BACKGROUND
It is estimated that 86 million, at least a third of the nation's adult population, are living with "'pre-diabetes,” placing them at high risk for developing type 2 diabetes (T2DM).1 T2DM accounts for 90% of all cases of diabetes, resulting in significant disability, premature mortality, and substantial healthcare costs.2 Genetic predisposition contributes to T2DM risk, but disease development is strongly linked to excess body weight and lifestyle factors. 2 Dietary restriction and participation in moderate physical activity (PA) that results in weight loss (5–7% of total body weight) has been shown to reduce diabetes risk by 58%.3 Additionally, PA has been shown to independently reduce cardiovascular events associated with pre-diabetes.4 Unfortunately, U.S. adults are not physically active; in contrast, they are spending increasingly more time in environments that limit PA and encourage, if not require prolonged sitting and other sedentary behaviors.5
A study from the National Health and Nutrition Examination Survey (NHANES) analyzed PA patterns (defined as moderate to vigorous physical activity, MVPA, in bouts of 8–10 minutes or longer) of a large population-based sample of U.S. adults and found that less than 5% of adults were meeting recommended levels of PA.6 Women were significantly less active than men however activity in both genders declined with age.6 Even though the American Diabetes Association recommends that individuals with pre-diabetes undergo lifestyle modifications that includes MVPA for 30 min/day,7 NHANES data revealed that at least 50% of participants with pre-diabetes were engaging in the least amount of PA (fewest bouts of accumulated PA).8 Additional analyses of NHANES data were performed to examine the prevalence of less intense PA performed in shorter durations and its effect on weight in U.S. adults.9 Most participants engaged in nonbouted (< 10 minutes of PA) rather than bouted activity (> 10 minutes of PA). Nonbouted activity is thought to reflect lifestyle PA, such as parking farther from your destination as opposed to taking a 30-minute walk. Findings showed that PA bout minutes and nonbout minutes were independently associated with BMI. Therefore accumulating PA in nonbouts might be a beneficial starting point to increase PA in sedentary populations at risk for diabetes.
Clearly there is a great need to intervene and forestall diabetes and its associated complications. The development and evaluation of community-based programs that translate proven lifestyle interventions to promote weight loss and PA among high-risk populations are a research priority. Our primary study, Reaching Out to Prevent Increases in Diabetes (RAPID) was a 2-year, NIH-funded study designed to evaluate a collaborative model to prevent T2DM by linking health system efforts to identify low-income adults with pre-diabetes with a community-based lifestyle intervention referred to as the YMCA adaptation of the U.S. Diabetes Prevention Program or YDPP.10 Lay health instructors employed by the YMCA delivered this group-based adaptation of the DPP lifestyle intervention. YDPP goals included losing 5% of baseline body weight and achieving at least 150 min/wk of moderate intensity PA. Twelve month outcomes revealed that RAPID participants achieved meaningful weight loss (2.3 kg (95% CI 1.1 to 3.4 kg). 11
Although it has been established that PA plays a key role in weight loss and weight loss maintenance 12 a recent review of lifestyle interventions revealed that despite including PA as a central intervention component, change in PA as a result of the intervention is rarely reported, making it impossible to determine the most effective features of these interventions on diabetes risk reduction.13 This study presents important information on the effectiveness of an adapted version of the DPP lifestyle intervention on change in PA.
PURPOSE
Therefore, the purpose of this secondary analysis using data from the RAPID study was to examine the ability of YDPP as compared to brief counseling to increase PA in a hard to reach sample of inner city adults over 24 months. Additionally, we examined the influence of psychosocial and community-level variables on PA change.
METHODS
Subjects and Recruitment
A description of the RAPID study protocol, design, and primary outcomes were previously reported.10,11 Briefly, RAPID (N=509) was a two-arm, individually randomized intervention trial comparing brief diabetes prevention counseling alone (control condition, n=252) versus brief counseling plus YDPP (n=257). A computer-generated list was used to identify patients at risk for diabetes from 9 metropolitan primary care clinics. Eligibility criteria included: 1) 18 years or older, 2) BMI ≥ 24 kg/m2, 3) no previous diagnosis of T2DM, 4) serum glucose test indicating high risk for the development of T2DM, (HbA1c level of 5.7–6.4% or fasting plasma glucose of 100–125 mg/dL), and 5) no condition that might contraindicate the gradual adoption of light-moderate PA (e.g. recent cardiovascular event, severe COPD, advanced arthritis, poorly controlled hypertension).
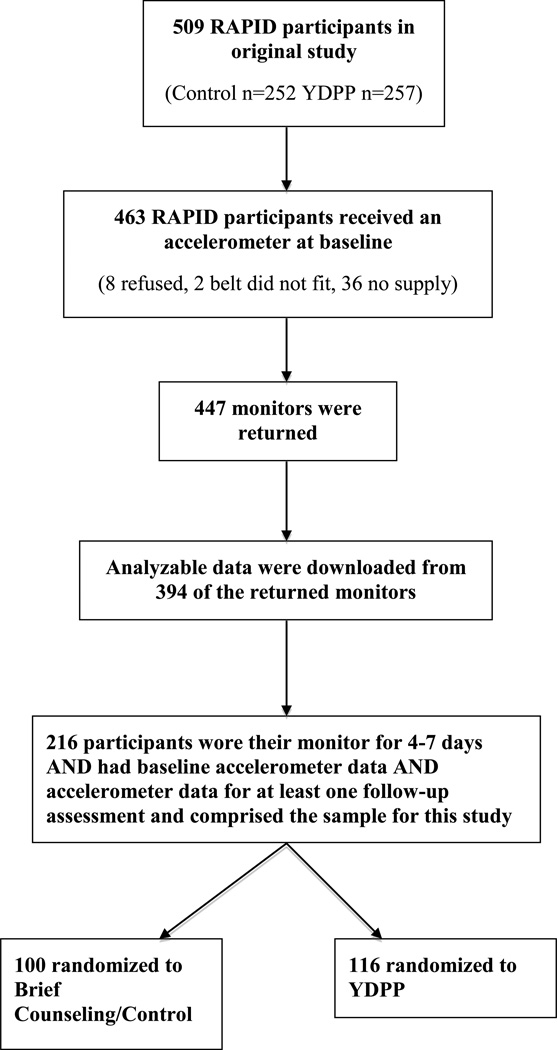
See Figure 1 for a description of the process used to identify participants for the current study. The Indiana University Purdue University Indianapolis and Northwestern University IRBs approved the study protocol and the procedure for obtaining informed consent.
Figure 1.
Study Flow.
Intervention
Brief Counseling (Control)
At all data collection points (baseline, 6, 12, and 24-months), a research assistant briefly delivered information on the importance of weight loss and PA on diabetes risk reduction. The conversation lasted no more than 5 minutes. Participants received a handout describing community resources that supported weight loss and PA programs. At each follow-up assessment, participants received repeat diabetes risk testing and brief lifestyle counseling.
Brief Counseling + YDPP (Intervention)
Participants randomized to the intervention arm received brief counseling plus YDPP. A thorough description of the intervention has been published.10 YDPP was based on the DPP lifestyle intervention14 with two important differences: 1) YDPP was delivered in a group format rather than on an individual basis and 2) YMCA wellness instructors rather than health care professionals delivered program content. YDPP goals included: 1) 7% weight loss and 2) engaging in 150 minutes/week of moderate PA (equivalent to walking). The curriculum included 16 classroom-style sessions that lasted about 60–90 minutes and were held at Y facilities and other community locations. Consistent with the DPP lifestyle intervention, the first eight sessions introduced goal setting, strategies to modify energy intake and output, and stressed the importance of self-monitoring. The latter eight sessions focused on maintaining weight loss and behavior change. Instructors followed specific, but flexible scripts and provided group-based counseling on goal setting, self-monitoring, and problem solving. Following completion of the core curriculum sessions, participants were offered monthly, 60-minute maintenance sessions for the full duration of the trial. YDPP attendance was encouraged but was not required for ongoing participation in the study, consequently we anticipated that a sizeable proportion of participants might not actually participate. On the basis of our pilot research, we anticipated that only 35–38% of YDPP participants would participate at a meaningful level.10 Completion of 9 or more YDPP sessions was defined as a meaningful dose of participation.
Participants were encouraged to engage in brisk walking. YDPP did not offer supervised PA sessions nor did it offer monetary assistance to meet PA goals. Instead, it relied on home-based forms of PA. Instructors worked both individually with participants to identify ways to increase PA in daily lives (i.e., using stairs rather than elevators, parking farther rather than closer to destination) and with groups to identify specific barriers to PA and possible solutions to these barriers.
Accelerometry
All RAPID participants were asked to wear an ActiGraph accelerometer (models GT1M or GT3X; Health Services, Fort Walton Beach, FL) at baseline and after each follow-up assessment. Accelerometers measure and record vertical acceleration as “counts,” providing an indication of the intensity of PA associated with locomotion. 15 Numerous studies have demonstrated that these monitors provide valid and reliable estimates of PA patterns and energy expenditure in a wide variety of populations 16–17 and that the vertical axis output between the GT1M and GT3X are similar.18
The accelerometers were programmed to collect data in 1-min intervals also referred to as epochs. Participants were instructed not to alter their typical pattern of PA during data collection. The monitors were to be worn over the right hip on an elasticized belt for at least 10 hours/day (during waking hours) for 7 consecutive days. Monitors were removed for swimming or bathing. Research assistants demonstrated proper placement for wear and provided instructions for completing a simple daily diary that recorded the number of hours the monitor was worn each day and any activity that took place when the monitor was removed. To reduce suspicion, participants were assured that the monitors only detected PA, not location and did not record conversations.
Preliminary analyses of a sample of the baseline RAPID accelerometer data revealed that 0 participants were engaging in MVPA defined using the “traditional” cut point of 2020–5999 counts/minute.19 Given the predominance of low intensity PA, we felt that it was inappropriate to use a cut point intended to capture high intensity PA; it was decided to use a cut point that could detect changes in low intensity PA in order to detect meaningful changes. Therefore, we decided to use a cut point of 760 counts/minute that has been shown to detect PA reflective of a typical older adult’s free-living activity. 9, 19 All accelerometer counts in this range were summed into nonbouted MVPA. Using nonbouted data allows for the capture of PA sessions as short as 1–3 minutes as opposed to bouted data that requires PA be accumulated into 8–10 minute sessions or bouts of activity. 9 Further, to gauge the extent of sedentary behavior, we examined the average across days of percent of day spent sedentary with a cut point < 100 counts/minute using 1 min epochs.20–21
Since time and transportation were potential barriers to compliance, participants were given a pre-addressed, postage-paid padded envelope to return their monitor by mail, thereby avoiding a return trip to the clinic. Research assistants attempted to contact participants by phone to provide encouragement and support for wearing the monitor, review return procedures, and answer any questions. Participants were mailed a $10.00 gift card upon return of the monitor. Incentives were not tied to the number of days the monitor was actually worn.
Psychosocial and Biometric Variables
At each data collection point (baseline, 6, 12, and 24 months), all participants were asked to complete a limited battery of baseline biometric, metabolic, and survey procedures (see Appendix). Trained research assistants measured height, body weight, blood pressure, hemoglobin A1c, total and HDL-cholesterol. Survey items included demographic and medical history information as well as partial or complete versions of previously developed instruments to assess behavioral mediators including readiness to change, 22–23 self-efficacy24–25, social support,26 diabetes risk perception,27 self-reported PA, 28–29 and perceived health status.30
Community-Level Data
A description of the types of objective community-level data gathered can be found in the Appendix. Community level datasets were developed by The Polis Center, a multi-disciplinary research center with expertise in the research, development and application of Geographical Information Systems technology (GIS). GIS is an integrative technology and mapping tool used to analyze and compare multiple data sets by specific geography. 31 Each participant’s home address was geo-coded using a statewide geo-coding service that returned geographic coordinates and blocked identification for each input address. Please see the Appendix for the definition, measurement, and interpretation of each variable. The returned geospatial attributes were used to link participants with information about the context and composition of their neighborhoods.
Data Analysis
The PA outcomes were calculated from accelerometer counts. The outcomes included: 1) average min/day of MVPA (using cut point of > 760 counts/minute)19 and 2) average across days of percent of day spent sedentary (using cut point < 100 counts/minute) in 1 min epochs20–21 both of which were derived from nonbouted 1-minute epochs
A multistep process was used to determine the validity of accelerometer data. First, both non-compliance and data corruption were assessed by analyzing the mean of the average amplitude between consecutive time points and the mean of the average distance between consecutive time points. Both values were used to formulate the K-statistic that was used to identify participants with invalid data.32 Secondly accelerometer data were considered valid if the mean number of counts/minute was < 800 and the participant wore the accelerometer for at least 10 hrs/day for 4 −7 days.19 Wear time was determined by subtracting non-wear time from 24 hours. Non-wear time was defined by an interval of at least 60 consecutive minutes of zero activity intensity counts, with allowance for 1–2 min of counts between 0 and 100. 33 Only those participants who had valid baseline data and valid data from at least one of the follow-up data collection points were included in the analyses (see Figure 1).
Participants with valid data were compared to non-participants to determine if there were any differences between the groups, using t-tests and Chi-Square tests, for continuous and categorical data respectively. Participants whose data was included in the analyses were slightly older (53.3 years vs. 50.0 years; p=0.0146) than those participants whose data was excluded from the analyses. There were no other significant demographic or anthropometric differences between these groups.
Repeated measures mixed effects models were used to analyze the outcome variables of interest, which were changes in PA since baseline, as measured by accelerometers at 6, 12, and 24 months. These analyses were used to determine the effects of treatment, baseline measures of outcome variables, demographic characteristics, and psychosocial and community-level measures on change in PA. Unadjusted, full, and reduced models were analyzed. All models included baseline measure of PA (whether change in >760 counts/per or change in percent of <100 counts/minute), treatment group, and follow-up assessment (6, 12, and 24 months). In order for parameter estimates to reflect this covariate, the number of follow-up assessments was included in the models. The repeated measures were performed using Restricted Maximum Likelihood (REML) methods, with person as a random effect, allowing for within-participant correlation, as well as the handling of missing data without dropping any participant without complete data throughout the study. Analyses were performed as intention-to-treat, keeping all participants in the original randomized treatment group placement, regardless of compliance. However, accelerometer data were excluded when determined invalid as explained above.
Covariates considered for full and reduced models were (a) baseline demographic and biometric measures (gender, race, age, weight, BMI, and HbA1c); (b) psychosocial measures (perceived health status, PA confidence, health-related quality of life, perceived risk of diabetes, social support for PA, and beliefs about preventing diabetes); and (c) objectively-measured community-level measures (food desert, walk score, crime, median household income, use of public transportation, and vegetation index). Any predictor correlated with either outcome at p-value < .20 for either outcome, MVPA or percent of day spent sedentary, was included in the full model for both outcomes. Correlations were calculated for 12-mo change when available, or the earliest of 6 and 24 mo when 12-mo was missing. Then final reduced models were calculated for each outcome to determine a parsimonious description of factors associated with change in PA, which included variables with p < .10 from the full model. With this method, full models were designed to have the same predictors for both outcomes, but the reduced models were allowed to have different predictors. Reduced models were used to determine the final results. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
See Figure 1 for a description of the study flow. At baseline, 463 of the 509 RAPID participants (91%) received an accelerometer (8 refused, 2 were unable to fit the accelerometer belt around their waists, and 36 participants were never offered an accelerometer due to low supply). Of the 463 monitors that were distributed at baseline, 447 (97%) were returned. Analyzable data were downloaded from 394 (88%) of the returned monitors. Further, 216 (55%) of the 394 participants with analyzable data, wore the monitor for 4–7 days and had valid accelerometer data from both baseline and at least one follow-up assessment. These 216 participants (and thus a total of 432 observations over time) comprised the sample for this study. Among the 216 participants, 100 were assigned to brief counseling and 116 to YDPP plus brief counseling.
Baseline demographic and anthropometric characteristics for participants are presented in Table 1. The pattern of completed follow-up assessments is presented in Table 2. Of the 216 participants in the current study, 17 participants (8%) completed baseline and 6-month assessment, 134 participants (62%) completed baseline, 6 and 12-month assessments, and 37 participants (17%) completed baseline and all follow-up assessments (6, 12, and 24 months). Mean weight loss at 12 months was 3.61±13.89 kg. There was no significant difference between YDPP participants and controls.
Table 1.
Characteristics of Study Populationa
| Characteristics of Study
Population (N = 216) a | |
|---|---|
| Age (years) | 53.3 (11.7) |
| Gender, Female (n=) | 155 (71.8%) |
| Race/Ethnicity (n=) | |
| African American | 108 (50.0%) |
| Non-Hispanic white | 93 (43.1%) |
| Other or Multi-race | 15 (6.9%) |
| BMI (kg/m2) | 36.2 (8.0) |
| Weight (lbs) | 218.3 (52.7) |
| HbA1c (%) | 6.0 (0.3) |
| Treatment Condition | |
| YDPP | 116 (53.7%) |
| Household income (n=186) | |
| < $10,000 | 56 (30.1%) |
| $10–14.999k | 31 (16.7%) |
| $15–24.999k | 36 (19.4%) |
| $25–34.999k | 22 (11.8%) |
| $35–49.999k | 18 (9.7%) |
| $50–74.999k | 6 (3.2%) |
| >$75,000 | 17 (9.1%) |
Data are means ± SD or n (%) unless otherwise stated. Percentages may not add up to 100 because of rounding and frequencies may be less than the study population due to participants not answering income questions.
Table 2.
Pattern of Completed Visits for Baseline and Follow-up Assessments (6, 12, and 24 months since baseline)
| Baseline | 6 mo | 12 mo | 24 mo | Frequency | Percent |
|---|---|---|---|---|---|
| x | x | 3 | 1.4 | ||
| x | x | 16 | 7.4 | ||
| x | x | x | 8 | 3.7 | |
| x | x | 17 | 7.8 | ||
| x | x | x | 2 | 0.9 | |
| x | x | x | 134 | 62.0 | |
| x | x | x | x | 36 | 16.7 |
x=observed visit
Compliance with Accelerometer Wear Schedule and Minutes of MVPA
See Table 3 for the number of valid days of monitor wear at baseline of participants included in statistical analysis. Participants who wore the accelerometer fewer days in the 4–7 day range were: 1) lower income (p<.0001, <25K vs. > 25K); 2) African American (p=.0003, African American vs. white and other); 3) younger (p=.01, for every 10 years younger, the number of days the monitor was worn decreased by 0.24 days); and 4) had a higher perceived risk of developing diabetes with less perceived personal control of diabetes (p=.04). Days of wear were not related to gender (p=0.91), number of children (p=0.30), or BMI (p=0.12); analyses were performed with Chi-Square and Spearman correlations, due to data non-normality.
Table 3.
Sample Response Rates for Number of Valid Days Wearing Accelerometer at Baseline a
| Number of Valid Days |
Frequency | Percent | Cumulative Frequency |
Cumulative Percent |
|---|---|---|---|---|
| 4 | 15 | 6.9 | 15 | 6.9 |
| 5 | 38 | 17.6 | 53 | 24.5 |
| 6 | 76 | 35.2 | 129 | 59.7 |
| 7 | 87 | 40.3 | 216 | 100 |
A valid day was defined as having 10 or more hours of accelerometer wear.
Accelerometer counts were based on PA strong enough to be recognized by the device and turned into electric signals for analysis. At baseline, accelerometers recorded a median of 227 counts per minute ((Inner quartile range (IQR)= (25th percentile, 176 counts; 75th percentile, 288 counts)); at 6 months a median of 233 counts per minute ((IQR=(171, 308)); at 12 months a median of 230 counts per minute ((IQR=(161, 294)); and at 24 months a median of 207 counts per minute ((IQR=(173, 263)). In terms of raw, non-change data across the study, both minutes of MVPA (>760 counts/minute) and percentage of the day spent sedentary (<100 counts/minute) remained constant over the course of the study. MVPA ranged from 64.2 to 67.3 minutes/day for the control care group and from 60.3 to 65.4 minutes/day for the YDDP group. Sedentary percent ranged from 62.0% to 63.5% for the control group and 62.6% to 64.8% for the YDDP group. There were no statistically significant changes in either measure or between treatment groups at each time point.
YDPP Attendance Rates
Additionally, among the 116 participants in the YDPP arm, 82 (70.7%) attended > 1 YDPP session and 60 (51.7%) completed > 9 sessions with a mean attendance of 8.4 (SD=7.1) out of 16 sessions. Change in MVPA and percent of day spent sedentary at 6, 12, and 24 months were not significantly correlated (p>0.05) with the number of YDPP sessions attended (correlations not shown).
Change in Physical Activity Unadjusted Treatment Effects
For both outcomes, neither treatment (control vs. YDPP) nor follow-up assessments (6, 12, and 24 months) main effects had a significant association. Baseline measures corresponding to the PA change outcome (MVPA or percent of day spent sedentary) were inversely related to change in PA exhibiting a regression to the mean effect: lower MVPA at baseline was associated with more increase in activity and higher percentage of day spent sedentary at baseline was associated with less increase in time sedentary (both p<.001). The number of follow-up assessments completed was not significant for either outcome.
Change in Physical Activity Final Models
MVPA
After adjusting for the covariates baseline PA, perceived health, worry, crime, and vegetation index, there was a marginal trend in treatment main effect on change in MVPA across time (p=.052, Table 4). Participants in the control group had no significant change in MVPA on average (least square mean= 2.0, SE=3.8) while on average those in the intervention group decreased the amount of MVPA (least square mean=− 7.1, SE= 3.3).
Table 4.
Repeated Measures Mixed Effect Model of Change Since Baseline in Moderate Vigorous Physical Activity (MVPA)*
| Effect | Estimate (Std Err) | p-value |
|---|---|---|
| Baseline MVPA ≥ 760 counts/min |
−0.33 (0.05) | <.001 |
| Follow-up Assessment* | 0.400 | |
| • 6 months | 0.34 (2.86) | |
| • 12 months | −3.91 (2.83) | |
| • 24 months | −4.02 (5.69) | |
| Treatment Assignment | 0.052 | |
| • Control | 2.00 (3.75) | |
| • YDPP | −7.06 (3.34) | |
| Perceived Health | 10.61 (3.13) | 0.001 |
| Worry | −4.12 (3.15) | 0.192 |
| Crime | −0.18 (0.08) | 0.019 |
| Vegetation Index | −115.03 (44.91) | 0.012 |
Values are means (standard deviations) of minutes/day participants had moderate-vigorous physical activity levels using accelerometer cut point of > 760.
Baseline MVPA was inversely associated (p<.001) with MVPA change since baseline, showing a regression to the mean after adjusting for other baseline covariates. The higher participants perceived their health, the less they increased MVPA (p=.001). The higher participants perceived their health, the less they increased MVPA (p=.001). Worry about health was retained for the final model, but was not significantly related (p=0.192). More crimes per 1,000 people in the geographic area were associated with lower MVPA over time (p=0.019). Higher vegetation index was related to increases in MVPA (p=.012).
Sedentary activity
For percent of day spent sedentary (Table 5) after controlling for the covariates, baseline percent of day spent sedentary, perceived health, crime, and vegetation index, the treatment effect was not statistically significant (p=.144). Participants in the control group had no significant change in percent of day spent sedentary at follow up (least square mean= −0.0, SE=0.1), nor did those in the intervention group (least square mean=0.1, SE=0.1). The higher participants perceived their health, the more they increased the percentage of day spent sedentary behavior (p=.025). A trend in crime (p=0.071) showed more crimes per 1,000 people in the geographic area were associated with an increase in percent of day spent sedentary on average. Lower Vegetation Index was associated with increasing percent of day spent sedentary (p=0.008).
Table 5.
Repeated Measures Mixed Effect Model of Change Since Baseline in Percent of Day Spent Sedentary*
| Effect | Estimate (Std Err) | p value |
|---|---|---|
| Baseline Percent of Time Sedentary |
−0.21 (0.05) | <.001 |
| Follow-up Assessments | 0.175 | |
| • 6 months | −0.01 (0.01) | |
| • 12 months | 0.00 (0.01) | |
| • 24 months | 0.01 (0.01) | |
| Treatment Assignment | 0.144 | |
| • Control | −0.00 (0.01) | |
| • YDPP | 0.01 (0.01) | |
| Perceived Health | −0.02 (0.01) | 0.025 |
| Crime | 0.00 (0.01) | 0.071 |
| Vegetation Index | −0.27 (0.10) | 0.008 |
Values are means (standard deviations) of percent of time participants had low physical activity levels using accelerometer cut point of < 100 counts/min
DISCUSSION
The PA outcomes for RAPID participants were disappointing. We offered a group-based adaptation of the DPP to a sample of hard to reach urban adults at high risk for developing T2DM. RAPID improved upon the design of many recent translational studies by using an objective measure of PA, by following changes in MVPA using a cut point analogous to our participants’ level of activity as well as the percentage of time spent sedentary over 24 months. Additionally, we examined the influence of social and physical environments on PA. RAPID participants did lose weight but they did not significantly change their PA. Despite assembling the required resources and implementing a ‘real-world’ translation of the DPP, YDPP failed to have a significant effect on increasing PA.
Numerous studies have developed and tested lifestyle interventions to decrease diabetes risk. Despite the fact that PA is routinely included as a component of these interventions, it is not consistently reported as a study outcome. In a recent review of DPP-translated lifestyle interventions, all of the reviewed studies included PA as a component of the interventions, yet only half (n=6) reported on changes in PA post-intervention. 13 Similarly, in a review of community-based, translational interventions, less than 1/3 of the studies reported PA outcomes. 34 Additional reviews describe the evidence for translated interventions to increase PA as weak.34–35 Subsequently, there is a need for such studies to report PA outcomes, even if the results are negative. This inadequate reporting of PA outcomes weakens the external validity of this literature and consequently limits both the generalizability of the findings and the ability to determine which factors are critical to T2DM prevention.35
We believe there are several factors that affected the results of our study. These include: compliance with accelerometer wearing schedule, limitations in the ability to measure PA in sedentary adults, issues related to YDPP, and environmental and personal factors.
Compliance with Accelerometer Wearing Schedule
It is recommended that accelerometer data be collected for at least 10 hours/day for 3 to 5 days 17 and since activity levels might vary between week and weekend days, 7 consecutive days of monitoring are preferred.19 Despite using several approaches to promote compliance (e.g., monitoring log, reminder phone calls, in-person instruction on protocol), only 62% of RAPID participants wore the monitor as recommended at baseline and this percentage fell over the course of the study. NHANES used data from individuals with just > 1 day of 10-hour wear time.6 Lowering the required number of days of wear increased the proportion of useable data from 26% (participants with 7 days of > 10-hour wear) to 68% (participants with > 1 day of 10-hour wear). Clearly lowering wearing requirements allows more data to be analyzed. However, adherence rates increase but the effect of fewer days of wear on the accuracy of PA estimates must be considered.
Tucker and Welk (American College of Sports Medicine 2010 Annual Meeting) examined the effect of adherence rates on estimates of PA. Researchers manipulated the minimum number of daily wear-hours (i.e., 8, 10, and 12 hours) and the minimum number of valid days required (i.e., 3, 5, and 7 days). A total of 9 wear-time scenarios were established, ranging from the least (8 hours/day for 3 days) to the strictest (12hours/day for 7 days). Depending on the wearing schedule, adherence rates ranged from 12.5% (n = 884) to 85.9% (n = 6,090), with rates declining as criteria for wear became more demanding. Most importantly, PA estimates (Mean ± SD) varied from 33.6 ± 52.7 min/d to 38.4 ± 66.0 min/d depending on the required amount of wear time, thereby affecting the reliability of the PA estimates.
Given the increasing popularity of accelerometer use, research is needed to identify factors related to monitor wear. Among a sample of older adults (N=201), wearing an accelerometer for > 7 days was associated with being Caucasian and reporting higher income, more education, better cognitive functioning, and less lower body dysfunction. 36 In the current study, African American, lower income, and younger participants were less likely to wear their accelerometer as instructed. Interestingly, participants with higher perceived risk of developing diabetes and who reported less perceived personal control of diabetes were also less likely to wear their accelerometers. BMI was not associated with compliance, discounting the idea that heavier participants would feel more self-conscious about wearing a waist-mounted monitor.
Defining physical activity for sedentary adults
RAPID participants spent a large portion of their day engaging in low intensity or sedentary activities. Although physical inactivity has been associated with health risks, few studies measure sedentary behavior. Instead study participants described as “sedentary” simply did not meet the criteria used to define MVPA.21 To derive estimates of percentage of day spent in PA with varying intensity, pre-defined accelerometer count cut-points or regression equations are applied to accelerometer data.33 These cut points and equations are largely based on tightly controlled treadmill-based protocols using healthy, physically active samples. As a result, there is skepticism surrounding the accuracy of estimates derived from these approaches for certain populations, particularly sedentary, unfit individuals.20, 37 As a result, the development and refinement of these methodologies are needed for high-risk sedentary populations.20,21 This will require developing cut points that have been validated using overweight and obese adults engaging in low intensity PA (i.e., slow walking, sitting and writing, cooking, and light housekeeping) and sedentary behaviors (i.e., sitting, lying down, or working on a computer). 21 Low intensity or lifestyle activities have been shown to increase the metabolic rate and contribute to the total daily energy expenditure. 9, 38 Since sedentary behavior has recently been proposed as a key factor in the obesity epidemic, increasing the amount of light activity may be a more realistic goal for individuals at risk for T2DM rather than engaging in MVPA.38 In addition to focusing on the intensity of the PA, it has also been recommended that cut points should take an individual’s age, 39 body weight, 40 and current cardiopulmonary fitness level 41 into consideration.
Program, personal, and environmental factors
RAPID participants lost a significant amount of weight despite increasing their level of PA (3.61±13.89 kg). Exercise self-efficacy levels were high at baseline and did not lower over the course of the study; but it is possible that participants’ overestimated their ability to change both dietary and PA behaviors. Further, the inherent flexibility that was part of YDPP might have made it easier to skip PA; there were no exercise classes to miss or walking groups to disappoint. It is also possible that since changes in diet were effective, participants had less interest in becoming physically active.
Can we expect that the success and results observed from large successful clinical trials be easily translated to community settings comprised of sedentary adults faced with numerous environmental, social, and health-related barriers? Past reviews of PA promotion among adults demonstrated that such interventions seemed to promote PA in certain populations, namely individuals who were not sedentary but those already slightly active and who were overweight but not obese. It is estimated that for every 17 sedentary adults referred to an exercise program only one becomes moderately active. 42 Even interventions that target disadvantaged groups are less effective in engaging ethnic minority or low-income populations as compared with middle-income white adults. 43 Clearly the search for more effective interventions in disadvantaged groups continues.
Attendance rates for the YDPP sessions were less than ideal (mean 8.4 +7.1). Despite their popularity, many community-based health promotion programs encounter similar problems of low recruitment and retention rates among low-income populations. 44 Commonly reported barriers to participation include cost, access to childcare, lack of time, and low awareness. It is possible that these typical concerns might mask real or more influential reasons for not participating.
Consistent with the literature, 44, 45 RAPID participants with stronger perceived health beliefs, who lived in neighborhoods with more green space and lower crime rates were more likely to engage in MVPA over time. Interestingly, exercise self-efficacy, social support and demographic factors such as age and gender were not significantly related to change in PA. It is possible that although these factors can affect physical activity behavior, there are additional variables keeping participants sedentary.
Perceived social stress may have adversely affected our results. It has been reported that sedentary individuals are more sensitive to criticism of their weight and fitness level and derive less affective pleasure and reinforcement from engaging in PA. 45 Additionally, the unpleasant physical sensations associated with PA, such as sweating, pain, and fatigue, and well as the risk of physical injury may be less tolerated. Participants might have been more receptive to support provided by peers who share characteristics, values, and lifestyles similar to their own. Peer mentors are role models who share experiential knowledge in overcoming comparable barriers to PA and have been shown to positively influence PA adoption. 46,47 Additionally since peer mentors typically volunteer their time or work for very little pay, they provide a cost effective way to provide the long-term support needed to adopt and maintain a PA program.
Limitations
The results of this study contribute important information to the PA literature but certain limitations must be acknowledged. First, participant compliance with accelerometer wear was low. Additionally, the cut-point values chosen for light MVPA and sedentary behavior were based on limited data although with suggestions from published articles. It is recognized that RAPID participants could be considered homogenous in that they were all overweight or obese, had pre-diabetes, and were relatively sedentary as shown by their less intense and low amount of MVPA. There may be additional demographic, behavioral, or environmental factors that are influencing PA in RAPID participants that will require further study. Finally, low number of completed YDPP sessions might have also adversely affected study results.
TRANSLATION TO HEALTH EDUCATION PRACTICE.
The results of this study expand the PA literature on the effectiveness of community-based lifestyle intervention on PA promotion. Participation in YDPP plus brief counseling compared with brief counseling alone was not associated with an increase in PA. RAPID participants live with significant personal, social, and environmental barriers to PA. We need interventions that not only take these factors into consideration, but also work so that participants become and remain physically active. It is possible that the “intensive” strategies borrowed from the DPP were not relevant to RAPID participants. We know that there are a myriad of factors that influence behavioral change, however we need a better understanding of which factors are most influential and when in the decision making process they exert the most influence on behavioral change. We advocate for additional qualitative work; examining the lived experience of individuals faced multiple PA barriers. The use of peer mentors might motivate and empower sedentary adults to become more physically active and be a cost-effective way to provide long-term support. Research is needed to identify accelerometer cut points for sedentary individuals with low cardiopulmonary fitness. It is important that more studies examine and report the effectiveness of lifestyle interventions to promote PA. Changes to the built environment or the provision of safe, low-cost, accessible places to exercise are needed in low-income, urban neighborhoods.
Acknowledgments
This work was supported by grants awarded to Dr. Hays [Indiana University School of Nursing T32 Postdoctoral Training Grant and Indiana CTSI Young Investigator Award, PHS (NCCR), KL2RR025760] and Dr. Ackermann [National Institute of Diabetes and Digestive and Kidney Diseases (R34 DK70702-S1)]. This study is registered in the National Clinical Trials Registry (NCT00656682).
We recognize James Colbert and Karen Comer from the Polis Center of Indianapolis, Indiana for their expertise in geospatial technologies and their assistance with this analysis. We also acknowledge Erin O’Kelly Phillips for her assistance with calibrating, downloading, and tracking our accelerometers.
Appendix. Description of Individual and Community-Level Variables Examined in Analyses
| Variable Name | Description |
|---|---|
| Individual-level Variables | |
| Gender | Male vs. female |
| Race | African American vs. white/other |
| Age | Years |
| Weight | Body weight was measured using a commercial-grade weight scale (lbs) |
| Height | Height was measured using a wall-mounted stadiometer (inches) |
| BMI | (kg/m2) |
| HbA1c | A1c was assessed from a fingerstick
capillary blood sample using a DCA 2000/Vantage portable bench-top analyzer. |
| Self-Efficacy for Exercise |
Self-Efficacy and Exercise Habits
Survey;4 12-items scored from 1 (I know I cannot) to 5 (I know I can); Subscales include: Making Time to Exercise, scored as the mean of 8 individual items (range 1 to 5); and Sticking to an Exercise Program, scored as the mean of 4 individual items (range 1 to 5)a |
| Perceived Health Status |
One item indicator of perceived health
status. “In general, would you say your health is…excellent, very good, good, fair, poor.” Lower scores indicate stronger health perceptions. b |
| Risk Perception Survey for Developing Diabetes (RPS- DD) |
The RPS-DD (Developing Diabetes) is a
43-item survey appropriate for people who are at risk for type 2 diabetes. RPS-DD assesses risk perceptions for developing diabetes and/or its complications, as well as environmental perceived risks. Subscales used: (1) Personal Control: (4 items) a higher score indicates greater perceived personal control over developing diabetes; (2) Worry about Developing Diabetes: (2 items) higher score indicates greater worry for developing diabetes; (3) Optimistic Bias: (2 items), a higher score describes more perceived risk for developing diabetes.c |
| Social Support and Exercise |
Abbreviated Social Support and
Exercise Survey;6 13-items each scored on a 1 (no support) to 5 (supported very often) Likert Scale; Family Participation scored as the sum of 10 items (range for total score 10 to 50); Family Punishment & Rewards scored as sum of 3 items (range for total score 3 to 15).d |
| Community-Level Variables | |
| Food desert | The online Food Desert Locator,
developed by the U.S. Department of Agriculture's (USDA) Economic Research Service (ERS) was used to assess the availability of nutritious food in food deserts, or low-income communities that lack ready access to healthy food. Census tracts in USDA’s food desert spreadsheet were compared to census tracts of study participants. Participants’ whose residential census tract matched a census tract in the USDA food desert dataset were flagged as living in a food desert. Data was downloaded in tabular form from the USDA Food Desert Locator Website in October of 2011. The data was obtained by using the “download the data” tool that leads to an Excel spreadsheet that has a list of all census tracts in the nation that USDA considers to be a food desert. The Healthy Food Financing Initiative Working Group considers a food desert as a low-income census tract (poverty rate is at least 20%) where a substantial number or share of residents has low access to a supermarket or large grocery store. Low access to a healthy food retail outlet is defined as more than 1 mile from a supermarket or large grocery store in urban areas.e |
| Walk Score | Walk Score’s mission is to
promote walkable neighborhoods. It uses a patent-pending system to measure the “walkability” of an address. The Walk Score algorithm awards points based on the distance to resources or amenities. The Walk Score website provides an interface that allows users to enter an address or a set of geographic coordinates and returns a walkability score between 0 and 100, with 100 representing the highest level of walkability and zero representing a location that is completely car-dependent. Walk Score measures resource proximity and density. Resources include grocery stores, bars, movie theaters, libraries, schools, and more. Scores do not distinguish “health-friendly” resources from other types, and do not incorporate aspects like area crime or sidewalk quality. Amenities within .25 miles receive maximum points and no points are awarded for amenities further than one mile. Walk Score uses a variety of data sources including Google, Education.com, Open Street Map, and Localeze. Walking score data were obtained from WalkScore.com’s interface tool during October 2011. We entered geographic coordinates directly into the webpage that returned a walkability score. WalkScore.com also has a batch tool available, but we did not use this for data retrieval for this project.f |
| Crime | Uniform Crime Report (UCR) data were
compiled by the Indianapolis Metropolitan Police Department (IMPD), Crime Analysis Section. These data were submitted by IPD as Part 1 crime statistics to the Federal Bureau of Investigations (FBI) for inclusion in the national Uniform Crime Report to assess and report crime trends in the IMPD jurisdiction.. We downloaded Uniform Crime Report data by 2000 census tracts for Marion County, IN from the SAVI Community Information System, October 2011. The original source was the Indianapolis Metropolitan Police Department (IMPD). Annual data for 2007 and for 2009 was downloaded. The downloaded census tract-level data were matched to the census tracts identified for each participant.g |
| Median Household Income and Use of Public Transportation |
The American Community Survey (ACS) is
a US Census Bureau program designed to supplement the decennial census. The advantage of ACS data is that it is released annually and is therefore more up-to-date than decennia census data. Median family income data was downloaded by 2000 census tracts for Marion County, October 2011. The original source is the 2005–2009 American Community Survey 5-year averages provided by the U.S. Census Bureau. The downloaded census tract data was matched to the census tracts identified for each participant.h |
| Vegetation Index | Normalized Differential Vegetation
Index (NDVI) is a measure of the amount and health of vegetation on the ground as measured by aerial or satellite photography. The value of the index varies from a minimum of negative one in spots where there is no vegetation to a maximum of positive one in areas that have lush, dense vegetation. The measure is sometimes referred to as “greenness.”The raw image was downloaded from IndianaView, a website developed by 14 universities and institutions in Indiana. The Polis Center received the NDVI image from the IUPUI Dept. of Geography. Using ESRI ArcMap software, Polis then calculated the mean NDVI within each of Marion County’s 212 census tracts. The calculation was made using the Zonal Statistics tool within ArcMap’s Spatial Analyst extension, with the Statistics Type option set to Mean. It should be noted that the NDVI only measures the amount and health of the vegetation, not it’s aesthetic values. The raw imagery was obtained by the Landsat 5 satellite on September 30th, 2010. The IUPUI Dept. of Geography then performed the necessary processing to create the NDVI image.I |
Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Educ Res. 1988; 3: 283–292.
Clark DO. Physical activity and its correlates among urban primary care patients aged 55 years and over. J Gerontol Soc Sci. 1999; 54B:S41–S48.
Walker EA, Mertz CK, Kalten MR, Flynn J. Risk perception for developing diabetes: comparative risk judgments of physicians. Diabetes Care. 2003; 26(9): 2543–8. PMID:12941716
Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. Nov 1987;16(6):825–836.
U.S. Department of Agriculture. Food Desert Locator, Economic Research Service. http://www.ers.usda.gov/data-products/food-access-research-atlas/documentation.aspx
Walk Score. Copyright 2012 Walk Score. https://www.walkscore.com/
Indianapolis Metropolitan Police Department. Crime Analysis Section, 50 N. Alabama Street, Room E303, Indianapolis, IN 46204, (317) 327–3525. www.indygov.org/eGov/City/DPS/IPD/home.htm
U.S. Census Bureau. American Community Survey: 5-year averages, 2005–2009. https://www.census.gov/programs-surveys/acs/
AmericaView. National Consortium for Remote Sensing Education, Research, and Applications. IndianaView. http://www.indianaview.org/pdf/IndianaView_FS_MultiSpec.pdf
Reference List
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koivula RW, Tornberg AB, Franks PW. Exercise and diabetes-related cardiovascular disease: systematic review of published evidence from observational and clinical trials. Curr Diab Rep. 2013;3:372–380. doi: 10.1007/s11892-013-0373-0. [DOI] [PubMed] [Google Scholar]
- 5.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Davidson MB, Defronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 8.Farni K, Shoham DA, Cao G, Luke AH, Hayden J, Cooper RS, Dugas LR. Physical activity and pre-diabetes: an unacknowledged mid-life crisis: findings from NHANES 2003–2006. PeerJ. 2014:e499. doi: 10.7717/peerj.499. https://doi.org/10.7717/peerj.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strath SJ, Holleman RG, Richardson CR, Ronis DL, Swartz AM. Objective physical activity accumulation in bouts and nonbouts and relation to rarkers of obesity in US adults. Prev Chronic Dis. 2008;5(4) http://www.cdc.gov/pcd/issues/2008/oct/07_0158.htm. [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann RT, Finch EA, Schmidt KK, et al. Rationale, design, and baseline characteristics of a community-based comparative effectiveness trial to prevent type 2 diabetes in economically disadvantaged adults: The RAPID study. Contemp Clin Trials. 2014;37(1):1–9. doi: 10.1016/j.cct.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann RL, Liss DT, Finch EA, et al. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015;105(11):2328–2334. doi: 10.2105/AJPH.2015.302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigal RJ, Kenny GP, Wasserman DK, Castandea-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 13.Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health. 2010;10:653. doi: 10.1186/1471-2458-10-653. http://www.biomedcentral.com/1471-2458/10/653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;12:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exer. 2005;37(11 Suppl):S582–S588. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- 16.Kong YC, Bassett DR. The technology of accelerometry-based activity monitors: Current and future. Med Sci Sports Exer. 2005;37(11 Suppl):S490–S500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- 17.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exer. 37(11 Suppl):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki JD, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 2011;14(5):411–416. doi: 10.1016/j.jsams.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Matthews CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–S522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 20.Atkin AJ, Gorely T, Clemes SA, et al. Methods of measurement in epidemiology: sedentary behaviour. Int J Epidemiol. 2012;41:1460–1471. doi: 10.1093/ije/dys118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pate RR, O’Neill JR, Lobelo F. The evolving definition of sedentary. Exerc. Sport Sci Rev. 2008;36(4):173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins DS, Hornsby PP, Schorling JB. Stages of change and weight loss among rural African American women. Obes Res. 2001;9(1):59–67. doi: 10.1038/oby.2001.8. [DOI] [PubMed] [Google Scholar]
- 23.Sarkin JA, Johnson SS, Prochaska JO, Prochaska JM. Applying the transtheoretical model to regular moderate exercise in an overweight population: validation of a stages of change measure. Prev Med. 2001;33(5):462–469. doi: 10.1006/pmed.2001.0916. [DOI] [PubMed] [Google Scholar]
- 24.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991;59(5):739–744. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 25.Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Educ Res. 1988;3:283–292. [Google Scholar]
- 26.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 27.Walker EA, Caban A, Schechter CB, et al. Measuring comparative risk perceptions in an urban minority population: the risk perception survey for diabetes. Diabetes Educ. 2007;33(1):103–110. doi: 10.1177/0145721706298198. [DOI] [PubMed] [Google Scholar]
- 28.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 29.Pereira MA, Fitzgerald SJ, Gregg EW, et al. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–S205. [PubMed] [Google Scholar]
- 30.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Humpel N, Owen N, Leslie E. Environmental factors associated with adults’ participation in physical in physical activity: a review. Am J Prev Med. 2002;22(3):188–199. doi: 10.1016/s0749-3797(01)00426-3. [DOI] [PubMed] [Google Scholar]
- 32.Slaven JE, Andrew ME, Violanti JM, Burchfiel CM, Vila BJ. A statistical test to determine quality of accelerometer data. Physiol Meas. 2006;27:413–423. doi: 10.1088/0967-3334/27/4/007. [DOI] [PubMed] [Google Scholar]
- 33.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exer. 2005;37:S582–S590. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- 34.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37:922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 35.Laws RA, St.George AB, Rychetnik L, Bauman AE. Diabetes prevention research a systematic review of external validity in lifestyle interventions. Am J Prev Med. 2012;43(2):205–214. doi: 10.1016/j.amepre.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Gemmill E, Bayles CM, McTigue K, Satariano W, Sharma R, Wilson JW. Factors associated with adherence to an accelerometer protocol in older adults. J Phys Act Health. 2011;8:1152–1159. doi: 10.1123/jpah.8.8.1152. [DOI] [PubMed] [Google Scholar]
- 37.Carr LJ, Mahar MT. Accuracy of intensity and inclinometer output of three activity monitors for identification of sedentary behavior and light-intensity activity. J Obes. 2012:2012. doi: 10.1155/2012/460271. http://dx.doi.org/10.1155/2012/460271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45(8):1493–1500. doi: 10.1249/MSS.0b013e318288a1e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller NE, Strath SJ, Swartz AM, Cashin SE. Estimating absolute and relative physical activity intensity across age via accelerometry in adults. J Aging Phys Act. 2010;18(2):158–170. doi: 10.1123/japa.18.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopes VP, Magalhaes P, Bragada J. Vasques C: Actigraph calibration in obese/overweight and type 2 diabetes mellitus middle-aged to old adult patients. J Phys Act Health. 2009;6(Suppl 1):S133–S140. doi: 10.1123/jpah.6.s1.s133. [DOI] [PubMed] [Google Scholar]
- 41.Ozemek C, Cochran HL, Strath SJ, Byun W, Kaminsky KA. Estimating relative intensity using individualized accelerometer cutpoints: the importance of fitness level. BMC Med Res Methodol. 2013;13:53. doi: 10.1186/1471-2288-13-53. http://www.biomedcentral.com/1471-2288/13/53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams NH, Hendry M, France B, Lewis R, Wilkinson C. Effectiveness of exercise-referral schemes to promote physical activity in adults: systematic review. Br J Gen Pract. 2007;545:979–986. doi: 10.3399/096016407782604866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yancey AK, Ory MG, Davis SM. Dissemination of physical activity promotion interventions in underserved populations. Am J Prev Med. 2006;31:S82–S91. doi: 10.1016/j.amepre.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Withall J, Jago R, Fox JR. Why some do but most don’t. Barriers and enablers to engaging low-income groups in physical activity programmes: a mixed methods study. BMC Public Health. 2011;11:507. doi: 10.1186/1471-2458-11-507. http://www.biomedcentral.com/1471-2458/11/507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sport Exer. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Tang TS, et al. A review of volunteer-based peer support interventions in diabetes. Diabetes Spectr. 2011;24:85–98. [Google Scholar]
- 47.Funnell MM. Peer-based behavioural strategies to improve chronic disease self-management and clinical outcomes: evidence, logistics, evaluation considerations and needs for future research. Fam Prac. 2010;27(suppl 1):i17–i22. doi: 10.1093/fampra/cmp027. [DOI] [PMC free article] [PubMed] [Google Scholar]