Abstract
High-dose interferon alfa-2b (IFN-α-2b) improves the survival of patients with high-risk melanoma. We aimed to identify baseline peripheral blood biomarkers to predict the outcome of acral melanoma patients treated with IFN-α-2b. Pretreatment baseline parameters and clinical data were assessed in 226 patients with acral melanoma. Relapse-free survival (RFS) and overall survival (OS) were assessed using the Kaplan-Meier method, and multivariate Cox regression analyses were applied after adjusting for stage, lactate dehydrogenase (LDH), and ulceration. Univariate analysis showed that neutrophil-to-lymphocyte ratio ≥2.35, platelet-to-lymphocyte ratio ≥129, systemic immune-inflammation index (SII) ≥615 × 109/l, and elevated LDH were significantly associated with poor RFS and OS. The SII is calculated as follows: platelet count × neutrophil count/lymphocyte count. On multivariate analysis, the SII was associated with RFS [hazard ratio (HR)=1.661, 95% confidence interval (CI): 1.066-2.586, P=.025] and OS (HR=2.071, 95% CI: 1.204-3.564, P=.009). Additionally, we developed a novel circulating T-cell immune index (CTII) calculated as follows: cytotoxic T lymphocytes/(CD4+ regulatory T cells × CD8+ regulatory T cells). On univariate analysis, the CTII was associated with OS (HR=1.73, 95% CI: 1.01-2.94, P=.044). The SII and CTII might serve as prognostic indicators in acral melanoma patients treated with IFN-α-2b. The indexes are easily obtainable via routine tests in clinical practice.
Introduction
Malignant melanoma is a highly aggressive skin cancer, and the global incident rate is increasing by 3% to 5% annually [1]. Patients with thick primary lesions, ulcerated lesions, or regional metastases have a high risk of relapse [1]. In particular, patients with stage IIB to IIIC have the highest recurrence risk, with postsurgical relapse rates of 40% to 55% and 40% to 80%, respectively [2]. The clinical characteristics and prognosis of Asian patients show significant variations from those of Caucasian patients [3], [4]. Acral melanoma is rarely observed in Caucasians but is the most commonly diagnosed pathological subtype in Asian, accounting for 47.5% to 65% of melanoma cases [5], [6]. Furthermore, non-Caucasian melanoma patients exhibit worse prognosis than Caucasian melanoma patients, which is still lack of effective adjuvant treatment strategy [7], [8]. Presently, interferon alfa-2b (IFN-α-2b) is the only drug approved by the US Food and Drug Administration for the adjuvant treatment of high-risk postoperative melanoma. A meta-analysis of 14 randomized controlled trials concluded that IFN-α-2b was significantly associated with improved disease-free survival and overall survival (OS) [9]. Moreover, 1-year administration of IFN-α-2b was clinically beneficial in Asian patients with stage IIIB to IIIC acral melanoma or with ≥3 nodal metastases, which is quite different from Caucasian population [10], [11]. However, there remain some controversies to using adjuvant interferon therapy, such as significant toxicities and financial burdens. Therefore, it is crucial to investigate prognostic biomarkers that can identify patients who are more likely to benefit from adjuvant interferon therapy.
It is clear that systemic inflammatory responses are a vital determinant of disease progression and survival in most cancers [12]. Infiltrating inflammatory cells in the immune system are increasingly recognized to be generic constituents of tumors that have opposing functions, as both tumor antagonists and promoters [13], [14]. Therefore, several immune-based prognostic scores, such as neutrophil count, lymphocyte count, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), prognostic nutritional index (PNI), and circulating CD4+T- and CD8+T-cell counts have been developed to predict the prognosis in several cancers, including melanoma [15], [16], [17], [18], [19], [20]. However, such parameters have never been utilized to predict outcome in acral melanoma patients treated with adjuvant interferon therapy. Moreover, the potential effects of peripheral lymphocytes, neutrophils, platelets, CD4+ regulatory T cells (CD4+Tregs), CD8+ regulatory T cells (CD8+Tregs), and cytotoxic T lymphocytes (CTLs) on melanoma recurrence and metastasis have not been explored.
In this study, we developed a novel index, the circulating T-cell immune index (CTII) that is based on CD4+CD25+regulatory T cells (CD4+Tregs), CD8+CD28− regulatory T cells (CD8+Tregs), and CD8+CD28+cytotoxic T lymphocytes (CTLs). We found that the SII and CTII were promising independent predictive factors of prognosis of the patients with acral melanoma who had undergone adjuvant interferon therapy.
Materials and Methods
Patients
The study was approved by the medical ethics committee of Peking University Cancer Hospital & Institute. Written informed consent was obtained from all participants. We retrospectively reviewed the medical records of 226 patients with high-risk acral melanoma who visited Peking University Cancer Hospital between October, 2010, and October, 2016. All patients diagnosed with melanoma were confirmed histopathologically. All methods were performed in accordance with the relevant guidelines and regulations. To ensure that the whole blood parameters were representative of normal baseline values, none of the patients had lymphatic system disorders or malignant hematologic diseases. Furthermore, all of the patients were treatment-naïve.
Study Design
This was a retrospective, single-center study. Patients were divided into two groups according to IFN-α-2b dose. Cohort A (152 patients) received 4 weeks of intravenous induction therapy of IFN-α-2b (15×106 U/m2/d, 5 days per week); Cohort B (74 patients) received 4 weeks of IFN-α-2b intravenous induction therapy (15×106 U/m2/d, 5 days per week), followed by 48 weeks of subcutaneous maintenance therapy at a dose of 9×106 U, 3 times per week. The dosage was based on that used in a previous clinical trial [11] as well as on our own clinical experience in Chinese melanoma patients [10]. The dosage was lower than the standard high-dose IFN dosage applied in the Eastern Cooperative Oncology Group trial [21], [22] but was more suitable for Chinese patients since they generally cannot tolerate the standard dosage owing to its toxicity.
The baseline parameters, including demographics, routine hematologic tests results, CD4+Tregs, CD8+Tregs, CTLs, liver function parameters, and clinical history, were all obtained. The following parameters were collected for analysis: age, sex, date of melanoma diagnosis and date of death or last follow-up, American Joint Committee on Cancer (AJCC) M stage, serum lactate dehydrogenase (LDH), ulceration, and clinical history. Parameters were collected from data on routine hematologic tests that were performed at the time of initial diagnosis and before the adjuvant high-dose interferon treatments. Six inflammatory factors (NLR, PLR, SII, MLR, PNI, and CTII) were included in this analysis. These inflammatory factors were calculated as follows: NLR = N/L; PLR = P/L; SII = P × N/L; MLR=M/L; PNI = albumin + 5 × L, and CTII= CTLs/(CD4+Tregs × CD8+Tregs), where N, L, M, and P are the peripheral neutrophil, lymphocyte, monocyte, and platelet counts, respectively.
Statistical Analysis
Two end points were analyzed: OS and relapse-free survival (RFS). OS was defined as the date of melanoma diagnosis to the time of death due to any cause or until October, 2016, for patients who remained alive (censored). RFS was calculated from the time of initial treatment until the time of disease relapse or death due to any cause, or until October, 2016, for patients who remained alive (censored).
Statistical evaluation was conducted with IBM SPSS statistical software (version 20.0). The t test was used to analyze mean values for normally distributed continuous variables, while the Mann-Whitney U test was used to compare mean values for abnormally distributed continuous variables. OS and RFS curves were estimated with the Kaplan-Meier method. Prognostic parameters associated with OS and RFS were assessed by both Cox univariate and multivariate analyses. Only possible prognostic factors associated with OS and RFS were subjected to Cox multivariable analysis. The R software was used to determine the cutoff values of the parameters associated with OS and RFS. The results are presented as hazard ratio (HR) with 95% confidence interval (CI). Receiver operating characteristic (ROC) curve analysis was used to evaluate predictive values of potential parameters for acral melanoma prognosis. For all statistical tests, P<.05 (two-tailed test) was considered statistically significant.
Results
Patient Characteristics
A total of 226 patients with acral melanoma were enrolled in this study; 152 patients received the 4-week regimen, and 74 patients received the 1-year regimen. The median RFS and OS rates were 22.3 and 47.2 months, respectively. Patient characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of Acral Melanoma Patients
| Variable | RFS (Months) | 95% CI | P Value | OS (Months) | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Total | 22.3 | (16.3-28.3) | 47.2 | (34-60.4) | ||
| Treatment | ||||||
| 4-week IFN-α-2b | 16.5 | (5.2-27.9) | .161 | 47.2 | (31.1-63.3) | .731 |
| 1-year IFN-α-2b | 25.4 | (16.3-34.6) | 55 | (35.6-74.4) | ||
| Gender | ||||||
| Male | 22.9 | (10.1-35.8) | .569 | 51 | (30.6-71.5) | .823 |
| Female | 20.8 | (15.6-26.0) | 44.8 | (23.2-66.3) | ||
| Age | ||||||
| <50 | 21.4 | (9.4-23.6) | .642 | 54.1 | (34.8-70.4) | .575 |
| ≥50 | 19 | (16.4-29.5) | 42.2 | (28.7-55.7) | ||
| AJCC M stage | ||||||
| II | 25.8 | (11.4-40.1) | <.001 | 62 | (43.9-80.0) | <.001 |
| III | 11.9 | (6.6-17.2) | 28 | (18.4-37.8) | ||
| Serum LDH | ||||||
| <ULN | 22.6 | (16.9-28.5) | <.001 | 54.1 | (37.9-70.3) | .024 |
| ≥ULN | 2.5 | (2.4-2.6) | 26.8 | (20.4-33.1) | ||
| Ulceration | ||||||
| Without ulceration | 22.3 | (16.4-28.2) | .042 | 51 | (37.2-64.9) | .037 |
| With ulceration | 4.3 | (2-17.1) | 28 | (3.7-34.7) | ||
| Lymphocyte cells count | ||||||
| <1.8×1010 | 22.3 | (16.5-28.1) | .918 | 55 | (36.7-73.2) | .772 |
| ≥1.8×1010 | 16.8 | (10.7-21.3) | 42.2 | (28.2-55.7) | ||
| Neutrophil cells count | ||||||
| <4×109 | 25 | (17.9-32.1) | .213 | 51 | (37.9-64.4) | .872 |
| ≥4×109 | 18.4 | (11.3-25.5) | 42.2 | (26.4-57.9) | ||
| NLR | ||||||
| <2.35 | 30.2 | (19.4-41.1) | .05 | 55 | (40.8-69.2) | .047 |
| ≥2.35 | 15 | (9.8-20.1) | 39.4 | (31.8-47) | ||
| PLR | ||||||
| <129 | 27.4 | (19.4-35.3) | .002 | 62 | (39.6-84.4) | .01 |
| ≥129 | 12 | (8-16) | 40.9 | (30.2-51.5) | ||
| SII | ||||||
| <615×109 | 30.2 | (20.3-40.2) | .029 | 62 | (41.1-82.9) | .006 |
| ≥615×109 | 14.8 | (11.1-18.4) | 34 | (24.5-43.7) | ||
| MLR | ||||||
| <0.26 | 22.6 | (13.7-31.7) | .365 | 54.1 | (33.6-74.6) | .461 |
| ≥0.26 | 22.3 | (13.9-30.7) | 47.2 | (31.3-63) | ||
| PNI | ||||||
| <54 | 18 | (9-27) | .649 | 51 | (36.8-65.2) | .759 |
| ≥54 | 23 | (17.1-28.8) | 47.2 | (25.5-68.9) | ||
| CD4+Tregs | ||||||
| <6.5 | 22.9 | (16.3-29.7) | .917 | 40 | (33-47) | .263 |
| ≥6.5 | 18.4 | (8.6-28.3) | 55.2 | (38.8-41.6) | ||
| CD8+Tregs | ||||||
| <21 | 17.9 | (8.4-27.5) | .182 | 40 | (35.5-44.5) | .221 |
| ≥21 | 25.4 | (16.7-34.2) | 55.2 | (31.8-78.7) | ||
| CTLs | ||||||
| <11 | 17.9 | (7.3-28.7) | .33 | 42.2 | (28.6-55.8) | .414 |
| ≥11 | 22.9 | (16.9-28.9) | 54.1 | (33.9-74.4) | ||
| CTII | ||||||
| <0.08 | 22.3 | (17.1-27.5) | .848 | 68.4 | (50-96.9) | .044 |
| ≥0.08 | 22.6 | (10.1-35.2) | 40 | (37.1-42.9) |
ULN, upper limit of normal.
There was no significant difference in OS and RFS rates between treatment arms. Therefore, all patients were subjected to prognostic factor analysis, regardless of their treatment arm.
Association of NLR, PLR, SII, MLR, PNI, and CTII with RFS and OS
We used the R software to determine the cutoff values of lymphocyte cells count, neutrophil cells count, NLR, PLR, SII, MLR, PNI, and CTII for the prediction of RFS and OS based on the data of the 226 melanoma patients. We transformed the continuous data to dichotomous data by employing cutoff values. On univariate Cox analyses, the NLR, PLR, SII, LDH, ulceration, and AJCC M stage were significantly associated with the RFS and OS of patients with acral melanoma (Figures 1 and 2). The CTII was only associated with the OS of patients with acral melanoma (P=.044). The results of the univariate analyses are shown in Table 2.
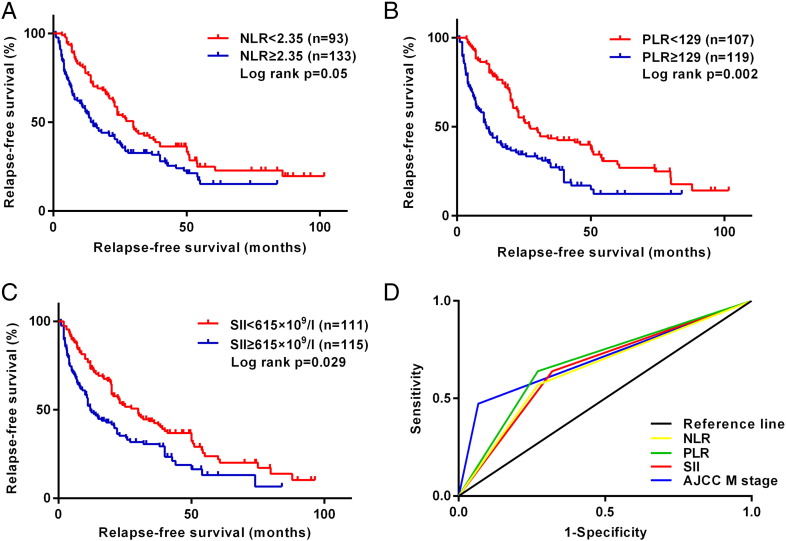
Figure 1.
Kaplan-Meier survival curves for RFS according to inflammation-based scores in 226 patients with acral melanoma. (A) Ninety-three patients with NLR ≥2.35 had shorter median RFS than 133 patients with NLR <2.35 (15 vs 30.2 months, P=.005). (B) One hundred seven patients with PLR ≥129 had shorter median RFS than 119 patients with PLR <129 (12 vs 27.4 months, P=.002). (C) One hundred eleven patients with SII ≥615×109/l had shorter median RFS than 115 patients with SII <615×109/l (14.8 vs 30.2 months, P=.029). (D) ROC curves of NLR, PLR, SII, and AJCC M stage for RFS, with a median survival time of 22.3 months.
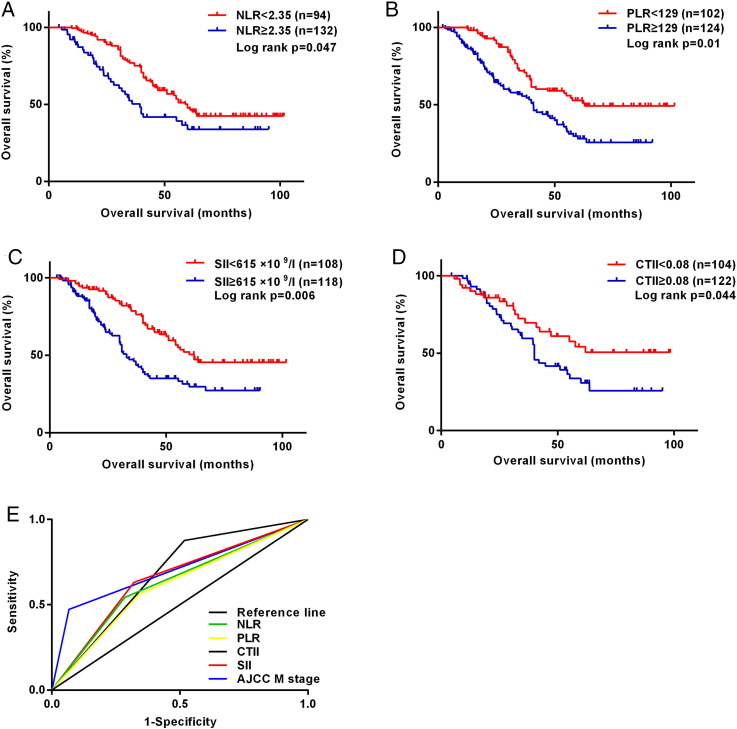
Figure 2.
Kaplan-Meier survival curves for OS according to inflammation-based scores in 226 patients with acral melanoma. (A) Ninety-four patients with NLR ≥2.35 had shorter median OS than 132 patients with NLR <2.35 (39.4 vs 55 months, P=.047). (B) One hundred two patients with PLR ≥129 had shorter median OS than 124 patients with PLR <129 (40.9 vs 62 months, P=.01). (C) One hundred eight patients with SII ≥615×109/l had shorter median OS than 118 patients with SII <615×109/l (34 vs 62 months, P=.006). (D) One hundred four patients with CTII ≥0.08 had shorter median OS than 122 patients with CTII <0.08 (40 vs 68.4 months, P=.044). (E) ROC curves of NLR, PLR, SII, CTII, and AJCC M stage for OS, with a median survival time of 47.2 months.
Table 2.
Association between Blood Routine Tests Parameters and RFS and OS of Acral Melanoma Patients in Univariate Cox Regression Analyses
| RFS |
OS |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| NLR, per increase of 1 unit | 1.83 (0.81-4.13) | .05 | 1.81 (0.69-4.76) | .047 |
| PLR, per increase of 1 unit | 1.18 (0.64-2.17) | .002 | 0.97 (0.46-2.02) | .01 |
| SII, per increase of 1 unit | 2.3 (1.02-5.21) | .029 | 3.7 (1.38-9.88) | .006 |
| AJCC M stage, stage II vs stage III | 2.59 (1.5-4.47) | <.001 | 3.78 (1.95-7.32) | <.001 |
| Serum LDH, <ULN vs ≥ULN | 2.33 (0.52-3.37) | <.001 | 2.96 (0.33-3.79) | .024 |
| Ulceration, without ulceration vs with ulceration | 1.04 (0.35-2.8) | .022 | 1.37 (0.03-3.21) | .017 |
| CTII, per increase of 1 unit | 1.41 (0.74-3.67) | .848 | 1.73 (1.01-2.94) | .044 |
Factors found significant on univariate analysis were subjected to multivariate Cox proportional hazards analysis. As shown in Table 3, only SII was significantly associated with RFS (HR=1.661, 95% CI=1.066-2.586, P=.025) and OS (HR=2.071, 95% CI=1.204-3.564, P=.009). Moreover, a higher AJCC M stage was a strong prognostic factor of RFS (HR=2.848, 95% CI=1.772-4.576, P<.001) and OS (HR=3.699, 95% CI=2.128-6.431, P<.001) in patients with acral melanoma.
Table 3.
Association between Blood Routine Tests Data and RFS and OS of Acral Melanoma Patients in Multivariate Cox Regression Analyses
| Variable | Category | RFS |
OS |
||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| SII | <615 vs ≥615 | 1.661 (1.066-2.586) | .025 | 2.071(1.204-3.564) | .009 |
| AJCC M stage | stage II vs stage III | 2.848 (1.772-4.576) | <.001 | 3.699(2.128-6.431) | <.001 |
Prognostic Influences of NLR, PLR, SII, and CTII on RFS and OS
We performed ROC analysis to evaluate the accuracy of SII in predicting RFS and OS in patients with acral melanoma. We found that elevated NLR, PLR, and SII predict RFS (area under the curve=0.565, 0.7, and 0.66, respectively; all P<.05). Moreover, elevated NLR, PLR, SII, and CTII values were associated with poor OS (area under the curve=0.629, 0.611, 0.655, and 0.68, respectively; all P<.05). We also performed Spearman’s chi-square analysis to test the prognostic values of NLR, PLR, SII, and CTII for RFS and OS in patients with acral melanoma; the data are shown in Tables 4 and 5.
Table 4.
Predictive Value of NLR, PLR, and SII for RFS of Acral Melanoma Patients
| Indexes | Cutoff | AUC (95%CI) | Sensitivity | Specificity | Accuracy | P |
|---|---|---|---|---|---|---|
| NLR | 2.35 | 0.565 (0.55-0.76) | 0.57 | 0.74 | 65.5 | .005 |
| PLR | 129 | 0.7 (0.59-0.79) | 0.64 | 0.74 | 69 | <.001 |
| SII | 615 | 0.66 (0.56-0.76) | 0.64 | 0.68 | 66.4 | <.001 |
AUC, area under curve.
Table 5.
Predictive value of NLR, PLR, SII and CTII for OS of acral melanoma patients
| Indexes | Cut-off | AUC(95%CI) | Sensitivity | Specificity | Accuracy | P |
|---|---|---|---|---|---|---|
| NLR | 2.35 | 0.629(0.526-0.732) | 0.544 | 0.714 | 62.8 | 0.018 |
| PLR | 129 | 0.611(0.507-0.715) | 0.561 | 0.661 | 61.1 | 0.042 |
| SII | 615 | 0.655(0.553-0.757) | 0.632 | 0.679 | 65.5 | 0.004 |
| CTII | 0.08 | 0.68(0.58-0.78) | 0.877 | 0.482 | 63.7 | <0.001 |
NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio; SII: systemic immune-inflammation index; AUC: area under curve; CI: confidence interval; CTII: circulating T cell immune index.
Comparison of SII and CTII in Different Acral Melanoma Subgroups
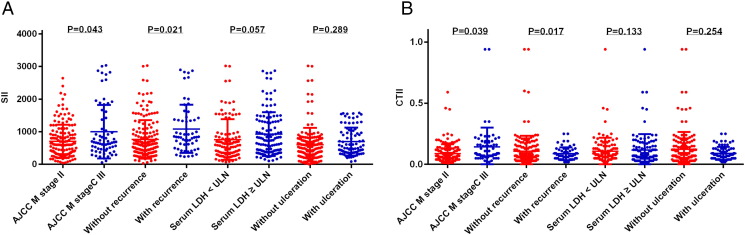
As AJCC M stage and tumor recurrence were significantly associated with prognosis in patients with acral melanoma, we compared SII and CTII in different patient subgroups that were created based on the clinicopathological features (Figure 3). We found that the SII and CTII in stage III patients as well as those who experienced recurrence were higher than stage II patients and those without recurrence (all P<.05). This indicated that SII and CTII may predict melanoma invasiveness and metastatic potential.
Figure 3.
Comparisons of SII (A) and CTII (B) in different subgroups of acral melanoma patients, including AJCC M stage, recurrence, serum LDH, and ulceration.
Discussion
We investigated potential prognostic biomarkers of IFN-α-2b therapy in Asian patients with acral melanoma to evaluate the clinical benefit of the therapy on OS and RFS. Several clinical trials have indicated that the median RFS ranged from 20.4 to 30 months for high-risk melanoma [22], [23]. Congruent with these studies, in the present study, the median RFS in acral melanoma patients treated with high-dose interferon was similar to the lower limit of the RFS range in Caucasian population [10], which partly confirms that acral melanoma subtype is associated with significantly inferior prognosis, as previously suggested [24]. Such prognostic differences might arise because of the variations in the genetics, pathogenesis, and immune microenvironment between different ethnic populations [15], [25], [26], [27], [28], [29].
Several studies have shown that pretreatment NLR, neutrophil counts, and lymphocyte counts in patients with melanoma are valid prognosticators [15], [16]. SII, which is based on lymphocyte, neutrophil, and platelet counts, has not been investigated extensively in melanoma patients; we are the first to verify its role in predicting RFS and OS in such patients. The SII prediction value was shown to be higher than that of the NLR, PLR, and other conventional parameters such as serum LDH and ulceration. Moreover, the SII value is based on measures that are easily obtained during routine laboratory tests in clinical practice. Therefore, the SII ought to be a simple, low-cost, and effective biomarker that may assist in the surveillance of patients most likely to relapse or to benefit from adjuvant interferon therapy. This might also contribute to early and accurate decision-making concerning the most effective treatment strategy.
Recent evidence indicates that infiltrating immune system cells present in the tumor microenvironment synergistically promote tumor progression. Tumor-promoting immune cells include macrophages, platelets, neutrophils, and T and B lymphocytes, which produce an attractive tumor microenvironment for tumor growth, metastasis, and facilitate angiogenesis [13], [30], [31], [32], [33]. Furthermore, some studies showed that immune cells facilitate tumor progression by releasing a series of molecules, such as the proangiogenic vascular endothelial growth factor, the proinvasive matrix degrading enzyme matrix metalloproteinase-9, and other cytokines [32], [34]. Meanwhile, activated T cells and other lymphocytes demonstrate potent antitumor effects [35]. The balance between these opposing immune inflammatory responses in tumors is likely to be crucial for accurate prognosis as well as for determining appropriate antitumor treatments [12]. A better understanding of the role of infiltrating immune system cells ought to help clarify the association between cancer, immunity, and inflammation [17].
In our study, Cox univariate and multivariate analyses indicated that the SII was significantly associated with the outcome of melanoma. The CTII was also shown to be a predictive factor for OS. Additionally, we found that elevated SII and CTII values were associated with tumor vascular invasion and recurrence, indicating a more aggressive phenotype [36], [37]. A recent study indicated that increased absolute lymphocyte counts concordant with delayed increases in CD4+ and CD8+ T cells are associated with positive outcome in advanced melanoma patients treated with ipilimumab [18]. Patients with metastatic melanoma and a high baseline NLR also appeared to benefit from immunotherapy with agents such as ipilimumab [16]. Therefore, the appropriate predictive biomarkers may help select the appropriate therapies (or sequences). Such predictive biomarkers may also serve to expedite decisions on whether to continue a particular therapy or switch to alternative options.
The limitations of this research include its retrospective nature and small sample size, which could produce selection biases. Moreover, NLR, PLR, SII, and CTII were not of powerful prognostic values in terms of outcome of melanoma patients. A recent study indicated that the underlying mechanism through which elevated SII is associated with poorer a prognosis is an increase in the dissemination of tumor cells into the circulation, allowing such cells to escape immune surveillance and increase peripheral circulating tumor cell levels [17]. Therefore, we hypothesized that additional biomarkers such as circulating tumor cell levels could be combined with SII and CTII in order to improve the prognostic accuracy. Measuring changes in specific immune-related parameters during therapy can improve the real-time assessment of the drug’s benefit. Martens et al. found that increases in absolute lymphocyte counts observed 2 to 8 weeks after ipilimumab initiation, combined with delayed increases in CD4+ and CD8+ T cell levels, are indicators of positive outcome in metastatic melanoma patients [18]. Thus, further prospective, well-designed studies with larger populations focused on changes in SII and CTII during therapy are warranted.
In conclusion, our study is the first to demonstrate the prognostic significance of the SII and CTII in high-risk acral melanoma patients treated with adjuvant IFN-α-2b. Both SII and CTII are easily assessable in clinical practice. Additional studies are required to clarify the mechanisms behind the association between elevated SII and CTII and poorer prognosis in melanoma patients.
Conflict of Interest
None.
Acknowledgement
This work was supported by grants from National Natural Science Foundation of China (81402264), Beijing Municipal Natural Science Foundation (7152033) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of special funding support (ZYLX201603).
Contributor Information
Yan Kong, Email: k-yan08@163.com.
Jun Guo, Email: guoj307@126.com.
References
- 1.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarhini AA, Kirkwood JM. Clinical and immunologic basis of interferon therapy in melanoma. Ann N Y Acad Sci. 2009;1182:47–57. doi: 10.1111/j.1749-6632.2009.05073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, Ross MI, Du XL. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907–1914. doi: 10.1001/archinte.166.17.1907. [DOI] [PubMed] [Google Scholar]
- 4.Bellows CF, Belafsky P, Fortgang IS, Beech DJ. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10–16. doi: 10.1002/jso.1116. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto T, Shibata S, Yasue S, Sakakibara A, Yokota K, Sawada M, Kono M, Kato K, Shimoyama Y, Tomita Y. Interval sentinel lymph nodes in patients with cutaneous melanoma: a single-institution study in Japan. J Dermatol. 2010;37:629–634. doi: 10.1111/j.1346-8138.2010.00856.x. [DOI] [PubMed] [Google Scholar]
- 6.Roh MR, Kim J, Chung KY. Treatment and outcomes of melanoma in acral location in Korean patients. Yonsei Med J. 2010;51:562–568. doi: 10.3349/ymj.2010.51.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rex J, Paradelo C, Mangas C, Hilari JM, Fernandez-Figueras MT, Ferrandiz C. Management of primary cutaneous melanoma of the hands and feet: a clinicoprognostic study. Dermatol Surg. 2009;35:1505–1513. doi: 10.1111/j.1524-4725.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 8.Chi Z, Li S, Sheng X, Si L, Cui C, Han M, Guo J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer. 2011;11:85. doi: 10.1186/1471-2407-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 10.Mao L, Si L, Chi Z, Cui C, Sheng X, Li S, Tang B, Guo J. A randomised phase II trial of 1 month versus 1 year of adjuvant high-dose interferon alpha-2b in high-risk acral melanoma patients. Eur J Cancer. 2011;47:1498–1503. doi: 10.1016/j.ejca.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Pectasides D, Dafni U, Bafaloukos D, Skarlos D, Polyzos A, Tsoutsos D, Kalofonos H, Fountzilas G, Panagiotou P, Kokkalis G. Randomized phase III study of 1 month versus 1 year of adjuvant high-dose interferon alfa-2b in patients with resected high-risk melanoma. J Clin Oncol. 2009;27:939–944. doi: 10.1200/JCO.2008.16.3121. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt H, Suciu S, Punt CJ, Gore M, Kruit W, Patel P, Lienard D, von der Maase H, Eggermont AM, Keilholz U. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol. 2007;25:1562–1569. doi: 10.1200/JCO.2006.09.0274. [DOI] [PubMed] [Google Scholar]
- 16.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis F, Marchetti P, Amato G. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112:1904–1910. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 18.Martens A, Wistuba-Hamprecht K, Yuan J, Postow MA, Wong P, Capone M, Madonna G, Khammari A, Schilling B, Sucker A. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22:4848–4858. doi: 10.1158/1078-0432.CCR-16-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Lolli C, Basso U, Derosa L, Scarpi E, Sava T, Santoni M, Crabb SJ, Massari F, Aieta M, Conteduca V. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016 doi: 10.18632/oncotarget.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkwood JM, Ibrahim J, Lawson DH, Atkins MB, Agarwala SS, Collins K, Mascari R, Morrissey DM, Chapman PB. High-dose interferon alfa-2b does not diminish antibody response to GM2 vaccination in patients with resected melanoma: results of the Multicenter Eastern Cooperative Oncology Group Phase II Trial E2696. J Clin Oncol. 2001;19:1430–1436. doi: 10.1200/JCO.2001.19.5.1430. [DOI] [PubMed] [Google Scholar]
- 22.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, Smith TJ, Rao U, Steele M, Blum RH. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 24.Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427–434. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93:273–278. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 29.Furney SJ, Turajlic S, Stamp G, Thomas JM, Hayes A, Strauss D, Gavrielides M, Xing W, Gore M, Larkin J. The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res. 2014;27:835–838. doi: 10.1111/pcmr.12279. [DOI] [PubMed] [Google Scholar]
- 30.Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010;176:1564–1576. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 33.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 36.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]