Abstract
BACKGROUND
Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting toxicity of several commonly used chemotherapy drugs including taxanes, vinca alkaloids, and platinum compounds. Development of CIPN is highly variable, both in self-reported symptoms and functional consequences, and can be severe enough to alter dose intensity.
PURPOSE
To describe the natural histories of both patient-reported symptoms of chemotherapy-induced peripheral neuropathy (CIPN) and functional impairments in breast cancer patients undergoing taxane-based chemotherapy.
METHODS
Thirty-three breast cancer patients (32 female/1 male; 47.8 ± 11.2 years; n=17 stage II/n=16 stage III) were enrolled. Patients completed self-reports of symptoms and function (e.g., EORTC QLQ-CIPN20) and objective measures of physical function (i.e., balance and gait testing) in an outpatient oncology clinic at five timepoints: (1) baseline - prior to starting chemotherapy, (2–4) before starting subsequent chemotherapy cycles, and (5) 1–3 months after receiving their last taxane infusion.
RESULTS
Significant negative changes in both patient-reported outcomes and objective functional measures were observed. Decreased balance was observed after the first chemotherapy cycle (28% increase in medial-lateral excursion of the center of pressure, p=0.016) and progressed with cumulative exposure (43% increase, p<0.001). Patients also demonstrated slower walking speeds (5% decrease, p=0.003) as they progressed through treatment. These functional deficits were mirrored with increased patient-reported symptom severity for all EORTC QLQ-CIPN20 subscales (all p<0.05).
CONCLUSION
This study longitudinally assessed patient-reported outcomes concurrently with balance and gait testing in patients undergoing taxane therapy. Taxane treatment was associated with the development of clinically-relevant problems in both CIPN symptoms and patient function.
Keywords: gait, balance, breast cancer, CIPN, neuropathies, taxane
Introduction
Significant progress has been achieved in breast cancer detection and treatment, with breast cancer patients comprising one of the largest groups of cancer survivors. However, chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting toxicity of several commonly used classes of chemotherapy drugs, including taxanes, vinca alkaloids and platinum compounds [1]. Peripheral neuropathies have been shown to lead to pain, falls, difficulty in walking and performing activities of daily living (ADLs) in many different populations including the elderly [2], diabetics [3], and cancer patients [4]. CIPN is a common toxicity experienced during and after chemotherapy, impacting up to 60% of patients receiving chemotherapy [5]. Although symptoms improve over time in many patients, full reversibility of symptoms does not always happen, as symptoms persist for at least 6 months in 30% of patients [5].
Development of CIPN due to chemotherapy is highly variable both in self-reported symptoms and functional consequences [4]. Predictive markers for development of CIPN and effective treatment strategies are not well-characterized [6]. Assessing CIPN is commonly performed either through self-reports of functional limitations or laboratory tests of individual nerve function rather than quantitative evaluation of whole-body function. Gait and balance deficits are well known to increase the risk of falls, including a 290% increase in elderly patients [7]. Decreased distal lower extremity strength and loss of sensation, including proprioception, are risk factors for increased falls and balance deficits [8–12]. Patients who undergo treatment with taxane drugs and/or develop CIPN often present with decreased postural stability [13–18,11]. Recent research suggests that CIPN may also be associated with functional impairments during ambulation [19]. Isolated studies of the natural histories of patient-reported outcomes [20] and balance impairments [21] have previously been reported in breast cancer patients undergoing taxane-based chemotherapy; however, little is known about the development of gait impairments in this population or the concomitant development of these varied quality-of-life impairing adverse effects.
The purpose of this study was to describe the natural history of these adverse effects to provide an initial step towards a more comprehensive understanding on how these varied symptoms develop throughout taxane therapy. Our central hypothesis was that cumulative taxane exposure would result in impaired gait and balance as well as self-reported symptoms of CIPN and functional declines in patients undergoing taxane therapy. To test this hypothesis, we evaluated longitudinal changes in objective gait and balance parameters as well as validated patient-reported outcomes in patients undergoing adjuvant/neoadjuvant taxane (paclitaxel or docetaxel) chemotherapy.
Materials and Methods
Patients
Thirty-three breast cancer patients (stages I–III) who were initiating taxane-based chemotherapy were enrolled into the study (Table 1). Patients unable to stand or walk without assistance, having a pre-existing diagnosis of neuropathy of any kind, or having previous exposure to taxane at any time were excluded from the study. Patients who had previous exposure to other chemotherapy or targeted therapy known to be associated with neuropathy (e.g., platinum therapy, bortezomib, vinblastine) within one year of starting in the study were also excluded. Prior exposure to other types of chemotherapy, such as doxorubicin and cyclophosphamide, were allowed. Patients who satisfied the inclusion criteria were enrolled in the study after providing institutional review board-approved informed consent.
Table 1.
Patient demographics and treatment characteristics
| Characteristic | |
|---|---|
| Enrolled No. of patients (%) | 33 (100%) |
| Completed Study | 28 (84%) |
| Dropped Out | 4 (13%) |
| Death | 1 (3%) |
| Sex (f/m) | 32/1 |
| Age (yr) | 47.8 ± 11.2 |
| Mass (kg) | 76.6 ± 22.7 |
| Height (m) | 1.63 ± 0.08 |
| BMI (kg/m2) | 28.9 ± 9.4 |
| Diabetes, No. of patients (%) | 1 (3%) |
| Cancer Stage at Diagnosis No. of patients (%) | |
| I | 0 (0%) |
| II | 17 (52%) |
| III | 16 (48%) |
| IV | 0 (0%) |
| Performance Status No. of patients (%) | |
| 0 | 31 (94%) |
| 1 | 2 (6%) |
| 2 | 0 (0%) |
| Pre-Taxane Treatment, No. of patients (%) Doxorubicin/Cyclophosphamide prior to taxane therapy | 26 (79%) |
| Taxane Treatment, No. of patients (%) | |
| Paclitaxel weekly | 18 (55%) |
| Paclitaxel every 2 weeks | 6 (18%) |
| Docetaxel every 3 weeks | 8 (24%) |
| Weekly Paclitaxel → Docetaxel every 3 weeks (switched after cycle 1) | 1 (3%) |
| Chemotherapy Dose Reductions and Holds | |
| Patients Receiving Dose Holds (n) | 6 (18%) |
| Patients Receiving Dose Reductions (n) | 5 (15%) |
| Pegfilgrastim, No. of patients (%) | |
| No | 19 (58%) |
| Yes | 14 (42%) |
Protocol
This study consisted of quantifying longitudinal changes in functional performance (i.e., standing balance and gait) and patient-reported outcomes as patients received taxane-based chemotherapy. Patient balance, gait, and self-reported outcomes were assessed at 5 timepoints: (1) prior to starting chemotherapy to provide a baseline, (2–4) before starting subsequent chemotherapy cycles, and (5) 1–3 months after receiving their last taxane infusion.
Clinic Measures
Standing Balance
Standing balance tasks were measured in the oncology clinic as previously reported for this patient population [21]. Briefly, 30 second quiet standing trials were performed on a firm surface with eyes closed and collected by trained clinical research coordinators using a custom data collection program and a balance plate (Bertec Corporation; Worthington, OH). We utilized the center of pressure (CoP), the location of the ground reaction force acting on the patients’ feet, to characterize postural stability. The root mean squared excursion in the medial-lateral direction (RMS-ml) characterizes the average lateral displacement of the CoP from its mean position during a trial. Higher RMS-ml values have been prospectively associated with increased risk of falling in an elderly population [22].
Gait
Patients were asked to walk as fast as they could for two 10 meter walking trials in a clinic hallway. All trials were recorded at 30 frames per second using an iPod touch (Apple, Inc.; Cupertino, CA). Custom-built timing gates placed 6 meters apart in the middle of the walkway produced signals visible in the video recordings as patients reached each gate, which were used to quantify time elapsed and steps taken between timing gates. Average step length and walking speed were selected to investigate changes in gait during treatment. Slower walking speed and decreased step length are characteristics of cautious gait and have been previously observed in fall-prone patient populations [23–25].
Modified Total Neuropathy Score (mTNS)
The mTNS is a validated tool that was performed by certified physical therapists on a subset of the patients to provide additional assessments of neuropathy symptoms [26]. This assessment includes patient-reported symptoms as well as objective measures administered by physical therapists to test for the presence and severity of deficits in vibration sensitivity, pinprick sensitivity, deep tendon reflexes, and muscle strength associated with peripheral neuropathy. The mTNS scores were left untransformed on its original 0 to 4 scale. Higher scores on this measure represent worse symptom severity.
Patient-Reported Outcomes (PRO)
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – Chemotherapy Induced Peripheral Neuropathy (EORTC QLQ-CIPN20)
The EORTC QLQ-CIPN20 instrument is a 20-item questionnaire that assesses sensory (nine items), motor (eight items), and autonomic (three items) system interference. Each item is rated on a 4-point scale regarding the extent to which patients experience a given symptom (1-not at all; 4-very much) [20,27]. Subscale scores (sensory, motor, autonomic) were transformed to a 0 to 100 scale, with higher scores indicating high symptom severity. Changes of at least 0.5 standard deviations are defined as being clinically significant [28].
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)
The EORTC QLQ-C30 is a 30-item questionnaire that assesses multiple aspects of health-related quality of life [29]. It is a well-validated measure comprised of six functional scales (physical, role, emotional, cognitive, social, and global health status/quality of life) and nine symptom scales/items (fatigue, nausea and vomiting, pain, dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, financial impact). This instrument was used to assess the impact of cancer and its treatments on the patients over time. The two global health/QOL subscale questions are rated 1-very poor to 7-excellent. All other questions are on a scale from 1 to 4 symptom (1-not at all; 4-very much). Scores for the EORTC QLQ-30 were transformed to a 0 to 100 scale with higher scores on the functional scales corresponding to higher (better) function, while higher scores on the symptom scales corresponding to more severe symptoms.
Brief Pain Inventory - Short Form (BPI-SF)
The BPI-SF was used to assess patient symptoms during the study period. The BPI-SF is valid for use in cancer patients and measures the presence, severity, and interference of pain [30]. BPI-SF scores were linearly transformed to a 0 to 100 scale. Higher scores represent worse symptom severity.
Gait testing and PRO measures for timepoints 1–4 were collected immediately prior to receiving the patients’ first (i.e., baseline) and subsequent taxane cycles (i.e., timepoints 2–4). Median time interval between the completion of chemotherapy and the last testing timepoint was 8.7 weeks (range: 3.1 – 18 weeks). All patient data were included on an intent-to-treat basis. Gait and balance assessments took less than 10 minutes to administer on average. A total of 131 balance and 133 gait tests were included in the statistical analyses.
Statistical Analysis
Linear mixed models for repeated measures with patients as random effects, and testing timepoint as the fixed effect, were used to estimate the changes in the various objective (e.g., balance and gait) and patient-reported (e.g., EORTC QLQ-CIPN20) parameters over time. The Mixed procedure in SAS (Version 9.4; SAS Institute Inc.; Cary, NC) was used to address the missing data in our study, assumed to be missing at random. Least squares means and corresponding contrasts of the fixed time parameters from the mixed models were used to estimate the differences between timepoints. Additionally, Spearman’s Rank-Order correlations were used to test for relationships between RMS-ml and the sensory subscale of the EORTC QLQ-CIPN20 to provide further insight into the relationship between patient physical function and sensory symptoms throughout patients’ chemotherapy treatment. The significance level for all statistical tests was set at α=0.05. Corrections for multiple comparisons were not made due to the exploratory nature of this study.
Results
Five of the 33 accrued patients did not complete the study. Four patients dropped out of the study (n=3 after completing baseline testing, n=1 after completing the 2nd timepoint). The stated reasons for dropping out were scheduling and time constraints. One additional patient died from neutropenia complications of chemotherapy before completing the 2nd timepoint. Thirteen patients received physical therapy while enrolled in the study; however, these treatments were largely isolated to upper extremities. Only one patient received physical therapy that involved the lower extremities, which was referred for muscle and joint pain and included two sessions of strengthening exercises for the upper extremities along with the hip and knee.
All patients received taxane-based chemotherapy. Patients were predominantly treated with weekly paclitaxel infusions (n=18, 55%). Other treatment regimens included dose dense every 2 weeks paclitaxel (n=6, 18%) and docetaxel plus cyclophosphamide (n=8, 24%), both with pegfilgrastim 24 hours after chemotherapy. Planned duration of taxane-based therapy was 12 weeks in all regimens. Six patients had delays and cancellations in their chemotherapy treatment days due to neutropenia (n=5) or neuropathy (n=1). Five patients received chemotherapy dose reductions, with one of these as a result of neuropathy symptoms beginning at cycle 4. This patient could not complete her last chemotherapy treatment due to complications of CIPN. Other reasons for dose reductions included diarrhea (n=1), fatigue (n=1), and neutropenia (n=2).
Changes in the primary outcomes compared to baseline values were observed in objective (i.e., balance and gait) and PRO measures with cumulative taxane exposure (Table 2). Physical function was negatively impacted by cumulative taxane exposure, which was detected by both objective measures. Previously reported balance changes in this patient population — including increased RMS-ml (mean±SD, p-value for maximum percent change from baseline: 43% ± 61%, p<0.001) [21] — were accompanied by decreased step length (4% ± 7%, p=0.004) and walking speed (5% ± 9%, p=0.003). PRO measures showed worsened physical functioning on the EORTC QLQ-C30 (7% ± 17%, p=0.04). Patients also demonstrated worsened sensory (12% ± 12%, p<0.001), motor (6% ± 10%, p=0.02), and autonomic symptoms (9% ± 17%, p=0.01) with cumulative taxane exposure, as measured by the EORTC QLQ-CIPN20. In addition, patient-reported pain, measured by the BPI-SF, increased with cumulative taxane exposure (83% ± 181%, p=0.03).
Table 2.
Primary objective and patient-reported outcomes throughout taxane therapy. Significant negative changes in objective and patient-reported measures were associated with taxane exposure. Means ± SD (p-value for contrast with timepoint 1)
|
|
||||||
|---|---|---|---|---|---|---|
| Outcome Measure | Timepoint 1 | Timepoint 2 | Timepoint 3 | Timepoint 4 | Timepoint 5 | |
|
|
||||||
| Objective Measures | Balance Parameters | |||||
| Patients Assessed (n) | 30 | 27 | 25 | 24 | 25 | |
| Root Mean Square Excursion - Medial-Lateral, RMS-ml (mm) | 3.3 ± 1.1 | 4.2 ± 2.3 (0.02)* | 4.0 ± 1.9 (0.04)* | 4.7 ± 2.1 (<0.001)* | 4.3 ± 1.9 (0.009)* | |
| Spatiotemporal Gait Parameters | ||||||
| Patients Assessed (n) | 33 | 27 | 25 | 22 | 26 | |
| Step Length (m) | 0.69 ± 0.07 | 0.68 ± 0.07 (0.4) | 0.66 ± 0.07 (0.003)* | 0.66 ± 0.07 (0.004)* | 0.67 ± 0.07 (0.2) | |
| Walking Speed (m/sec) | 1.5 ± 0.2 | 1.5 ± 0.3 (0.6) | 1.4 ± 0.3 (0.08) | 1.4 ± 0.3 (0.003)* | 1.5 ± 0.3 (0.7) | |
| Patient-Reported Outcomes | EORTC QLQ-C30 Functional | |||||
| Subscales | ||||||
| Patients Assessed (n) | 29 | 27 | 27 | 27 | 28 | |
| Global Health/QOL | 76.8 ± 16.6 | 70.9 ± 23.4 (0.1) | 76.3 ± 15.4 (0.9) | 76.1 ± 13.9 (0.8) | 80.9 ± 14.9 (0.3) | |
| Physical Functioning | 96.5 ± 7 | 91.8 ± 10.1 (0.02)* | 90.6 ± 12.4 (0.02)* | 91.7 ± 12.1 (0.02)* | 89.9 ± 16 (0.04)* | |
| EORTC QLQ-CIPN20 | ||||||
| Patients Assessed (n) | 33 | 28 | 27 | 27 | 28 | |
| Sensory- Raw Score | 99 ± 2.8 | 94.3 ± 13.7 (0.08) | 91.8 ± 11.6 (<0.001)* | 89.6 ± 12.1 (<0.001)* | 87.6 ± 13 (<0.001)* | |
| Motor- Raw Score | 98.6 ± 2.8 | 95.7 ± 7.8 (0.1) | 94.4 ± 8.3 (0.04)* | 93.1 ± 9.8 (0.02)* | 94.7 ± 8.1 (0.02)* | |
| Autonomic- Raw Score | 98.5 ± 4.8 | 94 ± 10.3 (0.06) | 89.3 ± 15.6 (0.01)* | 93.6 ± 10.1 (0.06) | 95.2 ± 8.5 (0.05) | |
| Brief Pain Inventory - Short Form | ||||||
| Patients Assessed (n) | 28 | 27 | 27 | 27 | 25 | |
| Patients Experiencing Pain (n) | 5 (17%) | 7 (26%) | 10 (37%) | 13 (48%) | 5 (20%) | |
| Mean Pain Severity | 7.3 ± 13.3 | 10.2 ± 15.8 (0.08) | 12.1 ± 18.4 (0.03)* | 13.3 ± 17.5 (0.03)* | 12 ± 14.7 (0.03)* | |
| Pain Interference with Walking | 7.8 ± 15.9 | 7.5 ± 14.2 (0.9) | 10.2 ± 17.3 (0.4) | 9.2 ± 13.6 (0.7) | 9 ± 15.8 (0.74) | |
|
|
||||||
EORTC QLQ-CIPN20 subscales are on a 0 to 100 scale with higher scores representing higher function
Brief Pain Inventory – Short Form values are on a 0 to 100 scale with higher scores representing worse symptoms
indicates significant difference from baseline value (p<0.05)
PRO measures generally reflected the objective functional measures (Fig. 1a), suggesting that cumulative taxane exposure is associated with worsened sensory symptoms and poorer postural control. Additionally, there was an association between patients’ balance and self-reported sensory symptoms 1–3 months after completing taxane therapy, (Spearman’s ρ = −0.51, p=0.010) (Fig. 1b). This relationship was not discernable at any of the other study timepoints (p>0.05).
Fig. 1.
Relationship between patient-reported sensory symptoms and objective balance measure throughout taxane therapy for the entire cohort (a), relationship between individual patients’ RMS-ml and the transformed CIPN20 sensory subscale values 1–3 months after completing taxane therapy (b)
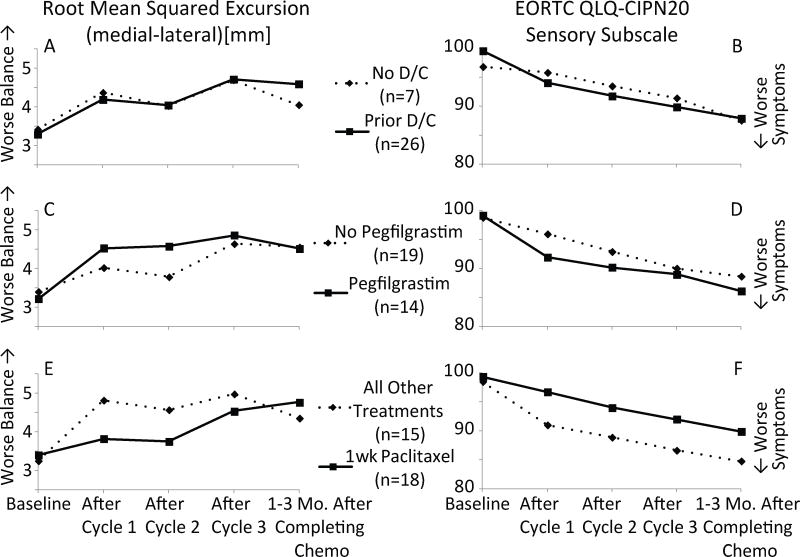
Exploratory analyses were also performed to investigate potential influences that treatment characteristics may have had on the effect of taxane exposure on patient balance (Fig. 2). Overall, none of the subgroup analyses (i.e., the effect of prior doxorubicin and cyclophosphamide exposure, pegfilgrastim, or weekly paclitaxel treatment) reached statistical significance.
Fig. 2.
Effects of treatment characteristics on patient balance during eyes closed quiet stance (a, c, e) and EORTC QLQ-CIPN20 sensory subscale (b, d, f). Rows correspond to subgroup analyses based on prior doxorubicin and cyclophosphamide (i.e., prior D/C) exposure (a, b), whether patients received pegfilgrastim (c, d), and whether patients received weekly paclitaxel vs. other treatment regimens (e, f)
In addition to the physical function related parameters, chemotherapy cycle dependent changes were also observed in specific symptoms (Online Resource 1). The only EORTC QLQ-C30 symptoms that demonstrated significant changes from baseline were increases in the pain (p=0.03), dyspnea (p=0.01), and diarrhea (p=0.04) subscales. Patient-reported fatigue did not change significantly during the study (p>0.1). mTNS subscale scores were dominated by patient scores of zero (i.e., no symptoms), which created convergence issues with the statistical model, and subsequently no statistical comparisons are presented. However, cumulative taxane exposure appeared to be associated with an increased number of patients who presented with symptoms (i.e., scores greater than zero) for the sum and sensory symptom subscales of the mTNS instrument (Online Resource 2).
Discussion
Cancer survivors are at an increased risk of falling and the incidence of falling after chemotherapy is a concern for survivors’ long-term quality of life [19,31,12,10,8]. This study aims to provide clinicians with a more comprehensive understanding of the development of adverse effects associated with taxane therapy including the timing of symptom onset during taxane-based chemotherapy. To the authors’ knowledge, this is the first study to report concurrent longitudinal changes in objective gait and balance measures, as well as established PROs in cancer patients receiving neurotoxic chemotherapy. Patients demonstrated balance and gait deficits which were reflected in the PRO measures (Table 2). These findings provide novel insight into how taxane therapy can impact fundamental aspects of patient function.
The changes observed in objective measures appear to be clinically relevant when compared to deficits previously reported for other fall-prone populations. The balance deficits that generally worsened with additional taxane exposure and persisted 1–3 months after completing chemotherapy exceeded values previously reported for a fall-prone elderly group [22]. Additionally, the decreases in step length and walking speed observed for the fast walking task are recognized as indicators of more cautious gait [25,32,33]. The decrease in walking speed associated with taxane therapy in this study appears to be clinically meaningful when considering the significance of similar decreases with respect to survival rate and falls in previous studies on the elderly [23,34]. Furthermore, it is possible that the deficits in gait parameters may be exaggerated in a more challenging walking environment (e.g., uneven ground), which has previously been observed in elderly patients with peripheral neuropathy [35,36]. These findings augment previous cross-sectional reports of balance and gait impairments associated with chemotherapy [18,13] by providing novel insight into the onset and development of these symptoms with cumulative taxane exposure.
Progressive deficits were also observed in PRO measures among patients with cumulative taxane exposure. The changes observed in EORTC QLQ-CIPN20 subscales in this study are consistent with those previously reported by Loprinzi et al., where sensory symptoms were reported to worsen more than motor or autonomic symptoms [20]. The increasing severity of sensory symptoms with cumulative taxane exposure in our study was corroborated by an increase in the presence of pain via BPI-SF as well as pin prick sensitivity and patient-reported sensory symptoms via mTNS. The physical functioning subscore of the EORTC QLQ-C30 was the only functional subscale that demonstrated a significant change with taxane treatment. This result indicates that patients reported functional deficits that were also quantified in the objective measures; however, the magnitude of the change from baseline to subsequent timepoints was less than for objectively-measured balance. Furthermore, these changes occurred without concomitant changes in fatigue, suggesting that factors other than fatigue likely contributed to these deficits.
The results of this study demonstrate the progression of functional deficits that patients treated with taxane-based chemotherapy may experience; however, future work is needed to establish the underlying mechanisms that drive these functional impairments. While the patient-reported sensory symptoms and decreased pin prick sensitivity may suggest that damage to peripheral somatosensory nerves contributed to these deficits, it is unknown whether previously reported cases of taxane toxicity to optic [37,38] and/or motor nerves [39] or the central nervous system [40] were also contributors. As these systems are all integral to postural control and physical function, future research is warranted to elucidate contributors to the functional deficits suggested by this study.
While the sizes of the subgroups were too small to power reasonable statistical comparisons between subgroups, these preliminary results may warrant further investigations with sufficient subgroup sample sizes (Fig. 2, c–f). Future work may elucidate the role that dose dense paclitaxel or docetaxel plus cyclophosphamide therapy as well as pegfilgrastim have with respect to the onset of balance deficits. In addition to the small subgroup sample sizes, it is difficult to gain insight into the specific nature of the contributions from each treatment characteristic in the current study because the patients comprising ‘non-weekly paclitaxel’ and ‘pegfilgrastim’ subgroups in this study nearly completely overlapped.
Additionally, this study provides initial support for the feasibility and potential utility of implementing objective measures of physical function into the oncology clinic. All tests were administered by trained clinical research coordinators in the clinic while patients waited for their chemotherapy infusion appointments. The comparatively large changes in balance parameters suggest that tracking patient balance may provide an objective measure to detect changes early in treatment. The utility of including an objective measure in clinical assessments of CIPN is supported by Nudelman et al., who suggested that the changes to cerebral structures associated with developing CIPN may increase the risk of patients to underreport symptoms on patient-reported outcomes [40]. By including an objective measure of patient function, clinicians may be better able to detect CIPN symptoms compared to relying solely on patient-reported measures. Functional measures, such as those described here, may also aid oncology rehabilitation clinics in reducing patients’ long-term increases in risk of falling by enabling therapists to establish objective goals for patients’ rehabilitation.
This study provides new insight into the natural history of functional deficiencies in breast cancer patients being treated with taxane-based chemotherapy; however, several limitations exist. Larger samples are needed to validate the current results and adequately establish how these relationships may differ between individuals with different characteristics (e.g., diabetes, elderly, chemotherapy regimen, etc.). However, the sample size of 33 patients enabled us to establish a starting point for understanding the concomitant changes associated with taxane treatment and for demonstrating the utility of objective measures of whole-body function measures in the oncology clinic. Additionally, future studies of the same nature could be strengthened by extending follow-up visits beyond 1–3 months to gain insight into the extent to which patients spontaneously recover from the deficits presented here. Finally, this study focused on one cancer type being treated with a single class of neurotoxic chemotherapy drug. Additional research is needed to understand the extent to which these results translate to patients of other cancer types being treated with different chemotherapy agents. Further research is also needed to validate the methods described here for clinical use.
Conclusion
In summary, the results of this longitudinal study demonstrated clinically relevant problems in both CIPN symptoms and patient function that were associated with cumulative taxane treatment. Taxane therapy was associated with negative changes in both objective, whole-body measures of patient function and patient-reported outcomes. These findings provide initial insight into the natural histories and severities of gait and balance impairments as well as their relationships with common patient-reported symptoms.
Supplementary Material
Acknowledgments
This research was supported by the National Cancer Institute (Grant No. R03 CA182165-01) and the National Science Foundation Graduate Research Fellowship Program (Grant No. DGE-1343012). The authors would also like to thank Tatiana Sedlak and Samuel Seelbach for their assistance with analyzing gait videos.
Footnotes
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Informed Consent: Informed consent was obtained from all participants that were included in this study.
References
- 1.Tofthagen C, Overcash J, Kip K. Falls in Persons with Chemotherapy-Induced Peripheral Neuropathy. Support Care Cancer. 2012;20:583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinetti ME. Preventing Falls in Elderly Persons. New England Journal of Medicine. 2003;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 3.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323–331. doi: 10.1097/01.NNR.0000289503.22414.79. [DOI] [PubMed] [Google Scholar]
- 5.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, Prevalence, and Predictors of Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12(9):619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18(2):141–158. doi: 10.1016/s0749-0690(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 8.Kolb NA, Smith AG, Singleton JR, Beck SL, Stoddard GJ, Brown S, Mooney K. The Association of Chemotherapy-Induced Peripheral Neuropathy Symptoms and the Risk of Falling. JAMA Neurol. 2016;73(7):860–866. doi: 10.1001/jamaneurol.2016.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macgilchrist C, Paul L, Ellis B, Howe TE, Kennon B, Godwin J. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med. 2010;27(2):162–168. doi: 10.1111/j.1464-5491.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 10.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159(2):327–333. doi: 10.1007/s10549-016-3939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miaskowski C, Mastick J, Paul SM, Topp K, Smoot B, Abrams G, Chen LM, Kober KM, Conley YP, Chesney M, Bolla K, Mausisa G, Mazor M, Wong M, Schumacher M, Levine JD. Chemotherapy-Induced Neuropathy in Cancer Survivors. J Pain Symptom Manage. 2017 doi: 10.1016/j.jpainsymman.2016.12.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gewandter J, Fan L, Magnuson A, Mustian K, Peppone L, Heckler C, Hopkins J, Tejani M, Morrow G, Mohile S. Falls and functional impairments in cancer survivors with chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study. Support Care Cancer. 2013;21(7):2059–2066. doi: 10.1007/s00520-013-1766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wampler MA, Topp KS, Maiskowski C, Byl NN, Rugo HS, Hamel K. Quantitative and Clinical Description of Postural Instability in Women With Breast Cancer Treated With Taxane Chemotherapy. Arch Phys Med Rehabil. 2007;88:1002–1008. doi: 10.1016/j.apmr.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Kneis S, Wehrle A, Freyler K, Lehmann K, Rudolphi B, Hildenbrand B, Bartsch HH, Bertz H, Gollhofer A, Ritzmann R. Balance impairments and neuromuscular changes in breast cancer patients with chemotherapy-induced peripheral neuropathy. Clin Neurophysiol. 2016;127(2):1481–1490. doi: 10.1016/j.clinph.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihorst G, Herich L, Grüssinger V, Gollhofer A, Bertz H. Exercise Program Improves Therapy-Related Side-Effects and Quality of Life in Lymphoma Patients Undergoing Therapy. Ann Oncol. 2014;25:493–499. doi: 10.1093/annonc/mdt568. [DOI] [PubMed] [Google Scholar]
- 16.Schwenk M, Garland LL, Grewal GS, Holloway D, Muchna A, Mohler J, Najafi B. Wearable sensor-based balance training in older adult cancer patients with chemotherapy-induced neuropathy: A randomized controlled trial. ASCO Annual Meeting Proceedings. 2015:195. doi: 10.1159/000442253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winters-Stone KM, Torgrimson B, Horak F, Eisner A, Nail L, Leo MC, Chui S, Luoh SW. Identifying factors associated with falls in postmenopausal breast cancer survivors: a multi-disciplinary approach. Arch Phys Med Rehabil. 2011;92(4):646–652. doi: 10.1016/j.apmr.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niederer D, Schmidt K, Vogt L, Egen J, Klingler J, Hubscher M, Thiel C, Bernhorster M, Banzer W. Functional capacity and fear of falling in cancer patients undergoing chemotherapy. Gait Posture. 2014;39(3):865–869. doi: 10.1016/j.gaitpost.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Winters-Stone KM, Hilton C, Luoh S-W, Jacobs P, Faithfull S, Horak FB. Comparison of physical function and falls among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. ASCO Annual Meeting Proceedings. 2016:130. [Google Scholar]
- 20.Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Novotny PJ, Lachance DH. Natural History of Paclitaxel-Associated Acute Pain Syndrome: Prospective Cohort Study NCCTG N08C1. J Clin Oncol. 2011;29(11):1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monfort SM, Pan X, Patrick R, Singaravelu J, Loprinzi CL, Lustberg MB, Chaudhari AM. Natural history of postural instability in breast cancer patients treated with taxane-based chemotherapy: A pilot study. Gait Posture. 2016;48:237–242. doi: 10.1016/j.gaitpost.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49(2):M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 23.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 25.MacAulay RK, Allaire TD, Brouillette RM, Foil HC, Bruce-Keller AJ, Han H, Johnson WD, Keller JN. Longitudinal assessment of neuropsychological and temporal/spatial gait characteristics of elderly fallers: taking it all in stride. Front Aging Neurosci. 2015;7:34. doi: 10.3389/fnagi.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EM, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33(3):173–183. doi: 10.1097/NCC.0b013e3181c989a3. [DOI] [PubMed] [Google Scholar]
- 27.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 28.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5(6):555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 29.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 30.Caraceni A, Cherny N, Fainsinger R, Kaasa S, Poulain P, Radbruch L, De Conno F. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23(3):239–255. doi: 10.1016/s0885-3924(01)00409-2. doi: S0885392401004092. [DOI] [PubMed] [Google Scholar]
- 31.Wildes TM, Dua P, Fowler SA, Miller JP, Carpenter CR, Avidan MS, Stark S. Systematic review of falls in older adults with cancer. J Geriatr Oncol. 2015;6(1):70–83. doi: 10.1016/j.jgo.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45(3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 33.Kirkwood RN, de Souza Moreira B, Vallone ML, Mingoti SA, Dias RC, Sampaio RF. Step length appears to be a strong discriminant gait parameter for elderly females highly concerned about falls: a cross-sectional observational study. Physiotherapy. 2011;97(2):126–131. doi: 10.1016/j.physio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64(8):896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. J Am Geriatr Soc. 2004;52(9):1532–1537. doi: 10.1111/j.1532-5415.2004.52418.x. [DOI] [PubMed] [Google Scholar]
- 36.Richardson JK, Thies SB, DeMott TK, Asthon-Miller JA. Gait Analysis in a Challenging Environment Differentiates Between Fallers and Nonfallers Among Older Patients with Peripheral Neuropathy. Arch Phys Med Rehabil. 2005;86:1539–1544. doi: 10.1016/j.apmr.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Capri G, Munzone E, Tarenzi E, Fulfaro F, Gianni L, Caraceni A, Martini C, Scaioli V. Optic nerve disturbances: a new form of paclitaxel neurotoxicity. J Natl Cancer Inst. 1994;86(14):1099–1101. doi: 10.1093/jnci/86.14.1099. [DOI] [PubMed] [Google Scholar]
- 38.Scaioli V, Caraceni A, Martini C, Curzi S, Capri G, Luca G. Electrophysiological evaluation of visual pathways in paclitaxel-treated patients. J Neurooncol. 2006;77(1):79–87. doi: 10.1007/s11060-005-9008-x. [DOI] [PubMed] [Google Scholar]
- 39.Argyriou AA, Kyritsis A, Matkatsoris T, Kalofonos HP. Chemotherapy-Induced Peripheral Neuropathy in Adults: A Comprehensive Update of the Literature. Cancer management and research. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nudelman KN, McDonald BC, Wang Y, Smith DJ, West JD, O’Neill DP, Zanville NR, Champion VL, Schneider BP, Saykin AJ. Cerebral Perfusion and Gray Matter Changes Associated With Chemotherapy-Induced Peripheral Neuropathy. J Clin Oncol. 2016;34(7):677–683. doi: 10.1200/JCO.2015.62.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.