||
| ODYSSEY-OUTCOMES | to evaluate the influence of alirocumab on the occurrence of cardiovascular events (composite endpoint of CHD death, non-fatal MI, ischemic stroke, unstable angina requiring hospitalization) in patients who have experienced an acute coronary syndrome event 4 to 52 weeks prior to randomization and are treated with evidence-based medical and dietary management of dyslipidemia | ||||
APOA1 – apolipoprotein A1, APOB – apolipoprotein B, CHD – coronary heart disease, CVD – cardiovascular disease, FH – familial hypercholesterolemia, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, Lp(a) – lipoprotein (a), MI – myocardial infarction, TC – total cholesterol, TG – triglycerides.
Bococizumab (RN316/PF-04950615)
Bococizumab is a humanized IgG2Δa monoclonal antibody that binds to PCSK9 and improves the LDL clearance by the same mechanism as evolucumab and alirocumab. The results of a phase II study and purposes of ongoing phase III trials are presented in Table III [90, 91].
Table III.
Clinical trials concerning bococizumab (PF-4950615, RN-316) – SPIRE Program
| Phase II | |||
|---|---|---|---|
| Study | Population | Dosage | LDL-C reduction |
| NCT01592240 (Ballantyne et al.) (n = 354, bococizumab = 253, placebo = 101) [90] | Patients with hypercholesterolemia (LDL-C ≥ 80 mg/dl) during statin therapy | 50, 100, or 150 mg every 2 weeks, 200 or 300 mg every 4 weeks (doses were reduced if LDL-C levels persistently decreased to ≤ 25 mg/dl); time of treatment = 24 weeks |
27–53% (vs. placebo) |
| Phase III – Ongoing | |||
| Study | Purpose | ||
| SPIRE-SI | to assess the lipid-lowering effect of bococizumab in subjects with statin intolerance | ||
| SPIRE-HF | to assess the efficacy, safety and tolerability of bococizumab in subjects with heterozygous FH who receive highly effective statins | ||
| SPIRE-LDL | to assess the efficacy, safety and tolerability of bococizumab in subjects with hypercholesterolemia who receive highly effective statins | ||
| SPIRE-LL | to access the efficacy, safety and tolerability of bococizumab in subjects with hyperlipidemia who receive background statin therapy | ||
| SPIRE-HR | to assess the efficacy, safety and tolerability of bococizumab in subjects with hypercholesterolemia who receive highly effective statins | ||
| SPIRE-1 | to evaluate bococizumab, compared with placebo, in reducing the occurrence of major cardiovascular events, including cardiovascular death, MI, stroke, and unstable angina requiring urgent revascularization, in high-risk subjects with hypercholesterolemia despite background lipid-lowering therapy (LDL-C ≥ 70 mg/dl and < 100 mg/dl or non-HDL-C ≥ 100 mg/dl and < 130 mg/dl) | ||
| SPIRE-2 | to evaluate bococizumab, compared with placebo, in reducing the occurrence of major cardiovascular events, including cardiovascular death, MI, stroke, and unstable angina requiring urgent revascularization, in high-risk subjects with hypercholesterolemia despite background lipid-lowering therapy (LDL-C ≥ 100 mg/dl or non-HDL-C ≥ 130 mg/dl) | ||
FH – familial hypercholesterolemia, MI – myocardial infarction.
PCSK9 antibodies in meta-analyses
Navarese et al. performed a meta-analysis of 24 randomized trials that evaluated the effects of anti-PCSK9 antibody administration in 10,159 adults with hypercholesterolemia [92]. In this heterogeneous population, PCSK9 inhibition caused a significant reduction in the LDL-C concentration, by 59% compared with placebo, and 36% compared with ezetimibe. The Lp(a) level decreased by approximately 25%, while HDL-C increased by 6% relative to both control groups [92]. Lipinski et al. obtained similar results in a meta-analysis of 17 randomized trials composed of 13,083 patients. Anti-PCSK9 antibodies reduced LDL-C by 57% compared with placebo (p < 0.001) and 36.1% compared with ezetimibe (p < 0.001) [93]. According to Li et al., PCSK9 inhibitors provided a substantial lowering effect on the levels of LDL-C, TC, TG, APOB, and Lp(a) and caused an increase in the concentrations of HDL-C and APOA1 [94]. The efficacy of PCSK9 inhibitors was also confirmed by Zhang et al., who analyzed the results of 25 randomized trials encompassing 12,200 patients [95]. Evolocumab administered as a 420 mg dose once a month significantly reduced LDL-C by an average of 78.9 mg/dl (54.6% vs. placebo and 36.3% vs. ezetimibe). An equal or even greater change was observed following biweekly 140 mg doses (60.4% vs. placebo). Moreover, the active treatment increased HDL-C by 7.6% vs. placebo, and 6.4% vs. ezetimibe. Evolocumab generated a significant reduction in TC, TC/HDL-C, non-HDL-C and very-low-density lipoprotein cholesterol (VLDL-C), by 36.7%, 41.3%, 52.1% and 22.8%, respectively, with a monthly 420 mg dose. It also lowered APOB, APOB/APOA1 and Lp(a). A significant decrease in TG and a rise in APOA1 were found in all dosages of evolocumab except for biweekly 105 mg administration. Monthly 420 mg and biweekly 140 mg treatment increased the APOA1 level by 5.2–6.3% and lowered the TG by 15.7–17.4% compared with placebo. Alirocumab administered at biweekly doses of 50 to 150 mg reduced LDL-C by 52.6% vs. placebo and 29.9% vs. ezetimibe, as well as increasing HDL-C by 8% [95].
The influence of PCSK9 inhibitors on the risk of cardiovascular events was indicated by Navarese et al. [92]. In a group of patients receiving anti-PCSK9 antibodies, the rates of all-cause mortality and MI occurrences were reduced by 55% (OR = 0.45; 95% CI: 0.23–0.86; p = 0.01) and 51% (OR = 0.49; 95% CI: 0.26–0.93; p = 0.03), respectively, relative to placebo. The difference in the risk of cardiovascular death was not significant (OR = 0.50; 95% CI: 0.23–1.10; p = 0.08) [92]. Lipiński et al. reported similar results [93]. Anti-PCSK9 antibodies reduced the incidence of all-cause mortality (OR = 0.43; 95% CI: 0.22–0.82; p = 0.01), whereas the change in risk of cardiovascular death (OR = 0.50; 95% CI: 0.22–1.13; p = 0.10) and cardiovascular events (OR = 0.67; 95% CI: 0.43–1.04; p = 0.07) did not reach statistical significance [93]. Large randomized trials are ongoing in order to assess the influence of evolocumab (FOURIER), alirocumab (ODYSSEY OUTCOMES) and bococizumab (SPIRE 1, SPIRE 2) on cardiovascular risk [96].
Safety concerns
In the trials conducted to date, anti-PCSK9 antibodies were generally safe and well tolerated. Some animal studies yielded concerns about higher risk for HCV infection, diabetes and impaired hepatic recovery abilities in PCSK9 deficiency. However, no human report currently supports these hypotheses. The theoretical induction of intestinal tumors by an excessive production of bile acid was not confirmed in practice. Due to the lack of safety evidence during pregnancy, PCSK9 inhibitors are contraindicated in this state [97]. Nevertheless, bococizumab did not affect the embryo-fetal development in an animal study [98]. A few trials revealed that PCSK9 inhibitors may increase the risk of neurocognitive adverse events (OR = 2.34; 95% CI: 1.11–4.93; p = 0.02) when compared with placebo [93]. These cases were rare (1.2% in ODYSSEY LONG TERM, 0.9% in OSLER) and not associated with on-treatment LDL-C levels (number needed to harm – 269) [99, 100]. The neurocognitive adverse effects of evolocumab are being examined in the ongoing EBBINGHAUS trial [96]. The influence of PCSK9 inhibitors on the concentration of fat-soluble vitamins and steroid hormones remained unresolved until the results of the DESCARTES study. It showed that among patients treated with evolocumab, vitamin E levels mirrored the changes in lipid fractions, while no dysfunction was observed in either adrenal or gonadal steroid hormone metabolism. The major source of cholesterol required for steroidogenesis may come from alternative LDL delivery pathways, such as endogenous synthesis and transport in HDL particles [101]. Anti-drug antibodies were detected in some cases, but their induction did not affect patient safety [88]. The majority of the treated population accepted the subcutaneous way of administration with self-injected pre-filled pen or syringe devices, which is very important for compliance [102].
Regarding safety analyses, Zhang et al. found no significant difference in the occurrence of AEs between anti-PCSK9 antibodies and agents used in the control groups (placebo or ezetimibe), except that alirocumab was associated with an increased rate of injection-site reactions (RR = 1.48; 95% CI: 1.05–2.09; p = 0.02) compared with placebo. Liver dysfunction occurred less often in patients treated with evolocumab (RR = 0.43; 95% CI: 0.20–0.93; p = 0.03) [95]. Prevalence of SAEs in subjects included in the meta-analysis conducted by Navarese et al. was comparable (9% in PCSK9 inhibitors and 8% in control groups, respectively (OR = 1.01; 95% CI: 0.87–1.18; p = 0.88). The authors reported a 30% reduction in the odds of increased creatine kinase activity among subjects using anti-PCSK9 antibodies, which may suggest that they even provide a muscle-sparing effect [92]. However, these findings must be confirmed in the ongoing large, long-term trials with prespecified primary CVD endpoints.
Approval status
Alirocumab and evolocumab have been recently registered by the US Food and Drug Administration (FDA) for treatment of patients with heterozygous FH or atherosclerotic CVD, who, despite the use of diet and statins in maximally tolerated doses, require an additional reduction of LDL-C levels [103]. The European Medicines Agency (EMA) has also approved evolocumab and alirocumab as the first PCSK9 inhibitors in Europe for treatment of adults and adolescents (over 12 years old) with primary hypercholesterolemia or mixed dyslipidemia, who are treated with lipid-lowering drugs but remain unable to achieve the recommended LDL-C goals. Moreover, they can be used in cases of statin intolerance or contraindications for their administration [103]. According to the recently published American Heart Association Statement for FH Management, PCSK9 inhibitors should be considered if LDL-C is not at goal level in spite of treatment consisting of high-intensity statin therapy and ezetimibe [104].
Future
Apart from evolocumab, alirocumab and bococizumab, the following PCSK9 targeted antibodies are under assessment: LGT209, 1D05-IgG2, LY3015014. RG-7652 was recently withdrawn from research for unknown reasons [105]. Other promising methods of limiting the PCSK9 production or autocatalytic cleavage include mimetic peptides, adnectin, small molecule inhibitors, antisense oligonucleotides and small interfering RNA oligonucleotides (siRNA) [106]. The siRNA molecules lead to degradation of PCSK9 messenger RNA (mRNA) and make the process of its translation impossible. Thus, they impair both intracellular and extracellular PCSK9 functions. In a phase I study, ALN-PCS, one of the siRNA-based drugs, was well tolerated and significantly reduced PCSK9 as well as LDL cholesterol levels after a single intravenous dose in healthy volunteers [107]. Galabova et al. proposed a novel approach by creating a peptide-based anti-PCSK9 vaccine. It may be an attractive therapy, but requires further investigation in clinical trials [108, 109]. In the future, an alternative option to PCSK9 inhibitors may be provided by APOB production inhibitors (mipomersen), microsomal triglyceride transfer protein inhibitors (lomitapide), cholesteryl ester transfer protein (CETP) inhibitors (anacetrapib) and dual modulator of AMP-kinase and ATP-citrate lyase (ETC-1002) [110–112].
Conclusions
The discovery of anti-PCSK9 antibodies seems to be a breakthrough for a wide range of patients with FH or statin intolerance or at high cardiovascular risk, who cannot achieve LDL-C target values with currently available drugs. The main advantages of evolocumab and alirocumab are their efficacy and safety, confirmed by many clinical trials. PCSK9 inhibitors have started a new era in the pharmacotherapy of lipid disorders, which will probably bring a remarkable improvement in CVD outcomes.
Conflict of interest
KJ and DAK declare no conflict of interest. PJ – advisory boards and travel grants from Amgen, KRKA, MSD, and Sanofi.
References
- 1.Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979;90:85–91. doi: 10.7326/0003-4819-90-1-85. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23:636–48. doi: 10.1177/2047487315569401. [DOI] [PubMed] [Google Scholar]
- 4.Jankowski P, Czarnecka D, Wolfshaut-Wolak R, et al. Secondary prevention of coronary artery disease in contemporary clinical practice. Cardiol J. 2015;22:219–26. doi: 10.5603/CJ.a2014.0066. [DOI] [PubMed] [Google Scholar]
- 5.Chiang CE, Ferrières J, Gotcheva NN, et al. Suboptimal control of lipid levels: results from 29 countries participating in the centralized pan-regional surveys on the undertreatment of hypercholesterolaemia (CEPHEUS) J Atheroscler Thromb. 2016;23:567–87. doi: 10.5551/jat.31179. [DOI] [PubMed] [Google Scholar]
- 6.Sahebkar A, Simental-MendÍa LE, Guerrero-Romero F, et al. Effect of statin therapy on plasma proprotein convertase subtilisinkexin 9 (PCSK9) concentrations: a systematic review and meta-analysis of clinical trials. Diabetes Obes Metab. 2015;17:1042–55. doi: 10.1111/dom.12536. [DOI] [PubMed] [Google Scholar]
- 7.Serban MC, Banach M, Mikhailidis DP. Clinical implications of the IMPROVE-IT trial in the light of current and future lipid-lowering treatment options. Expert Opin Pharmacother. 2016;17:369–80. doi: 10.1517/14656566.2016.1118055. [DOI] [PubMed] [Google Scholar]
- 8.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients-the PRIMOstudy. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–34. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banach M, Rizzo M, Toth PP, et al. Statin intolerance – an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myśliwiec M, Walczak M, Małecka-Tendera E, et al. Management of familial hypercholesterolemia in children and adolescents. Position paper of the Polish Lipid Expert Forum. J Clin Lipidol. 2014;8:173–80. doi: 10.1016/j.jacl.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Vallejo-Vaz AJ, KondapallySeshasai SR, Cole D, et al. Familial hypercholesterolaemia: a global call to arms. Atherosclerosis. 2015;243:257–9. doi: 10.1016/j.atherosclerosis.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Rynkiewicz A, Cybulska B, Banach M, et al. Management of familial heterozygous hypercholesterolemia: position paper of the Polish Lipid Expert Forum. J Clin Lipidol. 2013;7:217–21. doi: 10.1016/j.jacl.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 15.Timms KM, Wagner S, Samuels ME, et al. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum Genet. 2004;114:349–53. doi: 10.1007/s00439-003-1071-9. [DOI] [PubMed] [Google Scholar]
- 16.Shioji K, Mannami T, Kokubo Y, et al. Genetic variants in PCSK9 affect the cholesterol level in Japanese. J Hum Genet. 2004;49:109–14. doi: 10.1007/s10038-003-0114-3. [DOI] [PubMed] [Google Scholar]
- 17.Humphries SE, Whittall RA, Hubbart CS, et al. Genetic causes of familial hypercholesterolaemia in patients in the UK: relation to plasma lipid levels and coronary heart disease risk. J Med Genet. 2006;43:943–9. doi: 10.1136/jmg.2006.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J, Pertsemlidis A, Kotowski IK, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–5. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JC, Boerwinkle E, Mosley TH, Jr, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–23. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidah NG, Benjannet S, Wickham L, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl AcadSci U S A. 2003;100:928–33. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert G, Sjouke B, Choque B, et al. The PCSK9 decade. J Lipid Res. 2012;53:2515–24. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz R, SchlÜter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9) Basic Res Cardiol. 2015;110:4. doi: 10.1007/s00395-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashid S, Tavori H, Brown PE, et al. Proprotein convertase subtilisinkexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation. 2014;130:431–41. doi: 10.1161/CIRCULATIONAHA.113.006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persson L, Cao G, Ståhle L, et al. Circulating proprotein convertase subtilisinkexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. 2010;30:2666–72. doi: 10.1161/ATVBAHA.110.214130. [DOI] [PubMed] [Google Scholar]
- 26.Lakoski SG, Lagace TA, Cohen JC, et al. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–43. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui CJ, Li S, Li JJ. PCSK9 and its modulation. Clin Chim Acta. 2015;440:79–86. doi: 10.1016/j.cca.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 28.Richard C, Couture P, Desroches S, et al. Effect of the Mediterranean diet with and without weight loss on surrogate markers of cholesterol homeostasis in men with the metabolic syndrome. Br J Nutr. 2012;107:705–11. doi: 10.1017/S0007114511003436. [DOI] [PubMed] [Google Scholar]
- 29.Awan Z, Dubuc G, Faraj M, et al. The effect of insulin on circulating PCSK9 in postmenopausal obese women. Clin Biochem. 2014;47:1033–9. doi: 10.1016/j.clinbiochem.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Miao J, Manthena PV, Haas ME, et al. Role of insulin in the regulation of proprotein convertase subtilisin/kexin type 9. Arterioscler Thromb Vasc Biol. 2015;35:1589–96. doi: 10.1161/ATVBAHA.115.305688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonde Y, Breuer O, LÜtjohann D, Sjöberg S, Angelin B, Rudling M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55:2408–15. doi: 10.1194/jlr.M051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogacev KS, Heine GH, Silbernagel G, et al. PCSK9 plasma concentrations are independent of GFR and do not predict cardiovascular events in patients with decreased GFR. PLoS One. 2016;11:e014692. doi: 10.1371/journal.pone.0146920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feingold KR, Moser AH, Shigenaga JK, et al. Inflammation stimulates the expression of PCSK9. Biochem Biophys Res Commun. 2008;374:341–4. doi: 10.1016/j.bbrc.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walley KR, Thain KR, Russell JA, et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6:258. doi: 10.1126/scitranslmed.3008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walley KR, Francis GA, Opal SM, et al. The central role of PCSK9 in septic pathogen lipid transport and clearance. Am J Respir Crit Care Med. 2015;192:1275–86. doi: 10.1164/rccm.201505-0876CI. [DOI] [PubMed] [Google Scholar]
- 36.Alborn WE, Cao G, Careskey HE, et al. Serum proprotein convertase subtilisinkexin type 9 is correlated directly with serum LDL cholesterol. Clin Chem. 2007;53:1814–9. doi: 10.1373/clinchem.2007.091280. [DOI] [PubMed] [Google Scholar]
- 37.Walton TA, Nishtar S, Lumb PJ, et al. Pro-protein convertase subtilisin/kexin 9 concentrations correlate with coronary artery disease atheroma burden in a Pakistani cohort with chronic chest pain. Int J Clin Pract. 2015;69:738–42. doi: 10.1111/ijcp.12615. [DOI] [PubMed] [Google Scholar]
- 38.Abboud S, Karhunen PJ, LÜtjohann D, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) gene is a risk factor of large-vessel atherosclerosis stroke. PLoS One. 2007;2:e1043. doi: 10.1371/journal.pone.0001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner C, Hoffmann MM, Winkler K, et al. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol. 2014;62:94–102. doi: 10.1016/j.vph.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Careskey HE, Davis RA, Alborn WE, et al. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–8. doi: 10.1194/jlr.M700437-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Sahebkar A, Simental-MendÍa LE, Guerrero-Romero F, et al. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9(PCSK9) concentrations: a systematic review and meta-analysis of clinical trials. Diabetes Obes Metab. 2015;17:1042–55. doi: 10.1111/dom.12536. [DOI] [PubMed] [Google Scholar]
- 42.Dong B, Wu M, Li H, et al. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res. 2010;51:1486–95. doi: 10.1194/jlr.M003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor BA, Panza G, Pescatello LS, et al. Serum PCSK9 levels distinguish individuals who do not respond to high-dose statin therapy with the expected reduction in LDL-C. J Lipids. 2014;2014:140723. doi: 10.1155/2014/140723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kourimate S, Le May C, Langhi C, et al. Dual mechanisms for the fibrate-mediated repression of proprotein convertase subtilisin/kexin type 9. J Biol Chem. 2008;283:9666–73. doi: 10.1074/jbc.M705831200. [DOI] [PubMed] [Google Scholar]
- 45.Lambert G, Ancellin N, Charlton F, et al. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin Chem. 2008;54:1038–45. doi: 10.1373/clinchem.2007.099747. [DOI] [PubMed] [Google Scholar]
- 46.Sahebkar A. Circulating levels of proprotein convertase subtilisinkexin type 9 are elevated by fibrate therapy: a systematic review and meta-analysis of clinical trials. Cardiol Rev. 2014;22:306–12. doi: 10.1097/CRD.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 47.Khera AV, Qamar A, Reilly MP, Dunbar RL, Rader DJ. Effects of niacin, statin, and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 levels in patients with dyslipidemia. Am J Cardiol. 2015;115:178–82. doi: 10.1016/j.amjcard.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Berthold HK, Seidah NG, Benjannet S, Gouni-Berthold I. Evidence from a randomized trial that simvastatin, but not ezetimibe, upregulates circulating PCSK9 levels. PLoS One. 2013;8:e60095. doi: 10.1371/journal.pone.0060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konrad RJ, Troutt JS, Cao G. Effects of currently prescribed LDL-C-lowering drugs on PCSK9 and implications for the next generation of LDL-C-lowering agents. Lipids Health Dis. 2011;10:38. doi: 10.1186/1476-511X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res. 2007;48:763–7. doi: 10.1194/jlr.C600025-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Lindholm MW, Elmén J, Fisker N, et al. PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates. Mol Ther. 2012;20:376–81. doi: 10.1038/mt.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–20. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duff CJ, Scott MJ, Kirby IT, et al. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J. 2009;419:577–84. doi: 10.1042/BJ20082407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan JC, Piper DE, Cao Q, et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci U S A. 2009;106:9820–5. doi: 10.1073/pnas.0903849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni YG, Di Marco S, Condra JH, et al. PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo. J Lipid Res. 2011;52:78–86. doi: 10.1194/jlr.M011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang H, Chaparro-Riggers J, Strop P, et al. Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates. J Pharmacol Exp Ther. 2012;340:228–36. doi: 10.1124/jpet.111.187419. [DOI] [PubMed] [Google Scholar]
- 57.Ferri N. Proprotein convertase subtilisin/kexin type 9: from the discovery to the development of new therapies for cardiovascular diseases. Scientifica (Cairo) 2012;2012:927352. doi: 10.6064/2012/927352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–98. doi: 10.1016/j.jacc.2012.08.986. [DOI] [PubMed] [Google Scholar]
- 59.Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proproteinconvertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 60.Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–17. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 61.Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–17. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 63.Desai NR, Giugliano RP, Zhou J, et al. AMG 145, a monoclonal antibody against PCSK9, facilitates achievement of national cholesterol education program-adult treatment panel III low-density lipoprotein cholesterol goals among high-risk patients: an analysis from the LAPLACE-TIMI 57 trial (LDL-C assessment with PCSK9 monoclonal antibody inhibition combined with statin thErapy-thrombolysis in myocardial infarction 57) J Am Coll Cardiol. 2014;63:430–3. doi: 10.1016/j.jacc.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 64.Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128:962–9. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 65.Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–88. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Koren MJ, Giugliano RP, Raal FJ, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 2014;129:234–43. doi: 10.1161/CIRCULATIONAHA.113.007012. [DOI] [PubMed] [Google Scholar]
- 67.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 68.Stein EA, Giugliano RP, Koren MJ, et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J. 2014;35:2249–59. doi: 10.1093/eurheartj/ehu085. [DOI] [PubMed] [Google Scholar]
- 69.Stein EA, Honarpour N, Wasserman SM, et al. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–20. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- 70.Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk: primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073–82. doi: 10.1253/circj.cj-14-0130. [DOI] [PubMed] [Google Scholar]
- 71.Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–40. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 72.Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 73.Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–82. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 74.Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–8. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–19. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 76.Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–50. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 77.Kiyosue A, Honarpour N, Kurtz C, et al. A phase 3 study of evolocumab (AMG 145) in statin-treated Japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117:40–7. doi: 10.1016/j.amjcard.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 78.Langslet G, Emery M, Wasserman SM. Evolocumab (AMG 145) for primary hypercholesterolemia. Expert Rev Cardiovasc Ther. 2015;13:477–88. doi: 10.1586/14779072.2015.1030395. [DOI] [PubMed] [Google Scholar]
- 79.Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–18. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 80.McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–53. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- 82.Hopkins PN, Defesche J, Fouchier SW, et al. Characterization of autosomal dominant hypercholesterolemia caused by PCSK9 gain of function mutations and its specific treatment with alirocumab, a PCSK9 monoclonal antibody. Circ Cardiovasc Genet. 2015;8:823–31. doi: 10.1161/CIRCGENETICS.115.001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.KÜhnast S, van der Hoorn JW, Pieterman EJ, et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res. 2014;55:2103–12. doi: 10.1194/jlr.M051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lunven C, Paehler T, Poitiers F, et al. A randomized study of the relative pharmacokinetics, pharmacodynamics, and safety of alirocumab, a fully human monoclonal antibody to PCSK9, after single subcutaneous administration at three different injection sites in healthy subjects. Cardiovasc Ther. 2014;32:297–301. doi: 10.1111/1755-5922.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 86.Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized phase 3 trial. Int J Cardiol. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 87.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 88.Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906–15. doi: 10.1016/j.ahj.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. doi: 10.1093/eurheartj/ehv370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ballantyne CM, Neutel J, Cropp A, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–21. doi: 10.1016/j.amjcard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 91.Gencer B, Lambert G, Mach F. PCSK9 inhibitors. Swiss Med Wkly. 2015;145:w14094. doi: 10.4414/smw.2015.14094. [DOI] [PubMed] [Google Scholar]
- 92.Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:40–51. doi: 10.7326/M14-2957. [DOI] [PubMed] [Google Scholar]
- 93.Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J. 2016;37:536–45. doi: 10.1093/eurheartj/ehv563. [DOI] [PubMed] [Google Scholar]
- 94.Li C, Lin L, Zhang W, et al. Efficiency and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibody on hypercholesterolemia: a meta-analysis of 20 randomized controlled trials. J Am Heart Assoc. 2015;4:e001937. doi: 10.1161/JAHA.115.001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. doi: 10.1186/s12916-015-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cybulska B, Pająk A, Ponikowski P, et al. Severe hypercholesterolaemia – when to use the proprotein convertase subtilisin-kexin type 9 protease inhibitors (PCSK9 inhibitors)? Polish Society of Cardiology experts’ group statement. Kardiol Pol. 2016;74:394–8. doi: 10.5603/KP.2016.0051. [DOI] [PubMed] [Google Scholar]
- 97.Awan Z, Baass A, Genest J. Proprotein convertase subtilisin/kexin type 9 (PCSK9): lessons learned from patients with hypercholesterolemia. Clin Chem. 2014;60:1380–9. doi: 10.1373/clinchem.2014.225946. [DOI] [PubMed] [Google Scholar]
- 98.Campion SN, Han B, Cappon GD, et al. Decreased maternal and fetal cholesterol following maternal bococizumab (anti-PCSK9 monoclonal antibody) administration does not affect rat embryo-fetal development. Regul Toxicol Pharmacol. 2015;73:562–70. doi: 10.1016/j.yrtph.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Swiger KJ, Martin SS. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf. 2015;38:519–26. doi: 10.1007/s40264-015-0296-6. [DOI] [PubMed] [Google Scholar]
- 100.Santos RD. Review: PCSK9 inhibitors reduce mortality but increase neurocognitive events in hypercholesterolemia. Ann Intern Med. 2016;164:JC31. doi: 10.7326/ACPJC-2016-164-6-031. [DOI] [PubMed] [Google Scholar]
- 101.Blom DJ, Djedjos CS, Monsalvo ML, et al. Effects of evolocumab on vitamin E and steroid hormone levels: results from the 52-week, phase 3, double-blind, randomized, placebo-controlled DESCARTES study. Circ Res. 2015;117:731–41. doi: 10.1161/CIRCRESAHA.115.307071. [DOI] [PubMed] [Google Scholar]
- 102.Roth EM, Bujas-Bobanovic M, Louie MJ, Cariou B. Patient and physician perspectives on mode of administration of the PCSK9 monoclonal antibody alirocumab, an injectable medication to lower LDL-C levels. Clin Ther. 2015;37:1945–54. doi: 10.1016/j.clinthera.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Reiner Ž. PCSK9 inhibitors – past, present and future. Expert Opin Drug Metab Toxicol. 2015;11:1517–21. doi: 10.1517/17425255.2015.1075506. [DOI] [PubMed] [Google Scholar]
- 104.Gidding SS, Ann Champagne M, de Ferranti SD, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–92. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 105.Zhao HP, Xiang BR. Discontinued cardiovascular drugs in 2013 and 2014. Expert OpinInvestig Drugs. 2015;24:1083–92. doi: 10.1517/13543784.2015.1051619. [DOI] [PubMed] [Google Scholar]
- 106.Dragan S, Serban MC, Banach M. Proprotein convertase subtilisin/kexin 9 inhibitors: an emerging lipid-lowering therapy? J Cardiovasc Pharmacol Ther. 2015;20:157–68. doi: 10.1177/1074248414539562. [DOI] [PubMed] [Google Scholar]
- 107.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galabova G, Brunner S, Winsauer G, et al. Peptide-based anti-PCSK9 vaccines – an approach for long-term LDL-C management. PLoS One. 2014;9:e114469. doi: 10.1371/journal.pone.0114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crossey E, Amar MJ, Sampson M, et al. A cholesterol-lowering VLP vaccine that targets PCSK9. Vaccine. 2015;33:5747–55. doi: 10.1016/j.vaccine.2015.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ling H, Burns TL, Hilleman DE. An update on the clinical development of proprotein convertase subtilisinkexin 9 inhibitors, novel therapeutic agents for lowering low-density lipoprotein cholesterol. Cardiovasc Ther. 2014;32:82–8. doi: 10.1111/1755-5922.12056. [DOI] [PubMed] [Google Scholar]
- 111.Norata GD, Ballantyne CM, Catapano AL. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur Heart J. 2013;34:1783–9. doi: 10.1093/eurheartj/eht088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahebkar A, Watts GF. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc Drugs Ther. 2013;27:559–67. doi: 10.1007/s10557-013-6479-4. [DOI] [PubMed] [Google Scholar]