Abstract
Importance
Prior studies have demonstrated reduced dendritic spine density in the dorsolateral prefrontal cortex (DLPFC) in schizophrenia. However, it remains unclear how generalizable this finding is in schizophrenia and if it is seen in a historically distinct psychiatric condition, bipolar disorder.
Objective
To assess whether spine loss is present in the DLPFC of subjects with schizophrenia and bipolar disorder.
Design, Setting, and Participants
This study utilized postmortem human brain tissue from subjects with schizophrenia (n=14), bipolar disorder (n=9) and unaffected control subjects (n=19). Tissue samples containing the DLPFC (BA 46) were Golgi-stained, and basilar dendrites of pyramidal cells in the deep half of layer III were reconstructed.
Main Outcomes and Measures
The number of spines per dendrite, spine density, and dendrite length were compared across groups. We also assessed for the potential effects of clinical and demographic variables on dendritic parameters.
Results
Spine density was significantly reduced in bipolar disorder subjects. In schizophrenia subjects, spine density was also reduced, but just missed significance. Relative to control subjects, there was a significant reduction in the number of spines per dendrite in both schizophrenia and bipolar disorder subjects. In addition, both schizophrenia and bipolar disorder subjects had reduced dendrite length.
Conclusions and Relevance
Dendritic spine loss in the DLPFC was seen in both schizophrenia and bipolar disorder subjects suggesting that the two disorders may share some common pathophysiological features.
Introduction
Most excitatory synapses in the brain occur on dendritic spines. Thus, spines play a crucial role in a myriad brain functions1. Two studies observed spine loss on pyramidal cells in the dorsolateral prefrontal cortex (DLPFC) from schizophrenia (SZ) subjects. The first found reduced total spine density in layer III, whereas the second found lower basilar dendrite spine density in the deep half of layer III2,3. The DLPFC plays a key role in working memory4,5 which is commonly impaired in SZ6–8. Indeed, functional imaging studies implicate the DLPFC in the working memory deficits in SZ9–12.
Using similar methods, this study sought to replicate the findings of Glantz and Lewis (2000)3 to determine the generalizability of spine pathology in SZ. To determine if spine pathology occurs in a psychiatric disorder historically distinct from SZ, a cohort of bipolar disorder (BP) subjects was included. Although SZ and BP often differ clinically, they share many features. In both disorders, patients can develop psychosis and exhibit cognitive deficits13. Imaging studies reveal similar brain areas are affected in both disorders14. Genome-wide association studies (GWAS) have revealed multiple shared risk genes15,16. Although spine density has not been studied in BP previously, reduced spine density in the subiculum was observed in a mood disorder cohort17. Therefore, spine loss might be observed in the DLPFC from BP subjects. Similar to Glantz and Lewis (2000)3, we expected that reduced spine density would be observed among subjects with SZ. However, given the differences between the disorders, we hypothesized that any spine loss observed in BP would be, at most, intermediate between SZ and controls.
In addition to the generalizability of spine loss in SZ, the contribution of antipsychotic medication to spine pathology was unclear. In the current study, the potential confounding effects of antipsychotic medication was addressed by comparing SZ subjects who were on and off antipsychotics in the last year of life and by assessing the effects of chronic haloperidol and clozapine administration in the rat medial prefontal cortex (mPFC). We hypothesized that antipsychotic medications would have a minimal impact on spines in the DLPFC from SZ subjects and the mPFC of rats.
Methods
Subjects
Formalin-fixed, postmortem human brain tissue samples containing DLPFC (BA 46) were obtained from the Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA). The cohort included subjects with SZ (n=20), BP (n=18), and control subjects (n=20). Diagnoses were made using Feighner criteria for SZ18, and DSM-III-R (American Psychiatric Association 1987) for BP, and were based on medical records and a questionnaire completed by the donor’s family. In addition to the information required for diagnosis, subjects were assessed for suicide, a history of alcohol abuse or dependence, substance abuse or dependence, a history of cannabis use, antipsychotic medication treatment during the last year of life, and treatment with lithium or valproic acid at the time of death. Each brain was examined by a neuropathologist for gross and microscopic changes consistent with Alzheimer’s dementia, other neurodegenerative disorders, cerebrovascular disease, tumors, trauma and/or alcohol or drug abuse and was excluded from the current study if such changes were present.
Tissue Processing
Tissue samples were dissected to produce two tissue blocks measuring 1.5 cm2 × 0.5 cm and 1.5 cm2 × 0.2–0.3 cm, respectively. The first block was stained using the Golgi-Kopsch method19. In brief, tissue blocks were shaken in a 4% potassium dichromate in 5% paraformaldehyde solution at room temperature in the dark for 96 hours. The potassium dichromate/paraformaldehyde solution was replaced every 36 hours and acid-cleaned glassware was utilized. Tissue blocks were washed in increasing concentrations of silver nitrate (0.25, 0.5, 0.75 and 1%) and then shaken in 1% silver nitrate in acid-cleaned glassware at room temperature in the dark for 1 week. Stained tissue blocks were sectioned using a vibrating microtome (Vibratome, St. Louis, MO) at 100 μm, mounted on gelatin-coated slides, and briefly air-dried. Tissue sections were then dehydrated with a graded series of ethanol, cleared with xylene and coverslipped with Permount (Fisher Scientific). The second block was sectioned using a vibrating microtome at 40 μm, mounted on gelatin-coated slides, and air-dried. The tissue sections were then stained for cytoplasmic ribonucleic acid (Nissl substance) using thionin and cover-slipped.
Pyramidal Cell Reconstruction
Tissue for 4 SZ and 6 BP subjects could not be analyzed due to widespread precipitation and/or poor impregnation of staining reagents into pyramidal cells. All analyses were conducted by a single investigator (G.T.K.) blinded to subject number and diagnosis. Nissl-stained sections were used to confirm localization to DLPFC (BA 46) using cytoarchitectonic criteria20 and to ascertain the borders of layer III as a percentage of cortical thickness. Fifteen Golgi-stained pyramidal cells were selected for reconstruction per subject using the following criteria derived from Glantz and Lewis (2000)3: 1) Somata located in the bottom half of layer III and in the middle of the section thickness; 2) The pyramidal cell is fully impregnated; 3) Somata or dendrites are not obscured by large (>5 μm) staining opacities; 4) No morphological changes associated with postmortem interval (PMI)21; and 5) The presence of ≥3 basilar dendrites each branching at least once. For each selected pyramidal cell, the apparently longest basilar dendrite was selected visually and reconstructed using Neurolucida (version 11, MicroBrightfield Bioscience, Williston, VT) with a Zeiss Axioskop 2 Plus light microscope (Carl Zeiss, Germany) and a ×100 oil immersion objective (NA=1.4, working distance=0.17 mm). Reconstructions were done on live images captured using an CX9000 digital camera (MicroBrightfield Bioscience, Williston, VT) at a final resolution of 1600 × 1200. Each dendrite terminus was classified as ending naturally or artificially at the cut surface of the section.
Antipsychotic Administration in Rats
24 adult, male Sprague Dawley rats receiving haloperidol 1mg/kg/day, clozapine 25mg/kg/day, or sterile saline (n=8 per group) i.p. for 28 days22,23 were euthanized 24 hours after the last injection, and the frontal cortex dissected out. The frontal cortex was then stained using the Rapid Golgi Kit (FD NeuroTechnologies, Columbia, MD). Stained tissue blocks were sectioned using a vibrating microtome (Vibratome, St. Louis, MO) at 100 μm, mounted on gelatin-coated slides and cover-slipped. The longest basilar dendrite on 8 pyramidal cells, with their somata localized in the middle layers (III-V) of the mPFC, was reconstructed in manner similar to that of the postmortem human brain tissue.
Statistical Analyses
Analysis of subject data by one-way ANOVA revealed no differences in mean age, PMI, or pH across groups (p>0.05). However, storage time in formalin did differ across groups (p<0.01) and was included as a covariate in dendritic parameter analyses. To ensure that an optimal statistical model was utilized for each dendritic parameter, each of the following factors were systematically assessed alone and in combination with other factors using ANCOVA models with diagnosis and storage time in formalin included in each model. These included: age, sex, PMI, pH, hemisphere, suicide, history of alcohol abuse or dependence, history of substance abuse or dependence, history of cannabis use, treatment with antipsychotic medication in the last year of life, treatment with lithium at the time of death, and treatment with valproic acid at the time of death. An optimized model was selected for each dendritic parameter using the corrected Akaike’s Information Criterion (AICC)24,25 to identify the simplest, best-fitting model in each case. The AICC, which is the AIC corrected for small samples, resolves the “bias-variance tradeoff” in model selection by determining which covariates to include (to remove bias) and which to exclude (to minimize variance). A test-wise false-positive error rate was set at 0.05, thus controlling for potential experiment-wise errors. For any parameter having a significant ANCOVA effect for diagnosis (p<0.05), post-hoc pairwise comparisons were conducted using Dunnett’s method to control for multiple comparisons. Since we expected to replicate the findings of Glantz and Lewis (2000)3, post-hoc pairwise comparisons (again using Dunnett’s method) for spine density and dendrite length were conducted using one-tailed tests, all other analyses were conducted using two-tailed tests.
There were subjects in the SZ (n=2), BP (n=3) and control (n=1) groups who had incomplete substance abuse histories in the medical records, thus were not included in the dendritic parameter analyses. Table 1 and eTable 1 in the Supplement summarize the clinical and demographic data of the SZ (n=14), BP (n=9) and control (n=19) subjects included in the analyses.
Table 1.
Clinical and demographic data of subjects included in dendritic parameter analyses.
| Schizophrenia | Bipolar disorder | Control | p-value | |
|---|---|---|---|---|
| Sex (M/F) | 9/5 | 2/7 | 13/6 | |
| Age | 58.9±12.6 | 59.4±22.3 | 56.8±13.6 | n.s. |
| PMI (hours) | 24.9±8 | 23±6.2 | 22±3.8 | n.s. |
| Storage time in formalin (months) | 125.9±17 | 110.7±17.9 | 99±9 | <0.05 |
| pH | 6.7±1 | 6.5±0.3 | 6.4±0.2 | n.s. |
| Hemisphere (R/L) | 9/5 | 5/4 | 11/8 | |
| Suicide (Y/N) | 1/13 | 3/6 | 0/19 | |
| History of alcohol abuse or dependence (Y/N) | 5/9 | 2/7 | 2/17 | |
| History of cannabis use (Y/N) | 5/9 | 2/7 | 2/17 | |
| History of other substance abuse or dependence (Y/N) | 0/14 | 2/7 | 2/17 | |
| Antipsychotic medication (Y/N) | 12/2 | 9/0 | 0/19 | |
| Valproic acid (Y/N) | 5/9 | 2/7 | 0/19 | |
| Lithium (Y/N) | 3/11 | 4/5 | 0/19 |
Values are means ± S.D.
The relationship between the number of spines per dendrite, spine density (i.e., the number of spines per μm dendrite), dendrite length and clinical variables were assessed in SZ and BP groups. The following clinical variables were analyzed: history of alcohol abuse or dependence (yes/no), history of cannabis use (yes/no), history of other substance abuse or dependence (yes/no), suicide (yes/no), antipsychotic medication treatment in the last year of life (yes/no), lithium treatment at the time of death (yes/no) and valproic acid treatment at the time of death (yes/no). Clinical variables were analyzed with t-tests assuming unequal variances and p-values were corrected using the false discovery rate to control for multiple tests. Statistical analyses were conducted using STATA (v. 12, College Station, TX), and false discovery rate calculations were conducted using QVALUE (v. 1)26.
Results
DLPFC Layer III Borders
The upper border position for DLPFC layer III, as a percentage of the distance from the pia to white matter, was 19.3±1.9% for control, 20±2% for SZ, and 19.2±2.6% for BP subjects. The lower border position was 54.7±2.6% for control, 55.1±3% for SZ, and 55.5±4% for BP subjects. Analyses of the upper and lower border positions by one-way ANOVA revealed no differences across groups (p>0.05). Grey matter thickness was 2690.2±719.5 μm for control, 2550.8±343.1 μm for SZ and 2286±435.8 μm for BP subjects, analysis by ANCOVA revealed no differences between groups (p>0.05). Final section thickness was 74.7±14.3 μm for control, 72.6±12.1 μm for SZ and 69.9±10.1 μm for BP subjects, and one-way ANOVA revealed no differences across groups (p>0.05).
Dendritic Parameter Analyses
The Golgi-Kopsch staining method can produce very good staining of DLPFC deep layer III pyramidal cell basilar dendrites and spines in postmortem human brain tissue having prolonged storage in formalin (see Figure 1). However, several SZ and BP subjects had poor staining and were excluded. To elucidate potential sources of suboptimal staining, clinical and demographic variables were assessed between the included and excluded subjects. Continuous variables were assessed using t-tests assuming unequal variances and categorical variables were assessed using chi squared tests. No parameter was significantly different between groups, however pH trended toward significance. Excluded subjects tended to have lower tissue pH 6.2±0.4 vs. 6.4±0.6 (p=0.1). pH of staining solutions is an important parameter in the Golgi-Kopsch staining method19 and a low initial tissue pH might have produced poor staining in select subjects.
Figure 1.
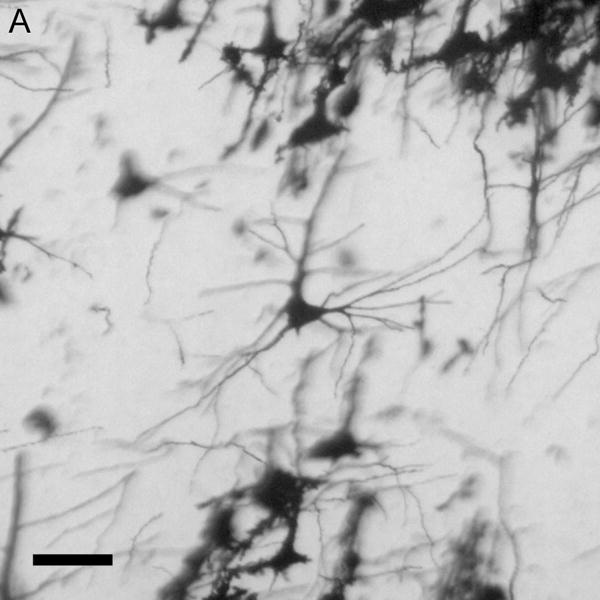
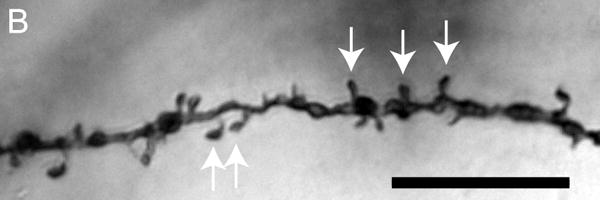
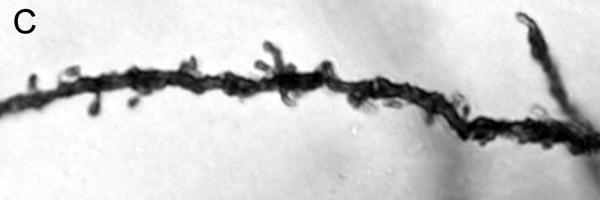
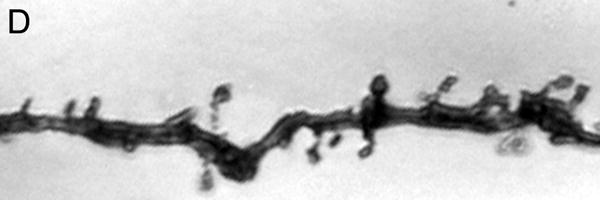
Brightfield photomicrographs of Golgi-stained pyramidal cells having their somata localized in the deep half of layer III. Low power image from a control subject (A). High power images of basilar dendrites from a control subject (B), schizophrenia subject (C) and a bipolar disorder subject (D). Scale bar=50 μm for panel A and 10 μm for panel B.
The ANCOVA model utilized for each dendritic parameter is given in eTable 2 in the Supplement. BP subjects had a significant 10.5% reduction in spine density relative to controls (p=0.024, one-tailed). Spine density was reduced by 6.5% in SZ subjects, which barely missed significance (p=0.061, one-tailed). Significant reductions in the number of spines per dendrite were observed in both SZ (21.6%, p=0.003) and BP (25.8%, p=0.005) subjects. Reduced dendrite length was observed in both SZ (18.3%, p=0.005, one-tailed) and BP (18.6%, p=0.05, one-tailed) subjects. Spine density per dendritic segment did not differ across groups for any segment (p>0.05, see Figure 2). Mean pyramidal cell somal area did not differ among groups, and there were no significant differences across groups for the other dendritic parameters (p>0.05, see Table 2).
Figure 2.

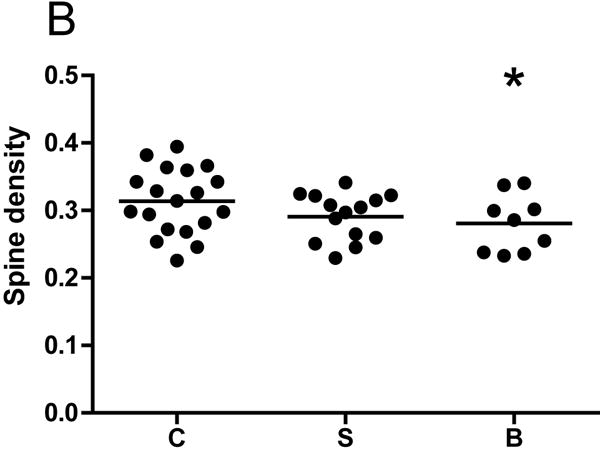
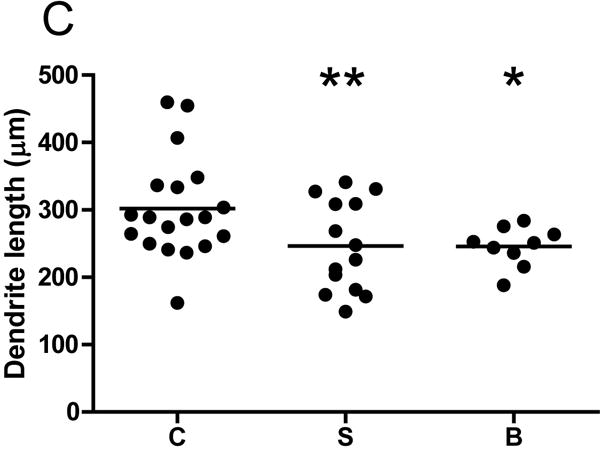
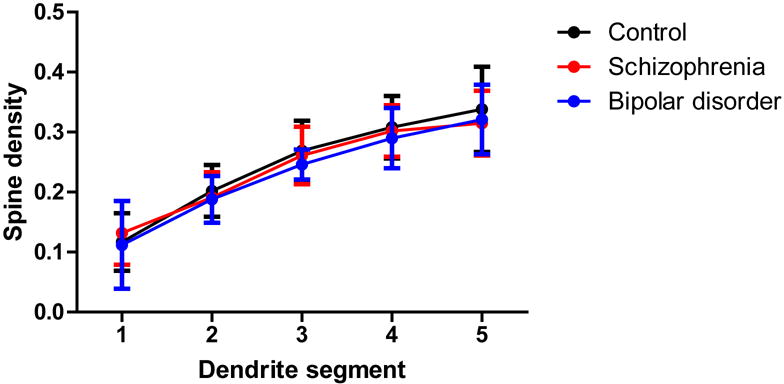
Graphs representing dendritic parameters for DLPFC deep layer III pyramidal cells. The number of spines per dendrite (A), spine density (B), dendrite length (C), and spine density per dendrite segment (D). Data were obtained from reconstructions of Golgi-stained pyramidal cells. C=control, S=schizophrenia, and B=bipolar disorder; * = p <0.05 relative to controls, ** = p <0.01 relative to controls.
Table 2.
Summary of dendritic parameter measurements.
| Parameter | Schizophrenia | Bipolar disorder | Control |
|---|---|---|---|
| Spines per dendrite | 72.8±24.9** | 68.9±12.9** | 92.8±31.1 |
| Spine density (spines per μm) | 0.29±0.03 | 0.28±0.04* | 0.31±0.05 |
| Dendrite length (μm) | 246.5±67.4** | 245.6±29.8* | 301.8±75.1 |
| Somal area (μm2) | 375.4±92.2 | 363.6±45.3 | 378.3±63.4 |
| Number branch segments | 14.8±3.2 | 14.3±2.2 | 14.9±2.1 |
| Max. branch order | 5.3±0.5 | 5.1±0.4 | 5.3±0.4 |
| Number artificial ends | 2.2±1.2 | 1.8±1.2 | 2.2±1.2 |
| Number natural ends | 5.7±1 | 5.9±0.7 | 5.7±0.7 |
Values are means ± S.D.;
=p<0.05 relative to controls,
=p<0.01 relative to controls.
Effects of Clinical Variables
There was no significant effect of any clinical variables on the number of spines per dendrite in either SZ or BP subjects. Among SZ subjects, a history of alcohol abuse or dependence and a history of cannabis use were associated with increased spine density. SZ subjects on lithium had longer dendrites relative to SZ subjects not treated with lithium (see Figure 3).
Figure 3.
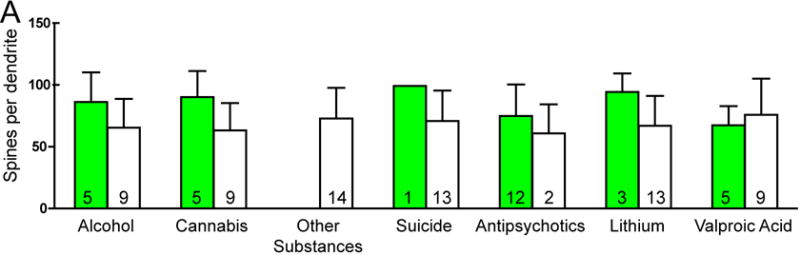
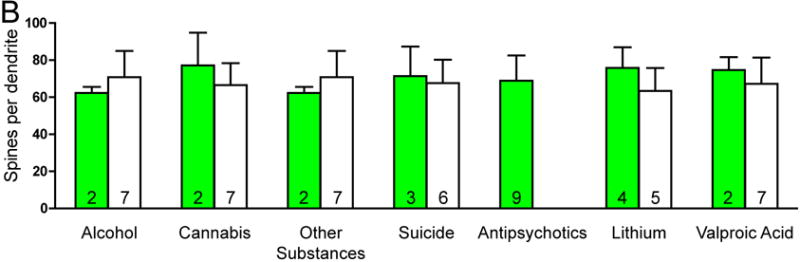
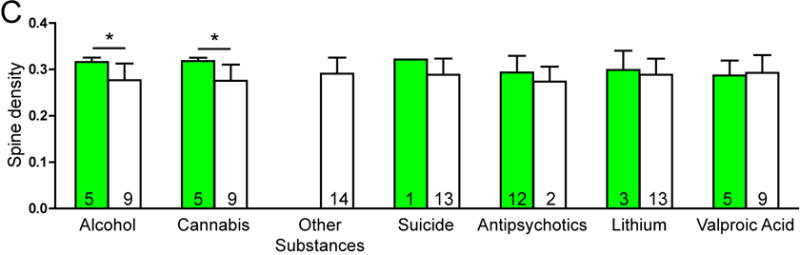
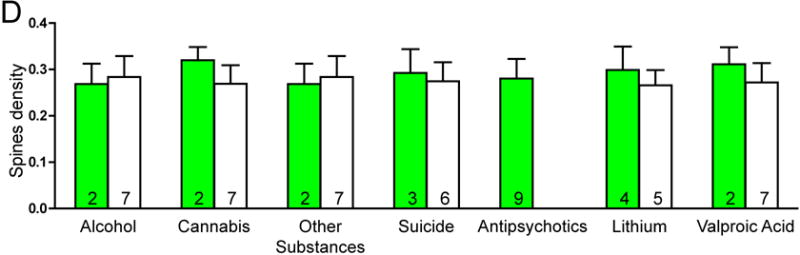
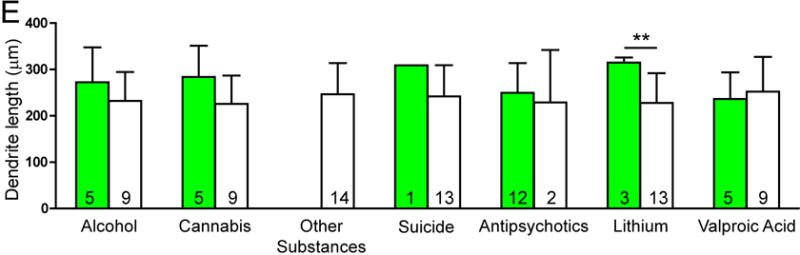
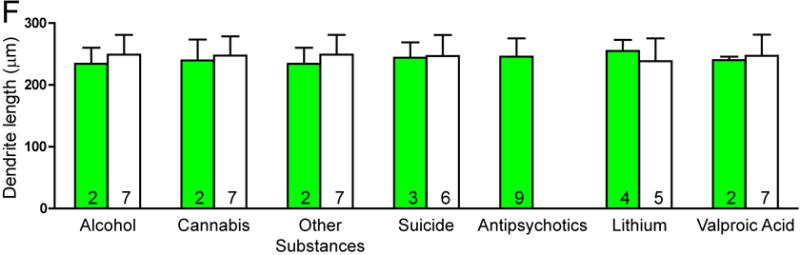
Graphs representing dendritic parameters for DLPFC deep layer III pyramidal cells. The number of spines per dendrite (A), spine density (B), dendrite length (C), and spine density per dendrite segment (D). Data were obtained from reconstructions of Golgi-stained pyramidal cells. C=control, S=schizophrenia, and B=bipolar disorder; * = p <0.05 relative to controls, ** = p <0.01 relative to controls.
Antipsychotic-Administered Rats
A 28-day administration of haloperidol or clozapine had no significant effect on the number of spines per dendrite, spine density, or dendrite length among pyramidal cells in the mPFC of rats (see eFigure 1 in the Supplement).
Discussion
Spine density was significantly reduced in BP subjects. Spine density was also reduced in SZ subjects, but it just missed significance. Both the number of spines per dendrite and dendrite length were significantly reduced in both SZ and BP. These findings are significant for two reasons: 1) spines appear similarly affected in both disorders; and 2) the magnitude of spine density reduction in SZ in the current study (6.5%) is much less than previously reported (23%)3 suggesting variability in spine pathology in SZ. In their excellent recent review, Glausier and Lewis (2013)27, discussed data on spine pathology in SZ, cortical spine density in the context of development, and possible mechanisms which might contribute to reduced spine density. The mechanisms discussed include dysregulation of the actin cytoskeleton, decreased presynaptic activity and/or deafferentation, and impaired energy metabolism among others. Our discussion will focus on synaptic activity and the regulation of the actin cytoskeleton given their potential relevance to both SZ and BP.
The actin cytoskeleton is highly dynamic and is modulated by synaptic activity. Through its effects on actin, synaptic activity regulates the formation, morphology, and maintenance of spines28. Several studies indicate that activity at NMDA-type glutamate receptors are particularly important in the regulation spines via the actin cytoskeleton29–32. Multiple mechanisms could alter NMDA receptor function including deafferentation33, decreased presynaptic release34, altered glutamate cycling and metabolism35, altered astrocyte activity36, and alterations in NMDA receptors or their downstream signaling partners37,38. An exploration of all these mechanisms is beyond the scope of this discussion, but the mechanism with the most relevance to both SZ and BP appears to be altered NMDA receptors and their signaling partners. A review of postmortem data from studies conducted in the DLPFC from subjects with SZ and BP reveals alterations in molecules involved in NMDA receptor signaling. mRNA expression for the NMDA subunit NR1 was reduced in both SZ and BP, and the NR2A subunit was reduced in SZ39. mRNA expression of the NR3A subunit was increased in SZ, but decreased in BP40. NF-L is a post-synaptic density molecule involved in NMDA receptor signaling which binds the NR1 subunit and PSD-95, a molecule involved NMDA receptor clustering. SAP102 also participates in the clustering of NMDA receptors. NF-L protein expression was reduced in SZ41,42 and SAP102 mRNA expression was reduced in BP39. The NR2B subunit and PSD-95 were found to have increased phosphorylation43 and several molecules involved in the trafficking of the NR2B subunit had altered expression in SZ44. Together, these data suggest that alterations in NMDA receptors and their associated signaling partners might contribute to spine pathology in both SZ and BP.
Previous studies have reported reduced somal size of layer III pyramidal cells in the DLPFC from SZ subjects45–47. In contrast to these studies, but in agreement with Glantz and Lewis (2000)3 no change in somal area was found across groups in the current study. This may reflect differences in staining methods48. Studies which found reduced somal size in SZ used Nissl staining, whereas Glantz and Lewis (2000)3 and the current study utilized Golgi staining.
Alcohol abuse or dependence and cannabis use were associated with increased spine density in SZ, but not in BP. Both chronic ethanol and Δ9-tetrahydrocannabinol (THC) reduced spine density in the rat hippocampus49,50. Moreover, cannabis use is associated with an increased risk of SZ in genetically predisposed individuals51–53 and with increased psychosis in SZ patients54. Cannabis appears to accelerate and alcohol might delay or have no effect on psychosis onset55,56. Alcohol use negatively impacts working memory function, but chronic cannabis might improve cognition in SZ patients57,58. Additional research is required to elucidate the potential impact of these substances on spines in SZ subjects.
Potential confounding effects of antipsychotic medications on dendritic parameters were assessed by comparing subjects with SZ off of antipsychotic medications for at least 1 year prior to death with those on antipsychotics (all BP subjects were on antipsychotics). The effects of chronic haloperidol and clozapine administration on pyramidal cell dendritic parameters were also assessed in the mPFC of rats. Antipsychotic medications had no effect on spines or dendrite length. The effects of antipsychotic medications cannot be ruled out entirely, and it is possible that a longer administration in rats (e.g. six months) might have produced an effect. Nevertheless, antipsychotic medications do not appear to be a significant factor in the observed spine pathology. The effects of mood stabilizers were also assessed by comparing SZ and BP subjects who were on and off lithium and valproic acid at the time of death. In SZ, lithium treatment was associated with longer basilar dendrites. Indeed, lithium prevents reductions in hippocampal dendrite length due to chronic stress in rats59. Given the small groups sizes no firm conclusions can be drawn without further research, but lithium treatment might be neuroprotective in SZ patients.
Historically, SZ and BP have been viewed as distinct entities. However, as stated previously, they share many features13–17 suggesting pathophysiological commonalities exist. Unlike GWAS studies, which include thousands of subjects, postmortem studies include orders of magnitude fewer. However, rigorously designed and executed postmortem studies are necessary to understand how risk genes might contribute to the pathophysiology of these disorders. The current study suggests that spine pathology is common to both SZ and BP. Moreover, the study of the mechanisms underlying the spine pathology might reveal additional similarities and differences between the two disorders which could lead to the development of novel biomarkers and therapeutics.
The current study has detected spine losses on pyramidal cell dendrites in deep layer III of the DLPFC in both SZ and BP subjects. These findings suggest that DLPFC spine loss may be a shared pathophysiological feature of both disorders. In addition, altered NMDA signaling might contribute to the observed spine pathology.
Supplementary Material
Acknowledgments
The authors would like to thank Susan Konopaske for reviewing this manuscript. Preliminary data from this manuscript were presented on December, 2013 at the American College of Neuropsychopharmacology.
Funding support: 1K08MH087640-01A1 (GTK), R01MH05190 and P50MH0G0450 (JTC), R24MH068855 and the William P. and Henry B. Test Endowment (FMB).
Footnotes
Potential conflicts of interest: GTK, NL, and FMB-nothing to declare. JTC served as a consultant for Abbvie Laboratories and En Vivo.
Bibliography
- 1.Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007 Jul-Sep;51(2–4):92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998 Oct;65(4):446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000 Jan;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999 Apr;81(4):1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- 5.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000 Jan 1;20(1):485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantelis C, Barnes TR, Nelson HE, et al. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997 Oct;120(Pt 10):1823–1843. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992 Dec;49(12):975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Puschel J, Sauter BH, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: a 4-month follow-up study. Biol Psychiatry. 1999 Aug 1;46(3):392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- 9.Manoach DS, Gollub RL, Benson ES, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000 Jul 15;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 10.Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009 Jan;35(1):19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998 Sep;155(9):1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 12.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001 Jul;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 13.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006 Oct;67(10):e12. [PubMed] [Google Scholar]
- 14.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010 Mar;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013 Apr 20;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schizophrenia Psychiatric Genome-Wide Association Study Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011 Oct;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosoklija G, Toomayan G, Ellis SP, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000 Apr;57(4):349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 18.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972 Jan;26(1):57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 19.Rosoklija G, Mancevski B, Ilievski B, et al. Optimization of Golgi methods for impregnation of brain tissue from humans and monkeys. J Neurosci Methods. 2003 Dec 30;131(1–2):1–7. doi: 10.1016/j.jneumeth.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999 Mar;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams RS, Ferrante RJ, Caviness VS., Jr The Golgi rapid method in clinical neuropathology: the morphologic consequences of suboptimal fixation. J Neuropathol Exp Neurol. 1978 Jan;37(1):13–33. doi: 10.1097/00005072-197801000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Merchant KM, Dobie DJ, Filloux FM, Totzke M, Aravagiri M, Dorsa DM. Effects of chronic haloperidol and clozapine treatment on neurotensin and c-fos mRNA in rat neostriatal subregions. J Pharmacol Exp Ther. 1994 Oct;271(1):460–471. [PubMed] [Google Scholar]
- 23.Linden AM, Vaisanen J, Lakso M, Nawa H, Wong G, Castren E. Expression of neurotrophins BDNF and NT-3, and their receptors in rat brain after administration of antipsychotic and psychotrophic agents. J Mol Neurosci. 2000 Feb-Apr;14(1–2):27–37. doi: 10.1385/JMN:14:1-2:027. [DOI] [PubMed] [Google Scholar]
- 24.Hurvich CM, Tsai CL. Regression and time series model selection in small samples. Biometrika. 1989;76(2):297–307. [Google Scholar]
- 25.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. International Symposium on Information Theory. 2nd. Budapest: Akademia Kiado; 1973. pp. 267–281. [Google Scholar]
- 26.Storey JD. A direct approach to false discovery rates. J Roy Statist Soc Ser B. 2002;64:479–498. [Google Scholar]
- 27.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013 Oct 22;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008 May;9(5):344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 29.Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998 Dec 1;18(23):9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003 May 8;38(3):447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 31.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999 Mar 19;283(5409):1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004 Oct;7(10):1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 33.Ulas J, Monaghan DT, Cotman CW. Plastic response of hippocampal excitatory amino acid receptors to deafferentation and reinnervation. Neuroscience. 1990;34(1):9–17. doi: 10.1016/0306-4522(90)90300-s. [DOI] [PubMed] [Google Scholar]
- 34.Baskys A, Malenka RC. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol. 1991 Dec;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibbs ME, O’Dowd BS, Hertz L, Robinson SR, Sedman GL, Ng KT. Inhibition of glutamine synthetase activity prevents memory consolidation. Brain Res Cogn Brain Res. 1996 Jul;4(1):57–64. doi: 10.1016/0926-6410(96)00020-1. [DOI] [PubMed] [Google Scholar]
- 36.Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008 Jun 25;28(26):6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Single FN, Rozov A, Burnashev N, et al. Dysfunctions in mice by NMDA receptor point mutations NR1(N598Q) and NR1(N598R) J Neurosci. 2000 Apr 1;20(7):2558–2566. doi: 10.1523/JNEUROSCI.20-07-02558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westphal RS, Tavalin SJ, Lin JW, et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999 Jul 2;285(5424):93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 39.Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008 Aug;33(9):2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- 40.Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res. 2004 Dec 1;71(2–3):361–370. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006 Aug;11(8):737–747. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 42.Pennington K, Beasley CL, Dicker P, et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008 Dec;13(12):1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- 43.Funk AJ, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology. 2012 Mar;37(4):896–905. doi: 10.1038/npp.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr Res. 2010 Jun;119(1–3):198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998 Mar;55(3):215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 46.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001 May;58(5):466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 47.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Somal size of prefrontal cortical pyramidal neurons in schizophrenia: differential effects across neuronal subpopulations. Biol Psychiatry. 2003 Jul 15;54(2):111–120. doi: 10.1016/s0006-3223(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 48.Maldonado-Aviles JG, Wu Q, Sampson AR, Lewis DA. Somal size of immunolabeled pyramidal cells in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2006 Aug 1;60(3):226–234. doi: 10.1016/j.biopsych.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Rubino T, Realini N, Braida D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009 Aug;19(8):763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- 50.King MA, Hunter BE, Walker DW. Alterations and recovery of dendritic spine density in rat hippocampus following long-term ethanol ingestion. Brain Res. 1988 Sep 6;459(2):381–385. doi: 10.1016/0006-8993(88)90656-7. [DOI] [PubMed] [Google Scholar]
- 51.Henquet C, Di Forti M, Morrison P, Kuepper R, Murray RM. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008 Nov;34(6):1111–1121. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987 Dec 26;2(8574):1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 53.Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005 Jul;31(3):608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 54.Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010 Aug;167(8):987–993. doi: 10.1176/appi.ajp.2010.09020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Compton MT, Kelley ME, Ramsay CE, et al. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009 Nov;166(11):1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011 Jun;68(6):555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 57.Yucel M, Bora E, Lubman DI, et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull. 2012 Mar;38(2):316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potvin S, Joyal CC, Pelletier J, Stip E. Contradictory cognitive capacities among substance-abusing patients with schizophrenia: a meta-analysis. Schizophr Res. 2008 Mar;100(1–3):242–251. doi: 10.1016/j.schres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 59.Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004 Mar 16;101(11):3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


