Abstract
During dusk and dawn, the ambient illumination undergoes drastic changes in irradiance (or intensity) and spectrum (or color). While the former is a well-studied factor in synchronizing behavior and physiology to the earth’s 24-h rotation, color sensitivity in the regulation of circadian rhythms has not been systematically studied. Drawing on the concept of color opponency, a well-known property of image-forming vision in many vertebrates (including humans), we consider how the spectral shifts during twilight are encoded by a color-opponent sensory system for non-image-forming (NIF) visual functions, including phase shifting and melatonin suppression. We review electrophysiological evidence for color sensitivity in the pineal/parietal organs of fish, amphibians and reptiles, color coding in neurons in the circadian pacemaker in mice as well as sporadic evidence for color sensitivity in NIF visual functions in birds and mammals. Together, these studies suggest that color opponency may be an important modulator of light-driven physiological and behavioral responses.
Keywords: Circadian rhythms, Sleep-wake cycles, Color opponency, Non-image-forming vision, Color vision, Retina
1. Introduction
Virtually all organisms modulate their behavior and physiology (including rest-activity cycles, feeding, metabolism, immune function, hormone secretion, and cognition) according to time of day, as defined by the Earth’s axial rotation. Critical components of this modulation are the sensory input pathways that entrain internal biological clocks to external time. The German word zeitgeber (‘time giver’) is used to refer to diurnal cues in the environment that are detected by these pathways and thus act to synchronize endogenous circadian rhythms to external time. Circadian rhythms persist in the absence of zeitgebers, but run at their own intrinsic period that is typically either slightly shorter or longer than 24 h (Wever, 1979). While temperature (e.g. Pittendrigh, 1960; Sweeney and Hastings, 1960), food availability (e.g. Brinkhof et al., 1998), and social interactions (e.g. Ehlers et al., 1988) can reset the circadian clock, light remains the best-studied and, under most circumstances, the most influential zeitgeber.
Studies of circadian photosensitivity have largely investigated the role of changes in light intensity, which is the more prominent diurnal characteristic of environmental illumination. Recent findings suggest that this is only one part of the picture: In mice, changes in the color of the ambient light can contribute to entraining circadian rhythms (Walmsley et al., 2015). This is accomplished using color opponency in which the signals from two different photoreceptor classes with different spectral tuning are subtracted, thereby encoding changes in the relative wavelength content of the light that occur during dusk and dawn.
While systematic comparative surveys of color sensitivity for image-forming-vision (e.g. Menzel (1979) for invertebrates, and Jacobs (1993) for mammals) show that color vision is extremely widespread, few investigators have examined color sensitivity for circadian regulation. That color opponency can be used to provide a marker of twilight for circadian entrainment has been suggested previously (Donley, 1975; Ekström and Meissl, 2010; Fleissner and Fleissner, 2002; Korf et al., 1981; Morita et al., 1987b; Roenneberg and Foster, 1997; Solessio and Engbretson, 1993). As discussed below, color opponency appears to be a pervasive feature of the sensory systems involved in non-image-forming (NIF) visual functions in many organisms, indicating that it may be a key adaptation to the signals available from the photic environment predictive of important diurnal events such as the timing of twilight.
Here, we synthesize disparate sources of evidence indicating that systems for measuring color may be a conserved component of the photic input pathways controlling circadian clocks across the animal kingdom. As a departure point, we will describe the changes in the intensity and color of natural illumination taking place throughout the day (Section 2). We will then consider how these changes could be encoded, and describe in detail the properties of a color opponent system (Section 3). Next, we will turn to organisms employing a color-opponent encode to regulate their diurnal behavior, or having color-opponent circuitry in organs thought to be involved in it, spanning fish, amphibians, reptiles, and birds, and point out that wavelength-specific opponent behavior already exists in unicellular organisms (Section 4). We will then review evidence for color coding in the photic control of the mammalian circadian system and consider in particular sensitivity to color in human circadian rhythms (Section 5). Finally, we consider future directions for studying color sensitivity in NIF visual functions in humans (Section 6).
2. The photic environment: light-dark cycles and spectral dynamics of twilight
As the Earth rotates around its own axis, the ambient light undergoes predictable changes in intensity and spectrum as a function of solar angle. During the day, the ambient light is 1,000,000 to 100,000,000 times brighter than at night. On a clear night, starlight provides an illuminance of about ~0.001 lx, while moonlight is about ~0.2 lx; by comparison, sunlight may reach up to 100,000 lx. The ambient light intensity at a given point during the day depends on the atmospheric conditions and may vary at fine time scales due to cloud cover and atmospheric turbidity. In addition, light availability will also fluctuate substantially as organisms move in and out of shaded areas (Peirson et al., 2009).
The ambient light intensity changes systematically around twilight (Daan and Aschoff, 1975; Kern, 1992): The intensity decreases (at dusk) or increases (at dawn) as a function of solar elevation angle relative to the observer (Fig. 1a, b). After the sun has set below the horizon during twilight (θs < 0°) and no provides direct illumination, the light in the sky results from refraction and scattering of sunlight in the upper atmosphere, providing nonetheless moderate illumination. Twilight can be separated into three distinct phases by solar angle and the prevailing visibility conditions due to the illumination level (Fig. 1a, b): Civil twilight (−6° < θs < 0°), when terrestrial objects can be identified and distinguished, nautical twilight (−12° < θs < −6°), when only the outlines of objects are visible, and astronomical twilight (−18° < θs < −12°), when the illumination is dark enough such that stars and other astronomical objects can be seen in the sky (United States Naval Observatory Astronomical Applications Department, 2005).
Fig. 1.
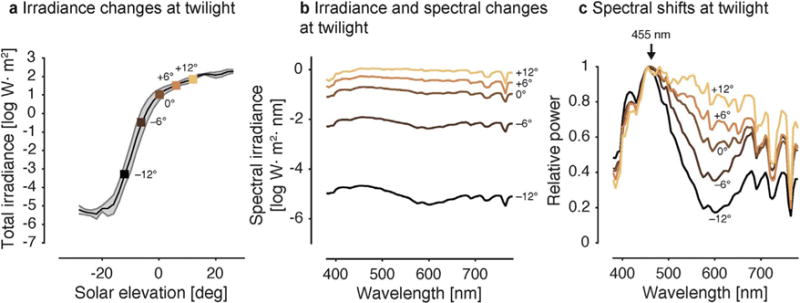
Changes in the photic environment at twilight. a. Changes in irradiance as a function of solar elevation. Key points are emphasized by square markers. b. Changes in irradiance and spectrum at twilight on logarithmic axis. Color coding of single lines follows colors of markers in panel a. c. Changes in spectrum (normalized to 455 nm), showing relative short-wavelength enhancement enhancement during twilight. Data from Spitschan et al. (2016).
During twilight, not only the intensity of the illumination changes, but also the spectral composition and apparent color (Fig. 1c), giving rise to colorful phenomena visible to the human eye at dusk and dawn such as the yellow arches during civil twilight, and the purple-red sky during nautical twilight (Lee, 1994; Lee and Hernandez-Andres, 2003; Lynch and Livingston, 2001). A typical twilight spectrum is blue shifted compared to the daylight spectrum (Le Grand, 1968), with peak power at around 455 nm (Fig. 1c; McFarland and Munz, 1976; Palmer and Johnsen, 2014; Spitschan et al., 2016; Sweeney et al., 2011; Walmsley et al., 2015). These spectral shifts are robust as a function of lunar phase until the sun has set 8° below the horizon (Palmer and Johnsen, 2014).
3. Resetting the circadian clock with color
3.1. Encoding ‘color’
Changes in the spectral composition of the ambient light are predictive of the onset of dawn and dusk. However, a single photopigment or photoreceptor class is unable to track changes in spectral composition and dissociate them from the changes in intensity which occur concurrently around dawn and dusk. This is a direct consequence of the principle of univariance formulated by Rushton (1972): A single photopigment class cannot distinguish between a change in intensity at a given wavelength and a change in wavelength at a given intensity. In other words, individual photopigments are “color blind”, as the generated output signal could be elicited by many different relative spectra of appropriately adjusted intensity, or in the more trivial case, two monochromatic lights of different wavelengths to which the photopigment is equally sensitive. To detect changes in spectral composition unambiguously, it is therefore necessary to compare the activity of two photopigments against each other. In such a color opponent system, outputs from different photopigments are subtracted from one another, such that stimulation by some narrowband wavelengths yields an excitatory response, while other wavelengths yield an inhibitory response. This enables a color opponent system to encode changes in the spectral composition of a spectrally complex light (such as daylight) (Fig. 2).
Fig. 2.
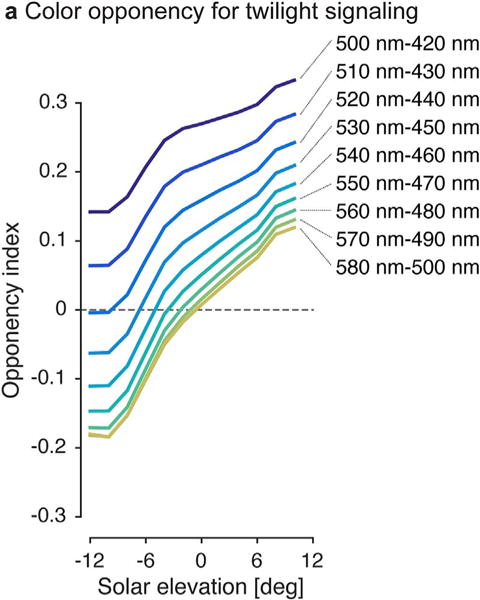
Color opponent coding of twilight. Twilight spectra between −12° and 12° of solar elevation were subjected to a simplified analysis of opponent processing as follows: Pairs of photopigment spectral sensitivities were obtained using the Stockman-Sharpe nomogram (Stockman and Sharpe, 2000) with the peak spectral sensitivities shown in the legend. An “opponency index” (R1 − R2)/(R1 + R2) (Sweeney et al., 2011) was calculated for 531 downwelling irradiance spectra for solar elevations −12° to 12° and averaged within 2° bins. Opponency index of 0 indicates a spectrum activating each pigment of the pair equally. Across all pigment pairs tested there is a clear relationship between opponency index and solar elevation, indicaing that time of day can be encoded by many different pigment pairs. For this simplified demonstration, we ignored the role of different photopigment proportions, bleaching, prereceptoral filtering by the ocular media and the effects of noise. Downwelling irradiance spectra from Spitschan et al. (2016).
One potential advantage of using a color opponent code to track twilight (Fig. 2) is that differencing signals from two photoreceptors eliminates shared noise (Buchsbaum and Gottschalk, 1983): A subtraction operation between two signals corrupted by the same noise (e.g. a variation in overall intensity of the signal) will remove the noise, a procedure termed common-mode noise rejection in engineering contexts. Such shared noise could arise from intensity fluctuations due to cloud cover or ‘behavioral noise’ introduced by moving between shaded and unshaded areas (Peirson et al., 2009). In turn, opponency effectively removes information that is common to both photoreceptors, thus reducing redundancy and maximizing information transmission (Atick et al., 1992; Buchsbaum and Gottschalk, 1983; Lee et al., 2002).
3.2. Color opponent codes in image-forming vision
Color opponent coding is well documented in the visual systems of many different species and in many visual structures associated with image-forming vision, including the retina, the lateral geniculate nucleus (LGN) and visual cortex (see Jacobs (2014) and Lee (2014) for a historical review). It is a characteristic of the visual systems of species of at least six mammalian orders (Jacobs, 2014). Beginning in the 1950s, color opponency was characterized in the fish retina by Svaetichin and co-workers (Daw, 1968; MacNichol and Svaetichin, 1958; Svaetichin, 1956; Svaetichin and Macnichol, 1959; Svaetichin et al., 1965; Wagner et al., 1960) (Fig. 3a), as well as single units in the LGN in primates characterized by De Valois and colleages (De Valois et al., 1966; De Valois et al., 1964; De Valois et al., 1958) and by Hubel and Wiesel (Wiesel and Hubel, 1966); early work also found color opponency in neurons in monkey visual cortex (Motokawa et al., 1962). Color opponency in primates arises as early as in the retinal ganglion cells (De Monasterio and Gouras, 1975; De Monasterio et al., 1975a,b; Gouras, 1968; Hubel and Wiesel, 1960). In many cold-blooded animals including turtles (Twig and Perlman, 2004; Ventura et al., 2001), frogs (Ogden et al., 1985), fish (Govardovskii et al., 1991), the phylogenetically older horizontal cells are color opponent (see Twig et al. (2003) for an extensive review). Horizontal cells in primates are color-selective (i.e. receive cone-class specific input), but not color-opponent (Dacey et al., 1996). Furthermore, bipolar cells in fish (Kaneko and Tachibana, 1983; Shimbo et al., 2000), turtles (Haverkamp et al., 1999) and mice (Breuninger et al., 2011) have been found to be color-opponent.
Fig. 3.
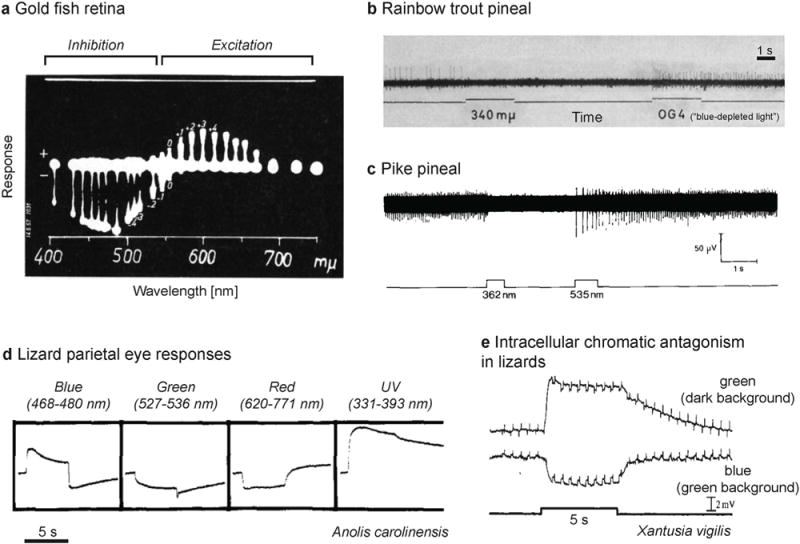
Electrophysiological studies of chromatic opponent neurons in retina and pineal. a. Blue-yellow opponent responses in the goldfish retina measured by MacNichol and Svaetichin (1958) (from their Fig. 7, top panel; p. 35; Copyright © 1958, Elsevier, reprinted with permission). The neutral point occurs at ~550 nm b. Inhibition of spiking activity by ultraviolet light and excitation by “blue-depleted” light in the rainbow trout (Salmo irideus) pineal measured by Morita (1966) (from their Fig. 5; p. 161; Copyright © 1966, Springer, reprinted with permission). c. Inhibition of spiking activity by ultraviolet light and excitation by green light in the pike (Esox Lucius) pineal measured by Falcón and Meissl (1981) (Fig. 5a; p. 132; Copyright © 1981, Springer-Verlag, reprinted with permission). Chromatic antagonism in parietal eyes of lizards. d. Effect of monochromatic light pulses in under adaptation to white light (5 min) studied by Jenison and Nolte (1980) (modified from their Fig. 1, p. 507; Copyright © 1980 Published by Elsevier B.V., reprinted with permission). e. Chromatic antagonism in the same photoreceptor cell. Blue flashes on a green background produce a hyperpolarizing response, while green flashes on a dark background produce a depolarizing response (modified from Solessio and Engbretson (1993); their Fig. 3a, p. 444; Copyright © 1993, Rights Managed by Nature Publishing Group, reprinted with permission). (This is a grayscale figure. All references to colors refer to the light used in the experiments described.)
It is worth noting that while most focus has been on opponency between different cone classes, there is emerging evidence suggesting that there might also be circuitry for opponency between rods and cones. A recent study (Joesch and Meister, 2016) found rod-cone opponency in transgenic mice; there is furthermore behavioral evidence for cone-rod opponent color discrimination under mesopic conditions in marine mammals (Oppermann et al., 2016). In humans, there is evidence for rod-based contributions to color vision in normal observers (Zele and Cao, 2014), and evidence for color matching and wavelength discrimination in blue cone monochromats under mesopic illumination mediated by the interaction of rods and S cones (Alpern et al., 1971; Reitner et al., 1991).
3.3. Evolution of color opponent processing
Behavior synchronized to the spectral composition of the ambient light is already present in simple unicellular organisms. The archaeon Halobacterium migrates vertically in water toward light, a behavior called phototaxis (Hildebrand and Schimz, 1986). This response depends on the spectral composition of the ambient light: The archae migrate towards the water surface in orange light and away from the surface in UV light, presumably to protect its DNA from damage from UV irradiation (Spudich, 1993). This behavioral phenotype is indicative of a simple form of “non-neural” color opponency, or at least the capability of controlling locomotion by some form of spectral discrimination to adapt to the ambient light. Halobacterium salinarium possesses two rhodopsins, which are coupled to a flagellar motor, in which phosphorylation of a protein causes switching of the rotation direction (Hoff et al., 1997), thus causing upward or downward vertical movement depending on the activated photopigment.
The dinoflagellate Gonyaulax polyedra also appears to be able to discriminate between long and shorter-wavelength light (Roenneberg and Hastings, 1988). Specifically, pulses of red light lengthen its circadian period, while pulses of blue light shorten it. This is consistent with the presence of two peaks (475 nm and 650 nm) in the action spectrum for circadian phase shifting of luminescence in these algae (Hastings and Sweeney, 1960). The presence of simple but spectrally specific and opposite responses in procaryotes indicates that using photopigment interactions to adjust behavior and/or physiology depending on the spectral composition of ambient light is an evolutionarily ancient strategy.
In vertebrates, at least two neural color opponent coding strategies have evolved to endow the sensory systems regulating circadian photoentrainment with color sensitivity. The first strategy is the more commonly found and studied one: photoreceptors with two spectrally different photopigments synapse onto an interneuron (e.g. bipolar cell, horizontal cell, or retinal ganglion cell) with opposing sign, such that one signal excites and the other signal inhibits the target neuron. The second strategy is far less common and has so far only been experimentally demonstrated in the lizard parietal eye and does not involve an interneuron (Solessio and Engbretson, 1993); instead, photopigments are co-localized within one cell and produce a hyperpolarizing or depolarizing response, respectively. Current evidence supporting the roles of such color-coding systems in vertebrate circadian photoentrainment is presented below.
3.4. Testing for color opponency in NIF visual function
There are multiple strategies for determining whether an organism is sensitive to color (Jacobs, 1993). At the neural level, the existence of neurons comparing signals from two photopigments (such as color-opponent retinal ganglion cells) is a prerequisite for all color vision. Importantly, the presence of multiple spectrally distinct photopigments is a necessary, but not a sufficient condition for color vision. For example, the mantis shrimp has 12 distinct photoreceptor classes spanning the entire spectrum (Cronin and Marshall, 1989), but remarkably poor wavelength discrimination (Thoen et al., 2014). Instead, direct psychophysical, physiological, and behavioral tests need to be carried out to establish that an organism is sensitive to color. Determining color sensitivity is complicated by the fact that it may depend on the overall light intensity or adaptation state of other photoreceptors (e.g. S-OFF inputs to luminance in humans (Ripamonti et al., 2009), ‘concealed color opponency’ in primate RGCs (De Monasterio et al., 1975a)).
A common experimental approach is action spectroscopy (Peirson et al., 2005), in which the spectral sensitivity of a criterion non-image-forming visual response (e.g. the suppression of the hormone melatonin by exposure to light) is measured using monochromatic or narrowband lights presented against a dark or very dim background. Owing to the possibility that chromatic sensitivity is only revealed under appropriate light or chromatic adaptation conditions, action spectroscopy may not represent an adequate tool to rule out the influence of photoreceptor inputs or combinations thereof into a given visual response. It is conceivable that chromatic effects would be masked when narrowband steps are presented against a dark background and would be revealed when incremental narrowband light pulses against a neutral or light-adapted background are measured. As a consequence, if the action spectrum (measured from the dark) of visual response is consistent with the action of only a given photopigment, it does not follow that this is the only photopigment contributing to the response in all situations (e.g. under different chromatic backgrounds).
4. Chromatic circadian photosensitivity in lower vertebrates
4.1. Pineal photosensitivity in fish and amphibians
Nearly all vertebrate species possess a pineal organ which contains extraretinal photoreceptors and provides a pathway through which light can regulate daily cycles in physiology and behavior (Dodt and Meissl, 1982). The existence of pineal photosensitivity driving circadian rhythms in vertebrate species suggests the semi-independent evolution of image-forming and non-image-forming photosensitive organs, although interestingly, pineal photosensitivity has been lost in mammals.
In the early 1960s, Dodt and Heerd (1962) investigated the photosensitive properties of pineal neurons in frogs (Rana temporaria) and found two classes of photosensitive cell types: chromatic cells which were inhibited in their spiking by short-wavelength (UV) light (~355 nm) and excited by long-wavelength light (~517 nm), and achromatic cells which respond in only one fashion to light (with peak sensitivity ~560 nm). This effect was also demonstrated for the slow potentials of pineal cells in frogs (Baumann, 1962; Donley, 1975). Later work in Rana esculenta found that the overall response of the frog pineal organ depends on the balance of inhibition and excitation from the two opponent photoreceptors (Baumann, 1962; Meissl and Donley, 1980). Color opponent neurons have also been demonstrated in the pineal of bullfrogs (Rana catesbyana; Morita, 1969) and toads (Xenopus laevis Daud; Korf et al., 1981).
In fish, chromatic responses have been documented in the pineal of rainbow trout (Salmo irideus; Morita, 1966), pike (Esox Lucius; Falcón and Meissl, 1981), and lamprey (Lampetra japonica; Morita et al., 1987a; Uchida and Morita, 1994) (Fig. 3b, c). The inhibitory component peaks at around 360 nm for trout, pike and lamprey, while the excitatory component has maximal sensitivity at 530 nm (trout), 620 nm (pike), and 525 nm (lamprey). In the lamprey, the proportion of color-opponent cells is much smaller than achromatic cells (e.g. two of 40 surveyed cells in Morita (1966)) and chromatic cells appear to be more common in the periphery (Uchida and Morita, 1994) of the lamprey pineal. Lampreys possess at least two paranopsins differing in their spectral sensitivity, providing a molecular basis for the chromatic antagonism (Koyanagi et al., 2015).
It is important to remember that the presence of chromatically sensitive neurons in the pineal organ or other neural structures does not necessarily mean that chromatic signals in the environment are used to adapt behavior to a diurnal rhythm or otherwise changing spectral information. Interestingly, pineal organs appear to project to downstream brain targets which are also innervated by signals from the lateral eyes (Korf et al., 1998). This suggests that the retinal photoreceptors also play a role in NIF visual functions and information from both retinal and extraretinal photoreceptors may take place to signal color and/or intensity of the ambient light. Thus, the in vivo response to changing color and intensity of the ambient light may be mediated by the mixed action of retinal and extraretinal photoreceptors.
Two studies examining the action spectrum for melatonin synthesis in the pineal in trout and zebrafish using monochromatic light against darkness found no strong evidence for an inhibitory interaction between two photopigments (Max and Menaker, 1992; Ziv et al., 2007). However, as mentioned above, action spectroscopy does not rule out chromatic sensitivity under light adaptation.
To examine whether changes in the color of ambient illumination can reliably entrain locomotor activity rhythms in fish (Pauers et al., 2012), fish of two cichlid species (Aequidens pulcher and Labeotropheus fuelleborni) were subjected to illumination cycles which were controlled in their chromatic content and luminance. Under naturalistic illumination conditions, in which both luminance and chromaticity underwent changes, the animals showed a diurnal or crepuscular pattern. When luminance (as defined by the photon catch of melanopsin) was held constant and only the color of the illumination (i.e. the balance between L + M and S excitation) changed, the animals surprisingly showed a similar pattern of activity, indicating that changes in luminance are not necessary to induce natural circadian behavior. When the fish were subjected to only intensity changes, but not color changes, they showed structured activity patterns but these were quite unlike the ones seen under natural illumination cycles, turning the fish into nocturnal (rather than diurnal or crepuscular) animals. This seems to suggest that in these animal species, spectral changes are necessary and sufficient to produce appropriately phased rhythms in behavior. Yet it is worth noting that the stimulation conditions in Pauers et al. (2012) only controlled the activation of the retinal photoreceptors, and did not consider extraretinal photoreceptors which are common in fish (see Kingston and Cronin (2016) for a review). As discussed above, both retinal and extraretinal photoreceptors may contribute to circadian regulation, and as a result, the effects attributed to changes in color may actually have been caused by modulations in effective irradiance.
In both amphibians and fish, circannual and even circalunar variations in the proportion of retinal visual pigments have been found, which may represent long-term adaptations to the changing spectral composition of the photic environment throughout the seasons (Allen, 1971; Allen et al., 1982; Allen and McFarland, 1973; Beatty, 1969; Bobbert et al., 1978; Himstedt, 1973; Himstedt et al., 1981; Lang, 2008; Muntz and Mouat, 1984; Nosaki, 1969; Whitmore and Bowmaker, 1989), but how these may influence the diurnal regulation of rest-activity cycles is unknown.
4.2. Chromatic antagonism in parietal eyes in lizards
Some lizard species have a parietal eye in addition to their lateral eyes (Dodt and Meissl, 1982). These eyes are located in a small foramen between the parietal bones and are equipped with a cornea, a lens and a retina. Cells in these parietal eyes exhibit color opponency in some species (Lacerta sicula campestris, Dodt and Scherer, 1968; Iguana, Hamasaki, 1969; Anolis carolinensis, Jenison and Nolte, 1980) (Fig. 3d). The opponency arises from the action of two pigments, a blue pigment (pinopsin) and a green pigment (parietopsin), and two G-protein signaling mechanisms (one for each pigment) in the photoreceptors themselves (Su et al., 2006). The blue-pigment pathway produces a hyperpolarizing response, while the green-pigment pathway produces a depolarizing response (Solessio and Engbretson, 1993; Su et al., 2006) (Fig. 3e). In iguana, pinopsin (blue-sensitive) is replaced by parapinopsin (UV-sensitive), endowing the animal with UV sensitivity (Wada et al., 2012). Notably, not all lizard species have parietal eyes, and the presence of a parietal eye seems to be correlated with the latitude in which they dwell (Gundy et al., 1975), suggesting that key information in the photic environments may be contained in the irradiance at high latitudes. We do not know (yet) whether the intracellular opponency is important for the diurnal control of physiology and/or behavior.
The presence of two photopigments in one cell with opposing outputs may be unique to lizards. Co-expression of two different photopigments in a single cone occurs in some mammals such as rabbits, guinea pigs and mice (Applebury et al., 2000; Lukats et al., 2002) as well as in the fetal retina in humans (Xiao and Hendrickson, 2000), but in none of these cases is there any evidence that the two photopigments yield opposing intracellular responses.
4.3. Birds
Birds under near-continuous lighting conditions in the Arctic exhibit a diurnal pattern of behavior (Marshall, 1938). In the high Arctic summer, color temperature undergoes predictable changes, ranging from approximately 7000K (‘cool white’) during the day to lower color temperatures around 3000K (yellowish) (Krüll, 1976), while the normal intensity changes occurring at twilight are largely attenuated. Both snowbuntings (Plectrophenax nivalis), local to the Arctic, and greenfinches (Carduelis chloris), which are native to lower latitudes, appear to synchronize their nest feeding activity with the solar cycle under these conditions (Krüll, 1976; Krüll et al., 1985). In a laboratory study, chaffinches (Fringilla coelebs) exhibit partial but incomplete synchronization to a light regimen with changing color temperature (2900K:3400K and 2900K:5600K at 2.5 lx, 12h:12h) (Krüll et al., 1985). Interestingly, the entrainment to the color temperature cycle appears to be mediated by the presence of sexual hormones: Castrated chaffinches under a 2900K:5600K cycle have a free-running rhythm, but entrain their activity to the color cycle upon treatment with testosterone (Krüll et al., 1985).
In the laboratory, the locomotor rest-activity patterns of zebra-finches (Taenipoygia guttata) (Demmelmeyer and Haarhaus, 1972) entrain to a color cycle of 2900K:3400K (400 lx, 12h:12h). Bramblings (Fringilla montifringilla) and common redpolls (Carduelis f. flammea), two bird species which breed in Scandianvia and Russia and migrate to Southern Europe for the winter, photoentrain to a schedule of lights at different color temperatures (2500K:5000K, 12h:12h), as well as narrowband blue (440 nm) and red (650 nm) at equal energy (Pohl, 1999). Lapland longspurs (Calcarius lapponicus), a species of arctic-breeding migratory songbirds, did not entrain to a similar light regime at relatively bright light (6500 K:3500 K at 780 lx, 12h:12h) (Ashley et al., 2014).
In summary then, there is evidence that daily changes in the color of ambient illumination could contribute to daily regulation of physiology and behavior in a range of avian species. An important caveat here, however, is that the lighting conditions employed by these studies were quantified solely in terms of color temperature and illuminance, both of which are meaningful only with respect to the human eye. Birds possess both extraretinal photoreceptos and have multiple retinal photoreceptor types whose spectral sensitivity deviates from the human cone-based visual system (Bennett and Théry, 2007). As such, the effective irradiance (or apparent brightness) of spectrally distinct lights could be quite different for birds even if matched for illuminance. Consequently, the lightning conditions employed by the above studies most likely provided changes in both apparent color and irradiance, rendering the specific contribution of putative color-opponent mechanisms uncertain.
5. Chromatic circadian photosensitivity in mammals
Color opponent cells have been found in the visual systems of at least six orders of mammals (Jacobs, 2014): Primates (e.g. vervet monkeys, baboons, squirrel monkeys, spider monkeys, marmosets, Cebus monkeys, and howler monkeys), rodents (e.g. ground squirrels, tree squirrels, guinea pigs, and mice), lagomorpha (e.g. rabbits); carnivora (e. g. domestic cats), scandetia (e.g. tree shrews), and diprodonta (e.g. tammar wallabies). Few studies have examined the role of color in circadian photosensitivity or photic control of the rest-activity cycle. There are, at present, only a small number of published studies (Geetha et al., 1995; Joshi and Chandrashekaran, 1985a; Nuboer et al., 1983; Walmsley et al., 2015) with varying depth and precision in controlling the excitation of the photoreceptors. These will be reviewed in the following.
5.1. Circadian phase shifting by color in lower mammals
In cave-dwelling bats (Hipposideros speoris), pulses of blue (430 nm) and green (520 nm) light matched in corneal irradiance have opposing effects at a given circadian time (Joshi and Chandrashekaran, 1985a). Circadian time (CT) in this context refers to the phase of an organism’s internal time: CT0 refers to the beginning of the subjective day, while CT12 is the beginning of the subjective night. At CT2, CT4, CT12 and CT18, blue light delays circadian phase in this species, while green light advances circadian phase. This is consistent with a color-opponent mechanism with two photopigments.
Later work found differences in circadian phase shifting for different types of white light of different spectral composition in the same species (Joshi and Chandrashekaran, 1985b). Fluorescent light and daylight delayed circadian phase when delivered during the subjective night, while, incandescent ‘white’ light evoked advances in circadian phase of approximately equal amplitude magnitude (~90 min) when delivered during the subjective night (CT12-24). While both natural daylight and the fluorescent lamp had relative peaks at around 430 nm and 600 nm, the incandescent light, though broadband, had peak power at around 600 nm and was relatively depleted of short-wavelength light. While light intensity in this study was matched in terms of illuminance for a human observer (~1000 lx), it seems implausible that an intensity-dependent mechanism insensitive to spectral shifts would yield phase advances for some values and phase delays in another when the light is delivered at the same CT.
At least some bats have both a long-wavelength sensitive pigment and a short/UV sensitive pigment (Muller et al., 2009; Wang et al., 2004) which may be the photoreceptive substrate for the color-dependent circadian phase shifting, but this is merely speculation. Notably, these bats are cave-dwelling and experience illumination only when they leave the cave for foraging in twilight. Locomotion within the dark cave and under starlight or moonlight condition is presumably mediated strongly by echolocation rather than image-forming vision. The presence of an opponent processing system for photoentrainment in a non-visual animal suggests the evolutionary eminence of such a color coding scheme (Joshi and Vanlalnghaka, 2005).
In wild rabbits (Oryctolagus cuniculus), increments of blue light are more effective in delaying and advancing locomotor activity than blue decrements, and vice versa for yellow increments and decrements (Nuboer et al., 1983). This is consistent with the presence of two classes of cones in the rabbit retina from which signals are compared in an opponent fashion. Indeed, the rabbit retina contains RGCs with S-ON center and M-OFF surrounds (Caldwell and Daw, 1978), as well as two types of RGCs with an S-OFF center and M-ON surround differing in their stratification (Mills et al., 2014; Vaney et al., 1981), providing a retinal mechanism for the sensitivity to blue/yellow decrements.
Most recently, Walmsley et al. (2015) set out to test the sensitivity of the mouse circadian system to changes in color information. A subset of neurons in the central circadian pacemaker (suprachiasmatic nucleus: SCN) of the mouse were found to be sensitive to color, receiving inputs from UV and M cones. Both blue-ON/yellow-OFF and yellow-ON/blue-OFF responses were found, though there was a bias towards cells of the former type in the sampled cell population. These color-opponent cells also integrate melanopsin signals, and respond to a simulated color and irradiance shift corresponding to the natural twilight dynamics by a proportional increase in firing as a function of solar elevation in the range −8° and 4°, indicating that these color-opponent cells integrate both color and irradiance to signal twilight. Importantly, it was also found that housing under a light regimen containing only the irradiance signal resulted in a different circadian phase in entrainment than under a light regimen including naturalistic changes in color as well. This effect was absent in mice lacking cone photoreception. This suggests that cone-mediated color information provides a strong signal for circadian phase entrainment.
Whether this color sensitivity is inherited from color-opponent RGCs or arises from local differencing with the SCN is not yet known. Interestingly, the importance of color information in circadian entrainment and driving SCN firing is not mirrored in the mouse image-forming visual system: Psychophysically, mice appear to have weak color sensitivity in wavelength discrimination (Jacobs et al., 2004), and the proportion of cone-opponent RGCs is very small (Ekesten and Gouras, 2005), though rod-cone opponent responses have recently been reported (Joesch and Meister, 2016).
There is likely considerable variation between mammalian species in terms of whether an animal’s circadian system is endowed with color sensitivity, and understanding the nature of such variability would yield valuable insight into the adaptation of their circadian visual system to the photic environments. How the organization of circadian photosensitivity differs across mammalian species and with differences in geographical and temporal niche is not known. It is estimated that ~90% of mammalian species contain at least two photopigments (Jacobs, 2013), which suggests that one prerequisite for color opponency, namely the presence of two spectral classes of photopigments, is at least widespread among mammals.
5.2. Spectral opponency in melanopsin-containing ipRGCs in primates
In the macaque retina, the melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs), which project (amongst other targets) to the SCN (Hannibal et al., 2014), are principally monostratified, sending their axons to either the inner or the outer stratum (Dacey et al., 2005; Hannibal et al., 2004; Hannibal et al., 2014; Liao et al., 2016). Electrophysiological recordings from macaque ipRGCs retrogradely labeled from the pretetcum and thalamus, revealed strong melanopsin-mediated intrinsic activity, but also positive synaptic inputs from L and M cones and rods, and negative synaptic input from S cones (Dacey et al., 2005), rendering them color-opponent in a yellow-ON, blue-OFF fashion. It should be noted, however, that there are various subtypes of ipRGCs in mice and rats which differ in their projection sites and physiological properties (Hu et al., 2013; Schmidt et al., 2011; Schmidt and Kofuji, 2010). Insofar as a full characterization of ipRGC subtypes and their heterogeneity in the primate retina is currently lacking, we do not know yet if all primate ipRGCs receive S opponent input, or if their synaptic inputs differ as a function of eccentricity.
In humans, the pupillary light reflex (PLR) is controlled by an S-cone opponent circuit, such that an increase in S cone stimulation leads to a paradoxical dilatatory response (Spitschan et al., 2014; Cao et al., 2015). Therefore, the S-cone opponency found in primate ipRGCs in is preserved in at least one non-image-forming visual function in humans. By contrast, in mice, S cones provide on-excitation to the pretectal olivary nucleus (PON) and drive pupil constriction (Allen et al., 2011), suggesting chromatic controls on the pupil may differ substantially across mammalin species. Nonetheless, based on the above, one way that color could contribute to primate circadian regulation is as follows: An increase in S cone excitation dilates the pupil, and therefore the retinal irradiance is larger than for stimuli providing weaker S cone activity. Given the relatively small dynamic range over which the human pupil can change retinal irradiance (< 1 log unit of change in retinal irradiance between maximally constricted and maximally dilated, Barlow, 1972), such a mechanism is unlikely to exert a major influence on circadian photosensitivity, however. As before, the demonstration of color-opponent circuitry is necessary but not sufficient for color sensitivity in circadian regulation. We do not know yet if the S cone-opponent ipRGCs project to the SCN and if this color sensitivity is preserved beyond the RGC level.
5.3. Photosensitivity in melatonin suppression and circadian phase shifting in humans
5.3.1. Melatonin suppression
Over the past 20years, considerable attention has been directed towards assessing the sensory signals regulating melatonin secretion in humans, an aspect of physiology under control of ipRGCs and the SCN (Foster et al., 2007). In the early 2000s, two independent efforts to determine the spectral sensitivity of acute melatonin suppression in people determined a peak spectral sensitivity (λmax) in the region 455–465 nm (Brainard et al., 2001; Thapan et al., 2001). This value is substantially short-wavelength shifted relative the peak spectral sensitivity (~480 nm) of melanopsin (Bailes and Lucas, 2013), suggesting a positive contribution of S-cones to this aspect of physiological regulation. It should be noted, however, that a more recent study of melatonin suppression using monochromatic lights with matched photon density found a peak at 480 nm (Najjar et al., 2014), suggesting that melanopsin makes the dominant contribution. Nonetheless, two studies investigating melatonin suppression by exposure to different polychromatic light sources have provided evidence for sub-additive responses that could be indicative of cone-opponent inputs (Figueiro et al., 2004; Revell et al., 2010), although a follow up study by the authors of one of those papers failed to find an opponent effect of combining short and long wavelength monochromatic stimuli (Papamichael et al., 2012). One model of the human circadian system explicitly includes an S-opponent input (Rea et al., 2010) but the evidence supporting such a role is far from conclusive. Red-green color-deficient people show no difference in acute melatonin suppression, indicating that a functional L/M-cone system is not a necessary condition for circadian phase effects (Ruberg et al., 1996).
5.3.2. Circadian phase shifting
Contributions of color opponency to circadian photoentrainment have never been directly investigated. A study comparing the effects of blue (460 nm) and green (555 nm) light administered in the evening indicate that the effects of the green light on both melatonin suppression and circadian phase shifting could not be accounted for by the activation of melanopsin only, suggesting a role for cones in these responses (Gooley et al., 2010). These influences were found to decay with continued exposure to the light. Importantly, this study and others (e.g. Najjar and Zeitzer, 2016) indicate that the sensory pathways mediating melatonin suppression and circadian phase shifting may be different in their temporal sensitivity and spectral sensitivity. Therefore, even if melatonin suppression was driven by an S-opponent pathway, this may not hold for phase shifting.
6. Future directions to study color sensitivity in human NIF visual function
6.1. Light adapted circadian photosensitivity
In people, prior light exposure strongly influences experimentally observed effects of light on both melatonin suppression (Gooley et al., 2011; Hebert et al., 2002; Smith et al., 2004) and circadian phase shifting (Chang et al., 2011). In particular, because of decreases in sensitivity due to prior exposure, studies examining the spectral sensitivity of the human circadian system have used light stimuli against a dark or dim background, thus producing an increase in all photoreceptors from their dark or dim adapted state. As discussed in detail above, this procedure may not reveal chromatic inputs into the visual response studied. In an alternative approach, the observer would be adapted to a large background field of known wavelength or spectral composition and sensitivity to flashes of different wavelengths is measured. This is equivalent, in logic, to action spectroscopy against a dark background except that the background is a light. This approach, developed by W. S. Stiles (Stiles, 1959, 1978), has unmasked the color sensitive mechanisms in human IF vision and could be adapted to measure incremental color thresholds of human NIF vision.
6.2. Method of silent substitution
Another approach to uncover color coding is the method of silent substitution (Estévez and Spekreijse, 1982), which allows for the selective stimulation of different photoreceptor classes, and may provide experimental leverage to study how photoreceptors combine to drive the effects of light on human chronobiology. In the method of silent substitution, pairs of lights are selected such that when alternating between them, the exitant light only stimulates the targeted photoreceptor class(es), while the change in light does not affect the silenced photoreceptor class(es) and is thus invisible to them (Estévez and Spekreijse, 1982). Using n light sources, it is possible to silence n–1 photoreceptor classes, and excite the remaining target class. In order to study the photoreceptor inputs to the circadian system in humans (three cone classes, rods, and melanopsin), at least five independent light sources are necessary. Although silent substitution has not to our knowledge been employed to study human circadian biology, it has been successfully employed to study the role of melanopsin, as well as the interactions between melanopsin and the cones (Barrionuevo and Cao, 2016; Cao et al., 2015; Fukuda et al., 2010; Spitschan et al., 2014; Tsujimura and Tokuda, 2011; Viénot et al., 2010), and may be a useful method to probe the photoreceptor mechanisms in the human NIF visual system.
7. Conclusion
Opposite responses to light of different wavelengths are present in unicellular organisms, suggesting that sensitivity to color in encoding diurnal variations in the light environment may be a key early evolutionary adaptation. Color-opponent responses have been well-documented electrophysiologically in the pineal/parietal organs of fish, amphibians and reptiles, in the SCN of mice, and are a property of melanopsin-containing retinal ganglion cells in primates. Color opponency for NIF vision represents an important emerging area of investigation.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. While at the University of Pennsylvania, author M.S. was partially supported through National Institutes of Health Grants R01 EY020516 (to Geoffrey K. Aguirre), R01 EY10016 (to David H. Brainard), and P30 EY001583 (Core Grant for Vision Research) and Deutscher Akademischer Austauschdienst. The author wishes to thank Drs. Geoffrey K. Aguirre, David H. Brainard, David F. Dinges and Andrew M. Stern for comments on an earlier draft of this manuscript.
References
- Allen DM, McFarland WN. The effect of temperature on rhodopsin-porphyropsin ratios in a fish. Vision Res. 1973;13:1303–1309. doi: 10.1016/0042-6989(73)90206-x. http://dx.doi.org/10.1016/0042-6989(73)90206-X. [DOI] [PubMed] [Google Scholar]
- Allen DM, Loew ER, McFarland WN. Seasonal change in the amount of visual pigment in the retinae of fish. Can J Zool. 1982;60:281–287. http://dx.doi.org/10.1139/z82-037. [Google Scholar]
- Allen AE, Brown TM, Lucas RJ. A distinct contribution of short-wavelength-sensitive cones to light-evoked activity in the mouse pretectal olivary nucleus. J Neurosci. 2011;31:16833–16843. doi: 10.1523/JNEUROSCI.2505-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.2505-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DM. Photic control of the proportions of two visual pigments in a fish. Vision Res. 1971;11:1077–1112. doi: 10.1016/0042-6989(71)90114-3. http://dx.doi.org/10.1016/0042-6989(71)90114-3. [DOI] [PubMed] [Google Scholar]
- Alpern M, Lee GB, Maaseidvaag F, Miller SS. Colour vision in blue-cone ‘monochromacy. J Physiol. 1971;212:211–233. doi: 10.1113/jphysiol.1971.sp009318. http://dx.doi.org/10.1113/jphysiol.1971.sp009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. http://dx.doi.org/10.1016/S0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Ashley NT, Ubuka T, Schwabl I, Goymann W, Salli BM, Bentley GE, Buck CL. Revealing a circadian clock in captive arctic-breeding songbirds, lapland longspurs (Calcarius lapponicus), under constant illumination. J Biol Rhythms. 2014;29:456–469. doi: 10.1177/0748730414552323. http://dx.doi.org/10.1177/0748730414552323. [DOI] [PubMed] [Google Scholar]
- Atick JJ, Li Z, Redlich AN. Understanding retinal color coding from first principles. Neural Comput. 1992;4:559–572. http://dx.doi.org/10.1162/neco.1992.4.4.559. [Google Scholar]
- Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G and Gi/o signalling cascades. Proc Biol Sci. 2013;280:20122987. doi: 10.1098/rspb.2012.2987. http://dx.doi.org/10.1098/rspb.2012.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. Dark and light adaptation: psychophysics. In: Jameson D, Hurvich LM, editors. Visual Psychophysics. Springer; Berlin/Heidelberg: 1972. pp. 1–28. [Google Scholar]
- Barrionuevo PA, Cao D. Luminance and chromatic signals interact differently with melanopsin activation to control the pupil light response. J Vis. 2016;16(29) doi: 10.1167/16.11.29. http://dx.doi.org/10.1167/16.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C. Lichtabhängige langsame Potentiale aus dem Stirnorgan des Frosches. Pflugers Arch. 1962;276:56–65. http://dx.doi.org/10.1007/bf00362462. [PubMed] [Google Scholar]
- Beatty DD. Visual pigments of the burbot, Lota lota, and seasonal changes in their relative proportions. Vision Res. 1969;9:1173–1183. doi: 10.1016/0042-6989(69)90107-2. http://dx.doi.org/10.1016/0042-6989(69)90107-2. [DOI] [PubMed] [Google Scholar]
- Bennett, Andrew TD, Théry M. Avian color vision and coloration: multidisciplinary evolutionary biology. Am Nat. 2007;169:S1–S6. http://dx.doi.org/10.1086/510163. [Google Scholar]
- Bobbert AC, Brandenburg J, Krul WH. Seasonal fluctuations of the circadian changes in rabbit visual evoked potentials. Int J Chronobiol. 1978;5:519–532. [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. J Neurosci. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.0616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhof MW, Daan S, Strubbe JH. Forced dissociation of food- and light-entrainable circadian rhythms of rats in a skeleton photoperiod. Physiol Behav. 1998;65:225–231. doi: 10.1016/s0031-9384(98)00051-1. http://dx.doi.org/10.1016/S0031-9384(98)00051-1. [DOI] [PubMed] [Google Scholar]
- Buchsbaum G, Gottschalk A. Trichromacy, opponent colours coding and optimum colour information transmission in the retina. Proc R Soc Lond Ser B. 1983;220:89–113. doi: 10.1098/rspb.1983.0090. http://dx.doi.org/10.1098/rspb.1983.0090. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW. New properties of rabbit retinal ganglion cells. J Physiol. 1978;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. http://dx.doi.org/10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Nicandro N, Barrionuevo PA. A five-primary photostimulator suitable for studying intrinsically photosensitive retinal ganglion cell functions in humans. J Vis. 2015;15 doi: 10.1167/15.1.27. http://dx.doi.org/10.1167/15.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. http://dx.doi.org/10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin TW, Marshall NJ. A retina with at least ten spectral types of photoreceptors in a mantis shrimp. Nature. 1989;339:137–140. http://dx.doi.org/10.1038/339137a0. [Google Scholar]
- Daan S, Aschoff J. Circadian rhythms of locomotor activity in captive birds and mammals: their variations with season and latitude. Oecologia. 1975;18:269–316. doi: 10.1007/BF00345851. http://dx.doi.org/10.1007/bf00345851. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. http://dx.doi.org/10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. http://dx.doi.org/10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Daw NW. Colour-coded ganglion cells in the goldfish retina: extension of their receptive fields by means of new stimuli. J Physiol. 1968;197:567–592. doi: 10.1113/jphysiol.1968.sp008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio FM, Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975;251:167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio FM, Gouras P, Tolhurst DJ. Concealed colour opponency in ganglion cells of the rhesus monkey retina. J Physiol. 1975a;251:217–229. doi: 10.1113/jphysiol.1975.sp011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio FM, Gouras P, Tolhurst DJ. Trichromatic colour opponency in ganglion cells of the rhesus monkey retina. J Physiol. 1975b;251:197–216. doi: 10.1113/jphysiol.1975.sp011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois RL, Smith CJ, Kitai ST, Karoly AJ. Response of single cells in monkey lateral geniculate nucleus to monochromatic light. Science. 1958;127:238–239. doi: 10.1126/science.127.3292.238. http://dx.doi.org/10.1126/science.127.3292.238. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Jacobs GH, Abramov I. Responses of single cells in visual system to shifts in the wavelength of light. Science. 1964;146:1184–1186. doi: 10.1126/science.146.3648.1184. http://dx.doi.org/10.1126/science.146.3648.1184. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Abramov I, Jacobs GH. Analysis of response patterns of LGN cells. J Opt Soc Am. 1966;56:966–977. doi: 10.1364/josa.56.000966. http://dx.doi.org/10.1364/JOSA.56.000966. [DOI] [PubMed] [Google Scholar]
- Demmelmeyer H, Haarhaus D. Die Lichtqualität als Zeitgeber für Zebrafinken (Taeniopygia guttata) J Comp Physiol. 1972;78:25–29. http://dx.doi.org/10.1007/bf00696478. [Google Scholar]
- Dodt E, Heerd E. Mode of action of pineal nerve fibers in frogs. J Neurophysiol. 1962;25:405–429. doi: 10.1152/jn.1962.25.3.405. [DOI] [PubMed] [Google Scholar]
- Dodt E, Meissl H. The pineal and parietal organs of lower vertebrates. Experientia. 1982;38:996–1000. doi: 10.1007/BF01955342. http://dx.doi.org/10.1007/bf01955342. [DOI] [PubMed] [Google Scholar]
- Dodt E, Scherer E. Photic responses from the parietal eye of the lizard Lacerta sicula campestris (De Betta) Vision Res. 1968;8:61–72. http://dx.doi.org/10.1016/0042-6989(68)90064-3. [Google Scholar]
- Donley CS. Color opponent slow potential interactions in the frontal organ of the frog: rana pipiens. Vision Res. 1975;15:245–251. doi: 10.1016/0042-6989(75)90214-x. http://dx.doi.org/10.1016/0042-6989(75)90214-X. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch Gen Psychiatry. 1988;45:948–952. doi: 10.1001/archpsyc.1988.01800340076012. http://dx.doi.org/10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- Ekesten B, Gouras P. Cone and rod inputs to murine retinal ganglion cells: evidence of cone opsin specific channels. Vis Neurosci. 2005;22:893–903. doi: 10.1017/S0952523805226172. http://dx.doi.org/10.1017/S0952523805226172. [DOI] [PubMed] [Google Scholar]
- Ekström P, Meissl H. Pineal photoreception and temporal physiology in fish. In: Kulczykowska E, Popek W, Kapoor BG, editors. Biological Clock in Fish. CRC Press; Boca Raton: 2010. pp. 35–70. [Google Scholar]
- Estévez O, Spekreijse H. The silent substitution method in visual research. Vision Res. 1982;22:681–691. doi: 10.1016/0042-6989(82)90104-3. http://dx.doi.org/10.1016/0042-6989(82)90104-3. [DOI] [PubMed] [Google Scholar]
- Falcón J, Meissl H. The photosensory function of the pineal organ of the pike (Esox lucius L.) Correlation between structure and function. J Comp Physiol. 1981;144:127–137. http://dx.doi.org/10.1007/bf00612806. [Google Scholar]
- Figueiro MG, Bullough JD, Parsons RH, Rea MS. Preliminary evidence for spectral opponency in the suppression of melatonin by light in humans. Neuroreport. 2004;15:313–316. doi: 10.1097/00001756-200402090-00020. [DOI] [PubMed] [Google Scholar]
- Fleissner G, Fleissner G. Perception of natural zeitgeber signals. In: Kumar V, editor. Biological Rhythms. Springer; Berlin/Heidelberg: 2002. pp. 83–93. [Google Scholar]
- Foster RG, Hankins MW, Peirson SN. Light, photoreceptors, and circadian clocks. Methods Mol Biol. 2007;362:3–28. doi: 10.1007/978-1-59745-257-1_1. http://dx.doi.org/10.1007/978-1-59745-257-1_1. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Tsujimura S, Higuchi S, Yasukouchi A, Morita T. The ERG responses to light stimuli of melanopsin-expressing retinal ganglion cells that are independent of rods and cones. Neurosci Lett. 2010;479:282–286. doi: 10.1016/j.neulet.2010.05.080. http://dx.doi.org/10.1016/j.neulet.2010.05.080. [DOI] [PubMed] [Google Scholar]
- Geetha L, Chandrashekaran MK, Subbaraj R. Intensity but not the colour temperature of light acts as an entraining agent for the circadian system of the tropical mouse Mus booduga. Biol Rhythm Res. 1995;26:541–552. http://dx.doi.org/10.1080/09291019509360357. [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000741. http://dx.doi.org/10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. http://dx.doi.org/10.1210/jc.2010–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968;199:533–547. doi: 10.1113/jphysiol.1968.sp008667. http://dx.doi.org/10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii VI, Byzov AL, Zueva LV, Polisczuk NA, Baburina EA. Spectral characteristics of photoreceptors and horizontal cells in the retina of the Siberian sturgeon Acipenser baeri Brandt. Vision Res. 1991;31:2047–2056. doi: 10.1016/0042-6989(91)90162-x. http://dx.doi.org/10.1016/0042-6989(91)90162-X. [DOI] [PubMed] [Google Scholar]
- Gundy GC, Ralph CL, Wurst GZ. Parietal eyes in lizards: zoogeographical correlates. Science. 1975;190:671–673. doi: 10.1126/science.1237930. http://dx.doi.org/10.1126/science.1237930. [DOI] [PubMed] [Google Scholar]
- Hamasaki DI. Spectral sensitivity of the parietal eye of the green iguana. Vision Res. 1969;9:515–523. doi: 10.1016/0042-6989(69)90139-4. http://dx.doi.org/10.1016/0042-6989(69)90139-4. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Ostergaard J, Georg B, Heegaard S, Larsen PJ, Fahrenkrug J. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004;45:4202–4209. doi: 10.1167/iovs.04-0313. http://dx.doi.org/10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D, Gamlin PD. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol. 2014;522:2231–2248. doi: 10.1002/cne.23588. http://dx.doi.org/10.1002/cne.23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JW, Sweeney BM. The action spectrum for shifting the phase of the rhythm of luminescence in Gonyaulax polyedra. J Gen Physiol. 1960;43:697–706. doi: 10.1085/jgp.43.4.697. http://dx.doi.org/10.1085/jgp.43.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Mockel W, Ammermuller J. Different types of synapses with different spectral types of cones underlie color opponency in a bipolar cell of the turtle retina. Vis Neurosci. 1999;16:801–809. doi: 10.1017/s0952523899164186. [DOI] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand E, Schimz A. Integration of photosensory signals in Halobacterium halobium. J Bacteriol. 1986;167:305–311. doi: 10.1128/jb.167.1.305-311.1986. http://dx.doi.org/10.1128/jb.167.1.305-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himstedt W, Helas A, Sommer TJ. Projections of color coding retinal neurons in urodele amphibians. Brain Behav Evol. 1981;18:19–32. doi: 10.1159/000121773. [DOI] [PubMed] [Google Scholar]
- Himstedt W. Die spektrale Empfindlichkeit von Urodelen in Abhangigkeit von Metamorphose, Jahreszeit und Lebensraum. Zoologische Jb (Allg Zool) 1973;772:246–274. [Google Scholar]
- Hoff WD, Jung KH, Spudich JL. Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu Rev Biophys Biomol Struct. 1997;26:223–258. doi: 10.1146/annurev.biophys.26.1.223. http://dx.doi.org/10.1146/annurev.biophys.26.1.223. [DOI] [PubMed] [Google Scholar]
- Hu C, Hill DD, Wong KY. Intrinsic physiological properties of the five types of mouse ganglion-cell photoreceptors. J Neurophysiol. 2013;109:1876–1889. doi: 10.1152/jn.00579.2012. http://dx.doi.org/10.1152/jn.00579.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH, Williams GA, Fenwick JA. Influence of cone pigment coexpression on spectral sensitivity and color vision in the mouse. Vision Res. 2004;44:1615–1622. doi: 10.1016/j.visres.2004.01.016. http://dx.doi.org/10.1016/j.visres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev Camb Philos Soc. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. http://dx.doi.org/10.1111/j.1469-185X.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Losses of functional opsin genes, short-wavelength cone photopigments, and color vision–a significant trend in the evolution of mammalian vision. Vis Neurosci. 2013;30:39–53. doi: 10.1017/S0952523812000429. http://dx.doi.org/10.1017/S0952523812000429. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. The discovery of spectral opponency in visual systems and its impact on understanding the neurobiology of color vision. J Hist Neurosci. 2014;23:287–314. doi: 10.1080/0964704X.2014.896662. http://dx.doi.org/10.1080/0964704X.2014.896662. [DOI] [PubMed] [Google Scholar]
- Jenison G, Nolte J. An ultraviolet-sensitive mechanism in the reptilian parietal eye. Brain Res. 1980;194:506–510. doi: 10.1016/0006-8993(80)91232-9. http://dx.doi.org/10.1016/0006-8993(80)91232-9. [DOI] [PubMed] [Google Scholar]
- Joesch M, Meister M. A neuronal circuit for colour vision based on rod-cone opponency. Nature. 2016;532:236–239. doi: 10.1038/nature17158. http://dx.doi.org/10.1038/nature17158. [DOI] [PubMed] [Google Scholar]
- Joshi D, Chandrashekaran MK. Spectral sensitivity of the photoreceptors responsible for phase shifting the circadian rhythm of activity in the bat, Hipposideros speoris. J Comp Physiol A: Sens Neuroethol Neural Behav Physiol. 1985a;156:189–198. http://dx.doi.org/10.1007/bf00610861. [Google Scholar]
- Joshi D, Chandrashekaran MK. White light of different spectral composition causes differential phase shifts of circadian rhythm of activity in a bat. Naturwissenschaften. 1985b;72:548–549. doi: 10.1007/BF00367607. http://dx.doi.org/10.1007/BF00367607. [DOI] [PubMed] [Google Scholar]
- Joshi DS, Vanlalnghaka C. Non-parametric entrainment by natural twilight in the microchiropteran bat, Hipposideros speoris inside a cave. Chronobiol Int. 2005;22:631–640. doi: 10.1080/07420520500180116. http://dx.doi.org/10.1080/07420520500180116. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. Double color-opponent receptive fields of carp bipolar cells. Vision Res. 1983;23:381–388. doi: 10.1016/0042-6989(83)90085-8. http://dx.doi.org/10.1016/0042-6989(83)90085-8. [DOI] [PubMed] [Google Scholar]
- Kern HE. Twilight zeitgebers for daily resetting of circadian pacemakers. In: Holick M, Klingman A, editors. Biologic Effects of Light. Walter de Gruyter; Berlin/New York: 1992. pp. 205–212. [Google Scholar]
- Kingston AC, Cronin TW. Diverse distributions of extraocular opsins in crustaceans, cephalopods, and fish. Integr Comp Biol. 2016;56:820–833. doi: 10.1093/icb/icw022. http://dx.doi.org/10.1093/icb/icw022. [DOI] [PubMed] [Google Scholar]
- Korf HW, Liesner R, Meissl H, Kirk A. Pineal complex of the clawed toad, Xenopus laevis Daud: structure and function. Cell Tissue Res. 1981;216:113–130. doi: 10.1007/BF00234548. http://dx.doi.org/10.1007/BF00234548. [DOI] [PubMed] [Google Scholar]
- Korf HW, Schomerus C, Stehle JH. The pineal organ, its hormone melatonin, and the photoneuroendocrine system. Adv Anat Embryol Cell Biol. 1998;146:1–100. doi: 10.1007/978-3-642-58932-4. http://dx.doi.org/10.1007/978-3-642-58932-4. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Wada S, Kawano-Yamashita E, Hara Y, Kuraku S, Kosaka S, Kawakami K, Tamotsu S, Tsukamoto H, Shichida Y, Terakita A. Diversification of non-visual photopigment parapinopsin in spectral sensitivity for diverse pineal functions. BMC Biol. 2015;13(73) doi: 10.1186/s12915-015-0174-9. http://dx.doi.org/10.1186/s12915-015-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüll F, Demmelmeyer H, Remmert H. On the circadian rhythm of animals in high polar latitudes. Naturwissenschaften. 1985;72:197–203. http://dx.doi.org/10.1007/BF01195761. [Google Scholar]
- Krüll F. Zeitgebers for animals in the continuous daylight of high arctic summer. Oecologia. 1976;24:149–157. doi: 10.1007/BF00572756. http://dx.doi.org/10.1007/bf00572756. [DOI] [PubMed] [Google Scholar]
- Lang HJ. Lunar periodicity of colour sense of fish. J Interdiscipl Cycle Res. 2008;8:317–321. http://dx.doi.org/10.1080/09291017709359593. [Google Scholar]
- Le Grand Y. Light, Colour and Vision, English. 2nd. Chapman & Hall; London: 1968. [Google Scholar]
- Lee RL, Hernandez-Andres J. Measuring and modeling twilight’s purple light. Appl Opt. 2003;42:445–457. doi: 10.1364/ao.42.000445. http://dx.doi.org/10.1364/AO.42.000445. [DOI] [PubMed] [Google Scholar]
- Lee TW, Wachtler T, Sejnowski TJ. Color opponency is an efficient representation of spectral properties in natural scenes. Vision Res. 2002;42:2095–2103. doi: 10.1016/s0042-6989(02)00122-0. http://dx.doi.org/10.1016/s0042-6989(02)00122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RL. Twilight and daytime colors of the clear sky. Appl Opt. 1994;33:4629–4638. doi: 10.1364/AO.33.004629. http://dx.doi.org/10.1364/AO.33.004629. [DOI] [PubMed] [Google Scholar]
- Lee BB. Color coding in the primate visual pathway: a historical view. J Opt Soc Am A Opt Image Sci Vis. 2014;31:A103–A112. doi: 10.1364/JOSAA.31.00A103. http://dx.doi.org/10.1364/JOSAA.31.00A103. [DOI] [PubMed] [Google Scholar]
- Liao HW, Ren X, Peterson BB, Marshak DW, Yau KW, Gamlin PD, Dacey DM. Melanopsin-expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. J Comp Neurol. 2016;524:2845–2872. doi: 10.1002/cne.23995. http://dx.doi.org/10.1002/cne.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukats A, Dkhissi-Benyahya O, Szepessy Z, Rohlich P, Vigh B, Bennett NC, Cooper HM, Szel A. Visual pigment coexpression in all cones of two rodents, the Siberian hamster, and the pouched mouse. Invest Ophthalmol Vis Sci. 2002;43:2468–2473. [PubMed] [Google Scholar]
- Lynch DK, Livingston WC. Color and Light in Nature. 2nd. Cambridge University Press; Cambridge, UK/New York: 2001. [Google Scholar]
- MacNichol EJ, Svaetichin G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958;46:26–40. doi: 10.1016/0002-9394(58)90053-9. http://dx.doi.org/10.1016/0002-9394(58)90053-9. (discussion 40–26) [DOI] [PubMed] [Google Scholar]
- Marshall AJ. Bird and animal activity in the Arctic. J Anim Ecol. 1938;7:248–250. http://dx.doi.org/10.2307/1158. [Google Scholar]
- Max M, Menaker M. Regulation of melatonin production by light, darkness, and temperature in the trout pineal. J Comp Physiol A: Sens Neural Behav Physiol. 1992;170:479–489. doi: 10.1007/BF00191463. http://dx.doi.org/10.1007/bf00191463. [DOI] [PubMed] [Google Scholar]
- McFarland WN, Munz FW. The visible spectrum during twilight and its implications to vision. In: Evans GC, Bainbridge R, Rackham O, editors. Light as an Ecological Factor. Blackwell; Oxford: 1976. pp. 211–247. [Google Scholar]
- Meissl H, Donley CS. Change of threshold after light-adaptation of the chromatic response of the frog’s pineal organ (Stirnorgan) Vision Res. 1980;20:379–383. doi: 10.1016/0042-6989(80)90027-9. http://dx.doi.org/10.1016/0042-6989(80)90027-9. [DOI] [PubMed] [Google Scholar]
- Menzel R. Spectral sensitivity and color vision in invertebrates. In: Autrum H, editor. Comparative Physiology and Evolution of Vision in Invertebrates. Springer; Berlin/Heidelberg: 1979. pp. 503–580. [Google Scholar]
- Mills SL, Tian LM, Hoshi H, Whitaker CM, Massey SC. Three distinct blue-green color pathways in a mammalian retina. J Neurosci. 2014;34:1760–1768. doi: 10.1523/JNEUROSCI.3901-13.2014. http://dx.doi.org/10.1523/JNEUROSCI.3901-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Samejima M, Uchida K. Pineal role in circadian locomotor rhythm and cellular basis of photic entrainment. In: Trentini GP, De Gaetani C, Pévet P, editors. Fundamentals and Clinics in Pineal Research. Raven Press; New York: 1987a. pp. 161–164. [Google Scholar]
- Morita Y, Samejima M, Uchida K. The role of directory photosensory pineal organ in the LD and circadian rhythm. In: Horishige T, Honma K, editors. Comparative Aspects of Circadian Clocks Hokkaido. University Press; Sapporo: 1987b. pp. 73–81. [Google Scholar]
- Morita Y. Entladungsmuster pinealer Neurone der Regenbogenforelle (Salmo irideus) bei Belichtung des Zwischenhirns. Pflugers Archiv. 1966;289:155–167. http://dx.doi.org/10.1007/bf00412906. [PubMed] [Google Scholar]
- Morita Y. Wellenlängen-Diskriminatoren im intrakranialen Pinealorgan von Rana catesbyana. Experientia. 1969;25:1277. doi: 10.1007/BF01897496. http://dx.doi.org/10.1007/bf01897496. [DOI] [PubMed] [Google Scholar]
- Motokawa K, Taira N, Okuda J. Spectral responses of single units in the primate visual cortex. Tohoku J Exp Med. 1962;78:320–337. doi: 10.1620/tjem.78.320. http://dx.doi.org/10.1620/tjem.78.320. [DOI] [PubMed] [Google Scholar]
- Muller B, Glosmann M, Peichl L, Knop GC, Hagemann C, Ammermuller J. Bat eyes have ultraviolet-sensitive cone photoreceptors. PLoS One. 2009;4:e6390. doi: 10.1371/journal.pone.0006390. http://dx.doi.org/10.1371/journal.pone.0006390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz WRA, Mouat GSV. Annual variations in the visual pigments of brown trout inhabiting lochs providing different light environments. Vision Res. 1984;24:1575–1580. doi: 10.1016/0042-6989(84)90315-8. http://dx.doi.org/10.1016/0042-6989(84)90315-8. [DOI] [PubMed] [Google Scholar]
- Najjar RP, Zeitzer JM. Temporal integration of light flashes by the human circadian system. J Clin Invest. 2016;126:938–947. doi: 10.1172/JCI82306. http://dx.doi.org/10.1172/JCI82306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar RP, Chiquet C, Teikari P, Cornut PL, Claustrat B, Denis P, Cooper HM, Gronfier C. Aging of non-visual spectral sensitivity to light in humans: compensatory mechanisms? PLoS One. 2014;9:e85837. doi: 10.1371/journal.pone.0085837. http://dx.doi.org/10.1371/journal.pone.0085837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaki H. Electrophysiological study of color encoding in the compound eye of crayfish, Procambarus clarkii. Z Vergl Physiol. 1969;64:318–323. http://dx.doi.org/10.1007/bf00340549. [Google Scholar]
- Nuboer JFW, van Nuys WM, van Steenbergen JC. Colour changes in a light regimen as synchronizers of circadian activity. J Comp Physiol. 1983;151:359–366. http://dx.doi.org/10.1007/bf00623911. [Google Scholar]
- Ogden TE, Mascetti GG, Pierantoni R. The outer horizontal cell of the frog retina: morphology, receptor input, and function. Invest Ophthalmol Vis Sci. 1985;26:643–656. [PubMed] [Google Scholar]
- Oppermann D, Schramme J, Neumeyer C. Rod-cone based color vision in seals under photopic conditions. Vision Res. 2016;125:30–40. doi: 10.1016/j.visres.2016.04.009. http://dx.doi.org/10.1016/j.visres.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Palmer G, Johnsen S. Downwelling spectral irradiance during evening twilight as a function of the lunar phase. Appl Opt. 2014;54:B85–B92. doi: 10.1364/AO.54.000B85. http://dx.doi.org/10.1364/ao.54.000b85. [DOI] [PubMed] [Google Scholar]
- Papamichael C, Skene DJ, Revell VL. Human nonvisual responses to simultaneous presentation of blue and red monochromatic light. J Biol Rhythms. 2012;27:70–78. doi: 10.1177/0748730411431447. http://dx.doi.org/10.1177/0748730411431447. [DOI] [PubMed] [Google Scholar]
- Pauers MJ, Kuchenbecker JA, Neitz M, Neitz J. Changes in the colour of light cue circadian activity. Anim Behav. 2012;83:1143–1151. doi: 10.1016/j.anbehav.2012.01.035. http://dx.doi.org/10.1016/j.anbehav.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Thompson S, Hankins MW, Foster RG. Mammalian photoentrainment: results, methods, and approaches. Methods Enzymol. 2005;393:697–726. doi: 10.1016/S0076-6879(05)93037-1. http://dx.doi.org/10.1016/S0076-6879(05)93037-1. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Halford S, Foster RG. The evolution of irradiance detection: melanopsin and the non-visual opsins. Philos Trans R: Soc Lond B: Biol Sci. 2009;364:2849–2865. doi: 10.1098/rstb.2009.0050. http://dx.doi.org/10.1098/rstb.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. http://dx.doi.org/10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Pohl H. Spectral composition of light as a Zeitgeber for birds living in the high Arctic summer. Physiol Behav. 1999;67:327–337. doi: 10.1016/s0031-9384(99)00070-0. http://dx.doi.org/10.1016/s0031-9384(99)00070-0. [DOI] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8(2) doi: 10.1186/1740-3391-8-2. http://dx.doi.org/10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitner A, Sharpe LT, Zrenner E. Is colour vision possible with only rods and blue-sensitive cones? Nature. 1991;352:798–800. doi: 10.1038/352798a0. http://dx.doi.org/10.1038/352798a0. [DOI] [PubMed] [Google Scholar]
- Revell VL, Barrett DC, Schlangen LJ, Skene DJ. Predicting human nocturnal nonvisual responses to monochromatic and polychromatic light with a melanopsin photosensitivity function. Chronobiol Int. 2010;27:1762–1777. doi: 10.3109/07420528.2010.516048. http://dx.doi.org/10.3109/07420528.2010.516048. [DOI] [PubMed] [Google Scholar]
- Ripamonti C, Woo WL, Crowther E, Stockman A. The S-cone contribution to luminance depends on the M- and L-cone adaptation levels: silent surrounds? J Vis. 2009;9(10):11–16. doi: 10.1167/9.3.10. http://dx.doi.org/10.1167/9.3.10. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Foster RG. Twilight times: light and the circadian system. Photochem Photobiol. 1997;66:549–561. doi: 10.1111/j.1751-1097.1997.tb03188.x. http://dx.doi.org/10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Hastings JW. Two photoreceptors control the circadian clock of a unicellular alga. Naturwissenschaften. 1988;75:206–207. doi: 10.1007/BF00735584. http://dx.doi.org/10.1007/bf00735584. [DOI] [PubMed] [Google Scholar]
- Ruberg FL, Skene DJ, Hanifin JP, Rollag MD, English J, Arendt J, Brainard GC. Melatonin regulation in humans with color vision deficiencies. J Clin Endocrinol Metab. 1996;81:2980–2985. doi: 10.1210/jcem.81.8.8768862. http://dx.doi.org/10.1210/jcem.81.8.8768862. [DOI] [PubMed] [Google Scholar]
- Rushton WA. Pigments and signals in colour vision. J Physiol. 1972;220:1P–31P. doi: 10.1113/jphysiol.1972.sp009719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci. 2010;30:16262–16271. doi: 10.1523/JNEUROSCI.3656-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.3656-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. http://dx.doi.org/10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbo K, Toyoda JI, Kondo H, Kujiraoka T. Color-opponent responses of small and giant bipolar cells in the carp retina. Vis Neurosci. 2000;17:609–621. doi: 10.1017/s0952523800174103. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. http://dx.doi.org/10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Solessio E, Engbretson GA. Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature. 1993;364:442–445. doi: 10.1038/364442a0. http://dx.doi.org/10.1038/364442a0. [DOI] [PubMed] [Google Scholar]
- Spitschan M, Jain S, Brainard DH, Aguirre GK. Opponent melanopsin and S-cone signals in the human pupillary light response. Proc Natl Acad Sci U S A. 2014;111:15568–15572. doi: 10.1073/pnas.1400942111. http://dx.doi.org/10.1073/pnas.1400942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitschan M, Aguirre GK, Brainard DH, Sweeney AM. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci Rep. 2016;6:26756. doi: 10.1038/srep26756. http://dx.doi.org/10.1038/srep26756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JL. Color sensing in the Archaea: a eukaryotic-like receptor coupled to a prokaryotic transducer. J Bacteriol. 1993;175:7755–7761. doi: 10.1128/jb.175.24.7755-7761.1993. http://dx.doi.org/10.1128/jb.175.24.7755-7761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles WS. Color vision: the approach through increment-threshold sensitivity. Proc Natl Acad Sci U S A. 1959;45:100–114. http://dx.doi.org/10.1073/pnas.45.1. 100. [Google Scholar]
- Stiles WS. Mechanisms of Colour Vision. Academic Press; London; New York: 1978. [Google Scholar]
- Stockman A, Sharpe LT. The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Res. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. http://dx.doi.org/10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Su CY, Luo DG, Terakita A, Shichida Y, Liao HW, Kazmi MA, Sakmar TP, Yau KW. Parietal-eye phototransduction components and their potential evolutionary implications. Science. 2006;311:1617–1621. doi: 10.1126/science.1123802. http://dx.doi.org/10.1126/science.1123802. [DOI] [PubMed] [Google Scholar]
- Svaetichin G, Macnichol EF., Jr Retinal mechanisms for chromatic and achromatic vision. Ann N Y Acad Sci. 1959;74:385–404. doi: 10.1111/j.1749-6632.1958.tb39560.x. http://dx.doi.org/10.1111/j.1749-6632.1958.tb39560.x. [DOI] [PubMed] [Google Scholar]
- Svaetichin G, Negichi K, Fatehchand R. Cellular mechanisms of a Young-Hering visual system. In: de Reuck AVS, Knight J, editors. CIBA Foundation Symposium: Colour Vision. Little Brown & Company; Boston: 1965. pp. 178–207. [Google Scholar]
- Svaetichin G. Spectral response curves from single cones. Acta Physiol Scand Suppl. 1956;134:17–46. [PubMed] [Google Scholar]
- Sweeney BM, Hastings JW. Effects of temperature upon diurnal rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:87–104. doi: 10.1101/sqb.1960.025.01.009. [DOI] [PubMed] [Google Scholar]
- Sweeney AM, Boch CA, Johnsen S, Morse DE. Twilight spectral dynamics and the coral reef invertebrate spawning response. J Exp Biol. 2011;214:770–777. doi: 10.1242/jeb.043406. http://dx.doi.org/10.1242/jeb.043406. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen HH, How MJ, Chiou TH, Marshall J. A different form of color vision in mantis shrimp. Science. 2014;343:411–413. doi: 10.1126/science.1245824. http://dx.doi.org/10.1126/science. 1245824. [DOI] [PubMed] [Google Scholar]
- Tsujimura S, Tokuda Y. Delayed response of human melanopsin retinal ganglion cells on the pupillary light reflex. Ophthalmic Physiol Opt. 2011;31:469–479. doi: 10.1111/j.1475-1313.2011.00846.x. http://dx.doi.org/10.1111/j.1475-1313.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- Twig G, Perlman I. Homogeneity and diversity of color-opponent horizontal cells in the turtle retina: consequences for potential wavelength discrimination. J Vis. 2004;4:403–414. doi: 10.1167/4.5.5. http://dx.doi.org/10.1167/4.5.5. [DOI] [PubMed] [Google Scholar]
- Twig G, Levy H, Perlman I. Color opponency in horizontal cells of the vertebrate retina. Prog Retin Eye Res. 2003;22:31–68. doi: 10.1016/s1350-9462(02)00045-9. http://dx.doi.org/10.1016/s1350-9462(02)00045-9. [DOI] [PubMed] [Google Scholar]
- Uchida K, Morita Y. Spectral sensitivity and mechanism of interaction between inhibitory and excitatory responses of photosensory pineal neurons. Pflugers Archiv. 1994;427:373–377. doi: 10.1007/BF00374547. http://dx.doi.org/10.1007/bf00374547. [DOI] [PubMed] [Google Scholar]
- United States Naval Observatory Astronomical Applications Department. Multiyear Interactive Computer Almanac, 1800–2050. Willmann-Bell; Richmond, VA: 2005. [Google Scholar]
- Vaney DI, Levick WR, Thibos LN. Rabbit retinal ganglion cells. Receptive field classification and axonal conduction properties. Exp Brain Res. 1981;44:27–33. doi: 10.1007/BF00238746. http://dx.doi.org/10.1007/bf00238746. [DOI] [PubMed] [Google Scholar]
- Ventura DF, Zana Y, de Souza JM, DeVoe RD. Ultraviolet colour opponency in the turtle retina. J Exp Biol. 2001;204:2527–2534. doi: 10.1242/jeb.204.14.2527. [DOI] [PubMed] [Google Scholar]
- Viénot F, Bailacq S, Rohellec JL. The effect of controlled photopigment excitations on pupil aperture. Ophthalmic Physiol Opt. 2010;30:484–491. doi: 10.1111/j.1475-1313.2010.00754.x. http://dx.doi.org/10.1111/j.1475-1313.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- Wada S, Kawano-Yamashita E, Koyanagi M, Terakita A. Expression of UV-sensitive parapinopsin in the Iguana parietal eyes and its implication in UV-sensitivity in vertebrate pineal-related organs. PLoS One. 2012;7:e39003. doi: 10.1371/journal.pone.0039003. http://dx.doi.org/10.1371/journal.pone.0039003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner HG, Macnichol EF, Jr, Wolbarsht ML. Opponent color responses in retinal ganglion cells. Science. 1960;131:1314. doi: 10.1126/science.131.3409.1314. http://dx.doi.org/10.1126/science.131.3409.1314. [DOI] [PubMed] [Google Scholar]
- Walmsley L, Hanna L, Mouland J, Martial F, West A, Smedley AR, Bechtold DA, Webb AR, Lucas RJ, Brown TM. Colour as a signal for entraining the mammalian circadian clock. PLoS Biol. 2015;13:e1002127. doi: 10.1371/journal.pbio.1002127. http://dx.doi.org/10.1371/journal.pbio.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Oakley T, Mower J, Shimmin LC, Yim S, Honeycutt RL, Tsao H, Li WH. Molecular evolution of bat color vision genes. Mol Biol Evol. 2004;21:295–302. doi: 10.1093/molbev/msh015. http://dx.doi.org/10.1093/molbev/msh015. [DOI] [PubMed] [Google Scholar]
- Wever RA. The Circadian System of Man. Springer; New York: 1979. [Google Scholar]
- Whitmore AV, Bowmaker JK. Seasonal variation in cone sensitivity and shortwave absorbing visual pigments in the rudd Scardinius erythrophthalmus. J Comp Physiol. 1989;166 http://dx.doi.org/10.1007/bf00190215. [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. J Comp Neurol. 2000;425:545–559. http://dx.doi.org/10.1002/1096-9861(20001002)425:4<545:aid-cne6>3.3.co;2-v. [PubMed] [Google Scholar]
- Zele AJ, Cao D. Vision under mesopic and scotopic illumination. Front Psychol. 2014;5:1594. doi: 10.3389/fpsyg.2014.01594. http://dx.doi.org/10.3389/fpsyg.2014.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv L, Tovin A, Strasser D, Gothilf Y. Spectral sensitivity of melatonin suppression in the zebrafish pineal gland. Exp Eye Res. 2007;84:92–99. doi: 10.1016/j.exer.2006.09.004. http://dx.doi.org/10.1016/j.exer.2006.09.004. [DOI] [PubMed] [Google Scholar]


