Abstract
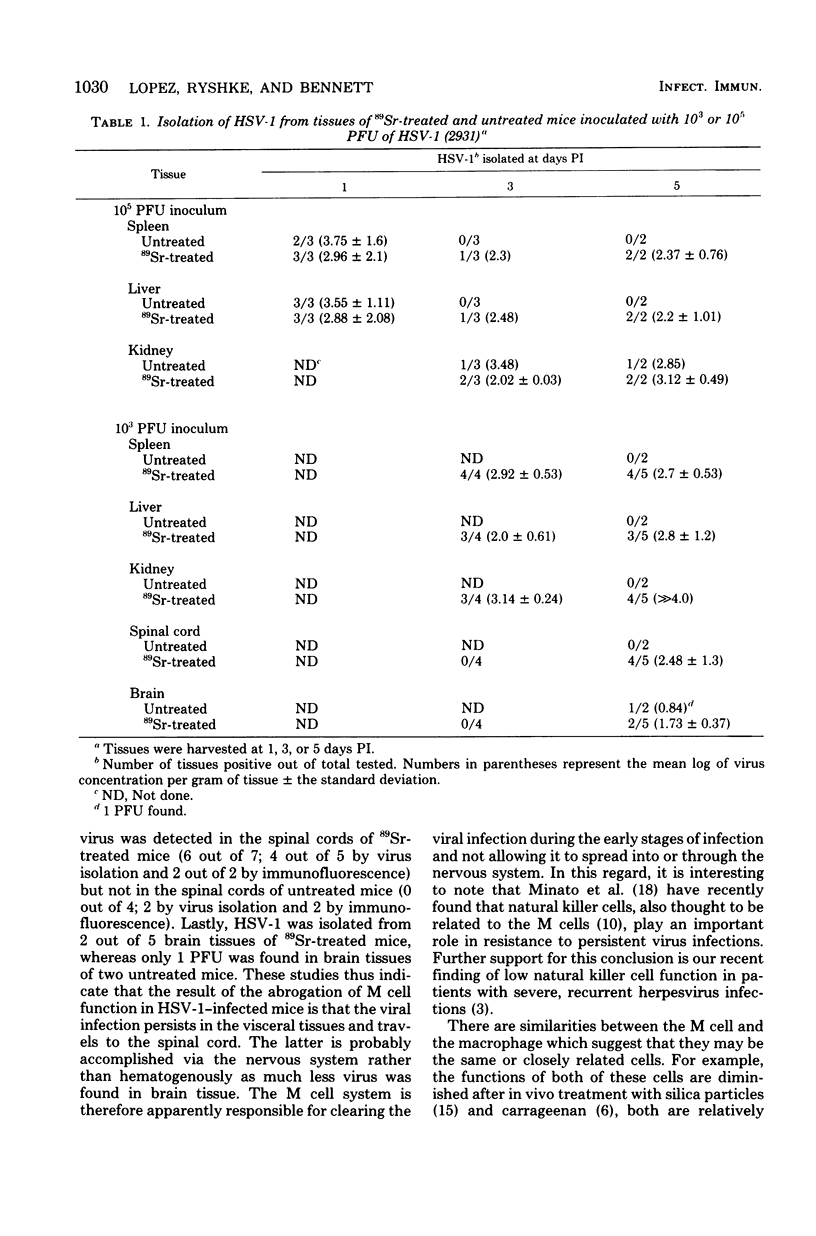
Adult mice resistant to infection with 10(6) plaque-forming units of a virulent strain of herpes simplex virus type 1 were treated with 89Sr to abrogate marrow-dependent cell functions. Treated mice were found to be much more susceptible to the herpes simplex virus type 1 infection than untreated mice. The virus persisted in the visceral tissues of 89Sr-treated mice for 3 or more days postinfection but not in those of untreated mice. The virus also spread to the spinal cords of treated but not untreated mice. A marrow-dependent cell appeared to mediate resistance to herpes simplex virus type 1 by controlling the infection early after inoculation and not allowing the infection spread to the central nervous system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M., Baker E. E., Eastcott J. W., Kumar V., Yonkosky D. Selective elimination of marrow precursors with the bone-seeking isotope 89Sr: implications for hemopoiesis, lymphopoiesis, viral leukemogenesis and infection. J Reticuloendothel Soc. 1976 Jul;20(1):71–87. [PubMed] [Google Scholar]
- Bennett M. Prevention of marrow allograft rejection with radioactive strontium: evidence for marrow-dependent effector cells. J Immunol. 1973 Feb;110(2):510–516. [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Bennett M. Peculiar immunobiology of bone marrow allografts. I. Graft rejection by irradiated responder mice. J Exp Med. 1971 Jul 1;134(1):83–102. doi: 10.1084/jem.134.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G. Genetic control of resistance to allogeneic and xenogeneic bone-marrow grafts in mice. Transplant Proc. 1975 Jun;7(2):155–159. [PubMed] [Google Scholar]
- Cudkowicz G., Yung Y. P. Abrogation of resistance to foreign bone marrow grafts by carrageenans. I. Studies with the anti-macrophage agent seakem carrageenan. J Immunol. 1977 Aug;119(2):483–487. [PubMed] [Google Scholar]
- Haller O., Wigzell H. Suppression of natural killer cell activity with radioactive strontium: effector cells are marrow dependent. J Immunol. 1977 Apr;118(4):1503–1506. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Hochman P. S., Haller O., Shearer G. M., Wigzell H., Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977 Sep;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Lopez C., Dudas G. Replication of herpes simplex virus type 1 in macrophages from resistant and susceptible mice. Infect Immun. 1979 Feb;23(2):432–437. doi: 10.1128/iai.23.2.432-437.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- Lopez C. Immunological nature of genetic resistance of mice to herpes simplex virus type 1 infection. IARC Sci Publ. 1978;(24 Pt 2):775–781. [PubMed] [Google Scholar]
- Lotzová E., Cudkowicz G. Abrogation of resistance to bone marrow grafts by silica particles. Prevention of the silica effect by the macrophage stabilizer poly-2-vinylpyridine N-oxide. J Immunol. 1974 Sep;113(3):798–803. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merluzzi V. J., Levy E. M., Kumar V., Bennett M., Cooperband S. R. In vitro activation of suppressor cells from spleens of mice treated with radioactive strontium. J Immunol. 1978 Aug;121(2):505–512. [PubMed] [Google Scholar]
- Minato N., Bloom B. R., Jones C., Holland J., Reid L. M. Mechanism of rejection of virus persistently infected tumor cells by athymic nude mice. J Exp Med. 1979 May 1;149(5):1117–1133. doi: 10.1084/jem.149.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Effect of silica on the pathogenic distinction between herpes simplex virus type 1 and 2 hepatitis in mice. Infect Immun. 1977 Aug;17(2):274–277. doi: 10.1128/iai.17.2.274-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Hayashi K., Katz B. J., Notkins A. L. Effect of immunization on acute and latent infections of vaginouterine tissue with herpes simplex virus types 1 and 2. J Infect Dis. 1977 May;135(5):744–752. doi: 10.1093/infdis/135.5.744. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]