Abstract
Background:
Teratoasthenozoospermia (TA) is a severe form of male infertility with no clear etiology.
Objective:
To compare the level of intracellular anion superoxide (O2–), heat shock protein A2 (HSPA2) and protamine deficiencies in ejaculated spermatozoa between teratoasthenozoospermic and normozoospermic men.
Materials and Methods:
In this case- control study, semen samples of 20 infertile men, with TA (with normal morphology lower than 4%_ and total motility lower than 40% ) as the case group and 20 normozoospermic fertile men as the control group were evaluated for intracellular O2– and HSPA2 by flow cytometry and protamine deficiency by Chromomycin A3 (CMA3) test.
Results:
The rate of CMA3+ spermatozoa in the case group was higher than controls (p=0.001). The percentages of HSPA2+ spermatozoa in the cases were significantly lower than controls (p=0.001). Also, intracellular O2– levels in the case group were significantly higher than controls (p=0.001) and had positive correlations with sperm apoptosis (r=0.79, p=0.01) and CMA3 positive sperm (r=0.76, p=0.01), but negative correlations with normal morphology (r=-0.81, p=0.01) and motility (r=-0.81, p=0.01). There was no significant correlation between intracellular O2– and HSPA2 in the case group (r=0.041, p=0.79).
Conclusion:
We suggest that the increase in intracellular O2–, decrease in spermatozoa HSPA2+, and high percentages of spermatozoa with immature chromatin might be considered as etiologies of infertility in TA patients.
Key Words: Male infertility, Sperm chromatin, HSPA2, Protamine deficiency
Introduction
Mammalian spermatozoa have the capability to produce various reactive oxygen species (ROS), which are involved in the physiological functions, such as sperm capacitation, oocyte fusion and acrosomal reaction (1). However, these short-lived and highly reactive radicals could potentially compromise lipid and protein content of human spermatozoa, sperm DNA and also sperm function (2). Overproduction of ROS and imbalance between ROS production and antioxidants in semen are the causing factors of oxidative stress, which in turn deteriorate spermatozoa function (4). In addition, there is a relationship between ROS generation and spermatozoa immaturity: there is also a relation between ROS-induced oxidative stress and male infertility (4-6). Infertility has an incidence of about 8% among reproductive age males, and there is a higher production rate of ROS by abnormal spermatozoa with poor motility and morphology in infertile patients than in fertile males with normal functional cells (3, 7, 8).
Anion superoxide (O2−) has been identified as the primary free radical of spermatozoa (2, 9). The O2− is produced by spermatozoa in a commonly rapid and temporary rate, and it is the main stimulator of lipid peroxidation in spermatozoa plasma membrane (2, 9). Mazzilli et al demonstrated that infertile males have a high level of O2−, and this radical exerts the toxic effect on spermatozoa. The severity of this effect depends on the exposure time period and concentration of O2− (10).
Heat shock protein A2 (HSPA2) is a molecular chaperone that assembles in mitochondria, cytoplasm, and reticulum endoplasmic. This molecule has been recognized to have a crucial role in male reproduction, including sperm-egg recognition, protein packaging, and transportation, substitution of histones by protamines during spermiogenesis, inhibition of apoptosis and removal of remained cytoplasm during sperm maturation (11-15). Several recent studies have shown the possible association between HSPA2 and male factor infertility (11, 16).
In fact, reduced expression of HSPA2 is associated with cytoplasmic retention and the resulting excess residual cytoplasm (ERC), has a positive correlation with ROS generation (15, 17). As a result, high levels of ROS, cause to a condition of oxidative stress which characterized by damage to mitochondrial and nuclear DNA (17, 18). HSPA2 has a probable ability to predict in vitro fertilization pregnancy outcomes (19). Also, it has been shown that the depression of HSPA2, is associated with spermatogenic and fertilization impairment in intracytoplasmic injection for azoospermic patients (20).
We suggest that the alternations in the level of intracellular anion superoxide, percentages of HSPA2 and protamine positive spermatozoa might be causes of infertility in terato-asthenozoospermic men. So, we aimed to compare these factors in ejaculated spermatozoa between normozoospermic and idiopathic TA patients.
Materials and methods
In this case-control study, semen samples were provided from 40 men referred to andrology laboratory of Royan Research and Clinical Center for Infertility (Tehran, Iran) from June 2014 to June 2016.
Semen samples were evaluated in two groups. The case group consisted of 20 infertile men with terato-asthenozoospremia (with normal morphology lower than 4% and total motility lower than 40%). A complete medical history was collected from each participant. Cases with leukocytospermia (>1× 106 WBC/ml), cancer, varicocele, genitourinary inflammation, endocrine disorders, autoimmune disease, cryptorchidism and subjects who were smoking or had alcohol consumption which may impact the intracellular O2− were excluded from this study. Also, at the time study began, none of the cases had been pre- treated with antioxidants. The control group consisted of fertile men comprised healthy, normozoospermic (with the same exclusion criteria) whose their partners had successful pregnancies within the last one year.
Sperm collection and analysis
Semen samples were obtained by masturbation after sexual abstinence of 2-4 days. After liquefaction, they evaluated for sperm parameters according to World Health Organization (WHO) criteria (21).
Assessment of sperm viability and morphology
Sperm viability and morphology in 200 spermatozoa per slide were assessed by Eosin/Nigrosin test and Papanicolaou staining, respectively.
Flow cytometry analysis for evaluation of intracellular O 2 − , HSPA2, and apoptotic spermatozoa
The o2− assessment was performed using DHE (Dihydroethidium) (22). Sperm suspension was incubated with 1.25 µM DHE (DHE; Sigma) at 25ºC for 20 min. The intracellular O2− oxidizes DHE and produces ethidium bromide which binds to the DNA and emits red fluorescence, then it analyzed via flow-cytometry (FACS Calibur; BD Biosciences, USA) between 590 and 700 nm. apoptotic spermatozoa were excluded by Yo- pro -1 Iodide (Y3603- Life Technology ) as a counterstaindye for the DHE (22). In this study at least 10000 spermatozoa were assessed for each sample, and data from flow- cytometry were interpreted using Flow-cytometry software (Flowjo 7.6.1) and expressed in percentage.
To analyze HSPA2, samples were washed twice with phosphate-buffered saline (PBS, Gibco, USA), fixed with 4% paraformaldehyde for 20 min at room temperature, and centrifuged for 5 min at 300g. After permeability in 0.5% Triton X-100 for 5 min, test fractions were incubated overnight with the primary anti-HSPA2 antibody (Santa Cruz Co.) at a dilution of 1:100 in 3% bovine serum albumin (BSA; Sigma Co.) at 4oC. Control samples were incubated under the same conditions with 3% BSA. Two samples were washed and incubated with PE- conjugated Donkey anti-goat IgG (1:200, Santa Cruz Co.) in 1.5% BSA at 4oC for 1 hr. After washing, BD FACS Calibur flow-cytometry was used for further analysis (16).
Chromomycin A3 (CMA3) staining for Protamine deficiency assessment
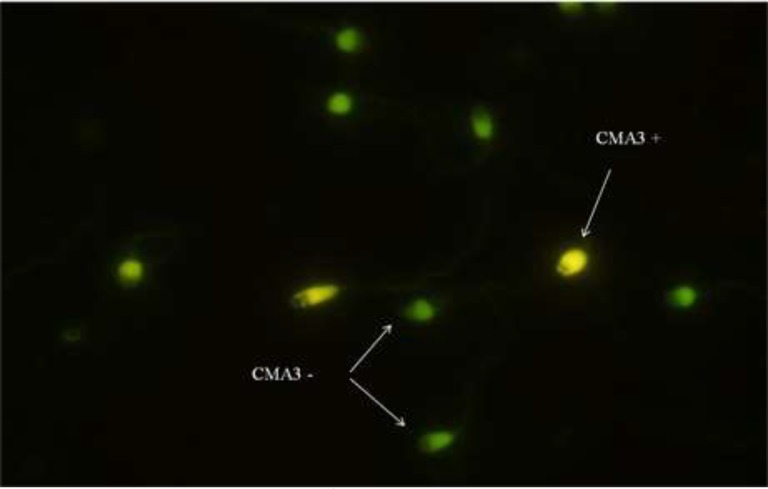
Chromomycin A3 ( CMA3), is an indirect assessment of protamine deficiencies, which competes with protamine to bind DNA (23). It is used for the estimate of the percentage of protamination in sperm chromatin. To do this test, semen samples were washed with PBS, and smears of samples were prepared and dried. Following fixation in Carnoy’s solution (Methanol/Glacial acetic: 3:1) at 4oC for 10 min, slides were stained with 100 µl of CMA3 (0.25 mg/ml) (Sigma Co.) for 25 min in dark at room temperature and mounted with buffered glycerol. Then, 200 spermatozoa were counted under a fluorescence microscope (BX51, Olympus, Tokyo, Japan) at 1000× magnification with oil immersion. Protamine deficient spermatozoa (CMA3-positive) and normal protamine spermatozoa (CMA3-negative) are staining bright yellow and a dull green (Figure1), respectively.
Figure 1.
CMA3 staining: Bright yellow sperm cells (CMA3+) show protamine deficiency and yellowish green sperm cells (CMA3-) show normal protamine content (fluorescent microscopy, ×100 eyepiece magnification).
Ethical consideration
This study was approved by institutional review board of Yazd Research and Clinical Center for Infertility and informs consent forms signed by all participants.
Statistical analysis
The data were presented as mean±SEM, and significant level was defined at p<0.05. Kolmogorov-Smirnov test (K-S, #1862) was used for evaluating normal distribution for quantitative data. Independent-samples t-test was applied in order to compare the parameters between experimental groups. Pearson test was performed for evaluation of the correlation between the percentage of O2− positive sperm and other variables. Statistical analysis was carried out using Stastical Package for the Social Sciences, version 16.0, SPSS Inc, Chicago, Illinois, USA ( SPSS software
Results
There was no significant difference between mean age in case and control groups (33.7±6.8 vs 32.1±5.7, respectively, p=0.173). Sperm concentration, total motility, morphology, vitality and total sperm count were analyzed according to WHO guidelines and reported in table I.
Table I.
The result of sperm parameters in terato- asthenozoospermic (case) and normozoospermic (control) men (n=20/each
| Variables | Control group | Case group | p-value* |
|---|---|---|---|
| Concentration (×106 ml) | 87.62±7.09 | 43.75±7.51 | 0.001 |
| Total motility (%) | 72.10±3.13 | 28.73±1.49 | 0.001 |
| Normal morphology (%) | 7.12±0.8 | 2.2±0.31 | 0.001 |
| Vitality (%) | 86.38±2.86 | 55.63±5.41 | 0.001 |
| Total sperm count (×106) | 121.54±30.38 | 97.32±24.33 | 0.01 |
Values are mean±SE.
independent-samples t-test
Flow cytometry analysis of O 2 − and apoptotic spermatozoa
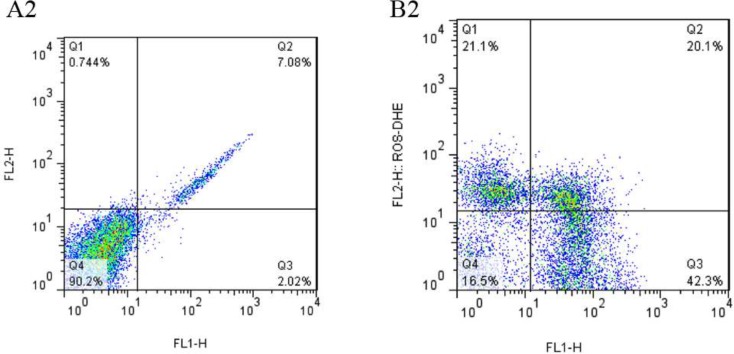
A minimum number of 10000 spermatozoa per sample were analyzed by flow cytometer FACS can (Becton Dickinson). Flow-cytometry data were analyzed with Flowcytometer Software (FlowJo 7.6.1). The percentage of apoptotic spermatozoa (YO+) and level of intracellular O2− were significantly higher in the case group than controls (Table II). Dot plot flow-cytometry histograms by flow-cytometry in two groups are shown in figure 2.
Table II.
The results of the level of interacellular O2- (DHE+), percentage of spermatozoa CMA3+, HSPA22 and apoptotic spermatozoa (YO+) in teratoasthenozoospermic (case) and normozoospermic (control) men ((n=20/each
| Variables | Control group | Case group | p-value* |
|---|---|---|---|
| Spermatozoa CMA3+ | 20.71±0.68 | 36.67±1.69 | 0.001 |
| Spermatozoa HSPA2+ | 46.56±1.81 | 22.86±2.87 | 0.001 |
| Intracellular O2- | 16.35±2.7 | 33.69±1.43 | 0.001 |
| Apoptotic spermatozoa | 20.43±1.89 | 40.58±3.80 | 0.001 |
Values are mean±SE.
independent-samples t-test
Figure 2.
Dot plot flow- cytometry histograms, showing the intracellular O2− levels measured by DHE in normozoospermic (control) (A2), and terato- asthenozoospermic (case) men (B2). The lower left quadrant represents viable, non-stained sperm and lower right represents apoptotic sperm. The upper left quadrant represents viable sperm with high intracellular O2−, and upper right shows apoptotic sperm with high intracellular O2
Flow cytometry analysis of HSPA2, protamine deficiency and semen parameters
The percentage of spermatozoa HSPA2+ was significantly lower in the case group than controls, while intracellular O2− was higher in case group (Table II). Also, the percentage of spermatozoa with protamine deficiencies was higher in the case group than controls (Table II). Intracellular O2− revealed positive correlations with CMA3_positive sperm (r=0.76, p=0.01) and sperm apoptosis (r=0.79, p=0.01) but, negative significant correlations with sperm normal morphology (r=-0.81, p=0.01), viability (r=-0.83, p=0.01), motility (r=-0.81, p=0.01), sperm count (r=-0.83, p=0.01) and sperm concentration (r=-0.052, p=0.05).
Discussion
Infertility is a major reproductive health problem that affects about 15% of all reproduction age couples. Male factors are responsible for half of the cases (24). Despite the development of science in diagnostic methods, in some cases, the etiology of male infertility is still unknown. The present study conducted a comparison between the protamine deficiency, percentage of spermatozoa HSPA2+, and level of intracellular O2− in patients suffering from terato-asthenozoospermic and normozoospermic men.
Our findings showed a statistically significant difference between case and control groups in terms of HSPA2. Case group experienced a significant decrease in the percentage of spermatozoa HSPA2+ in comparison to control group. The majority of other studies, such as studies conducted on infertile idiopathic oligo-teratozoospermia and patients with varicocele have shown a decrease in the level of HSPA2 (25, 26).
Tian et al study indicated the correlation between the levels of HSPA2 expression in testis with spermatogenic impairment, and fertilization rate following intracytoplasmic injection treatment for azoospermic patients (20). Also, in a similar study, Moteiti et al have shown a decrease in the percentage of HSPA2 positive spermatozoa in infertile men by flow cytometry (16). Normally, residual cytoplasm is removed from spermatozoa during spermiation and failure in the release of excess residual cytoplasm (ERC) could cause cytoplasm retention (27). HSPA2 is involved in cytoplasmic extrusion during sperm maturation, and a decrease in the level of HSPA2 leads to cytoplasmic retention and abnormal spermatozoa morphology (15, 28). A positive correlation between ERC and ROS production has been shown by Gomez et al (17).
It has also been reported that infertile men with varicocele have an increased level of excess residual cytoplasm and stimulated ROS production in semen (29). In fact, ERC activates the NADPH (Nicotinamide adenine dinucleotide phosphate) system through the hexose monophosphate shunt; this shunt is used by spermatozoa to supply electrons for ROS production, which further leads to oxidative stress (30). One of the main objectives of the present study was to investigate the intracellular O2− in TA patients through flow cytometry. The results showed the higher levels of intracellular O2− in the case group in comparison to control group. Multiple studies have shown similar results in both infertile patients (31) and experimental varicocele model (32).
According to Pearson coefficient, there was a negative correlation between intracellular O2− and sperm motility which could be due to the plasma membrane deficiency following oxidative stress. The excess in ROS levels can damage the sperm plasma membrane and its fluidity, thereby reducing membrane integrity and sperm motility (4, 33). According to the findings of the present study, decrease in HSPA2 and increase in intracellular O2−, might account as two characteristics of spermatozoa in TA patients.
Apoptosis and abnormal chromatin condensation may have a negative effect on potential infertility. Therefore, we used CMA3 staining for detection of sperm protamine deficiency and yo - pro -1 was used to assess sperm apoptosis. The findings of this study demonstrated that TA patients have higher percentages of sperm apoptosis and spermatozoa with protamine deficiencies in comparison to control group. Multiple studies have shown the protamine deficiency in infertile or subfertile men (34, 35). The protamination process occurs during sperm nuclear compaction; protamine protects sperm DNA against the toxic effects of ROS, and protamine deficiencies may be considered as one of the main risk factors for sperm DNA fragmentation (36, 37).
Also, DNA damage is a critical step in apoptosis; many studies have shown the relationship between DNA damage and abnormal sperm chromatin condensation (38, 39). According to our results, abnormal sperm chromatin condensation was another characteristic of spermatozoa from TA patients. On the other hand, HSPA2 plays a vital role in histon- protamine replacement; thus, one of the assumptions of the research was that the reduction of HSPA2 might cause chromatin abnormalities in TA samples (13). The results of a study showed a significant positive correlation between the percentage of CMA3 -positive sperm and HSPA2 in fertile men; however, the findings of the present research showed no correlation between these factors in two groups (16). Further studies with larger sample size covering different groups of infertile men are required to achieve more authentic, precise, and reliable findings.
The findings of the present research showed increased rate of intracellular O2− and decreased the percentage of sperm HSPA2+ in the samples of TA patients; however, there was no statistically significant correlation between them, which might be due to the other mechanisms for intracellular O2− production in studied cases.
Conclusion
The findings of the present study showed that TA samples contain the lower percentage of HSPA2– positive sperm, higher level of intracellular anion superoxide, and higher proportion of spermatozoa with abnormal chromatin packaging compare with normozoospermic men. Therefore, these factors might be considered as possible causes of infertility in these patients; it is worth mentioning that the assessment of these three factors is not only applicable to diagnostic design and treatment strategies in TA patients, it might also improve assisted reproductive success rates.
Acknowledgments
This study was supported financially by Research and Clinical Center for Infertility, Yazd Reproductive Science. The authors thank Anatomy Department of Tehran University of Medical Sciences as well as Royan Institute for their cooperation in doing this work.
Note
This article extracted from the Ph.D. thesis. (Parvin Sabeti)
Conflict of interest
There is no conflict of interest in this article.
References
- 1.Ferramosca A, Zara V. Bioenergetics of mammalian sperm capacitation. BioMed Res Int. 2014;2014:902953. doi: 10.1155/2014/902953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013;14:158–172. [PMC free article] [PubMed] [Google Scholar]
- 3.Moazamian R, Polhemus A, Connaughton H, Fraser B, Whiting S, Gharagozloo P, et al. Oxidative stress and human spermatozoa: diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod. 2015;21:502–515. doi: 10.1093/molehr/gav014. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Men's Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015;32:509–520. doi: 10.1007/s10815-014-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 7.Esteves SC, Agarwal A. Novel concepts in male infertility. Int Braz J Urol. 2011;37:5–15. doi: 10.1590/s1677-55382011000100002. [DOI] [PubMed] [Google Scholar]
- 8.Tremellen K. Oxidative stress and male infertility-a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-j, Allam J-P, Duan Y-g, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013;288:191–199. doi: 10.1007/s00404-013-2801-4. [DOI] [PubMed] [Google Scholar]
- 10.Mazzilli F, Rossi T, Marchesini M, Ronconi C, Dondero F. Superoxide anion in human semen related to seminal parameters and clinical aspects. Fertil Steril. 1994;62:862–868. doi: 10.1016/s0015-0282(16)57017-4. [DOI] [PubMed] [Google Scholar]
- 11.Redgrove KA, Nixon B, Baker MA, Hetherington L, Baker G, Liu D-Y, et al. The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS One. 2012;7:e50851. doi: 10.1371/journal.pone.0050851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huszar G, Stone K, Dix D, Vigue L. Putative creatine kinase M-isoform in human sperm is identifiedas the 70-kilodalton heat shock protein HspA2. Biol Reprod. 2000;63:925–932. doi: 10.1095/biolreprod63.3.925. [DOI] [PubMed] [Google Scholar]
- 13.Gaucher J, Reynoird N, Montellier E, Boussouar F, Rousseaux S, Khochbin S. From meiosis to postmeiotic events: the secrets of histone disappearance. FEBS J. 2010;277:599–604. doi: 10.1111/j.1742-4658.2009.07504.x. [DOI] [PubMed] [Google Scholar]
- 14.Scieglinska D, Krawczyk Z. Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans. Cell Stress Chaper. 2015;20:221–235. doi: 10.1007/s12192-014-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovanci E, Kovacs T, Moretti E, Vigue L, Bray-Ward P, Ward DC, et al. FISH assessment of aneuploidy frequencies in mature and immature human spermatozoa classified by the absence or presence of cytoplasmic retention. Hum Reprod. 2001;16:1209–1217. doi: 10.1093/humrep/16.6.1209. [DOI] [PubMed] [Google Scholar]
- 16.Motiei M, Tavalaee M, Rabiei F, Hajihosseini R, Nasr‐Esfahani MH. Evaluation of HSPA2 in fertile and infertile individuals. Andrologia. 2013;45:66–72. doi: 10.1111/j.1439-0272.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- 17.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276–287. [PubMed] [Google Scholar]
- 18.Jena N. DNA damage by reactive species: Mechanisms, mutation and repair. J Biosci. 2012;37:503–517. doi: 10.1007/s12038-012-9218-2. [DOI] [PubMed] [Google Scholar]
- 19.Ergur AR, Dokras A, Giraldo JL, Habana A, Kovanci E, Huszar G. Sperm maturity and treatment choice of in vitro fertilization (IVF) or intracytoplasmic sperm injection: diminished sperm HspA2 chaperone levels predict IVF failure. Fertil Steril. 2002;77:910–918. doi: 10.1016/s0015-0282(02)03073-x. [DOI] [PubMed] [Google Scholar]
- 20.Tian Y, Zhang F, Zhang X, Li L, Wang L, Shi B, et al. Depression of HspA2 in human testis is associated with spermatogenic impairment and fertilization rate in ICSI treatment for azoospermic individuals. J Assist Reprod Genet. 2014;31:1687–1693. doi: 10.1007/s10815-014-0360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Agarwal A. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Mahfouz RZ, du Plessis SS, Aziz N, Sharma R, Sabanegh E, Agarwal A. Sperm viability, apoptosis, and intracellular reactive oxygen species levels in human spermatozoa before and after induction of oxidative stress. Fertil Steril. 2010;93:814–821. doi: 10.1016/j.fertnstert.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 23.Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–1126. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 24.De Kretser D. Male infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 25.Cedenho AP, Lima SB, Cenedeze MA, Spaine DM, Ortiz V, Oehninger S. Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa. Hum Reprod. 2006;21:1791–1794. doi: 10.1093/humrep/del055. [DOI] [PubMed] [Google Scholar]
- 26.Esfahani MHN, Abbasi H, Mirhosseini Z, Ghasemi N, Razavi S, Tavalaee M, et al. Can altered expression of hspa2 in varicocele patients lead to abnormal spermatogenesis? Int J Fertil Steril. 2010;4:104–113. [Google Scholar]
- 27.Cooper TG. Epididymal Secretion and Resorption of Proteins. The Epididymis, Sperm Maturation and Fertilisation. Springer; 1986. pp. 200–230. [Google Scholar]
- 28.Nixon B, Bromfield EG, Dun MD, Redgrove KA, McLaughlin EA, Aitken RJ. The role of the molecular chaperone heat shock protein A2 (HSPA2) in regulating human sperm-egg recognition. Asian J Androl. 2015;17:568. doi: 10.4103/1008-682X.151395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zini A, Buckspan M, Jamal M, Jarvi K. Effect of varicocelectomy on the abnormal retention of residual cytoplasm by human spermatozoa. Hum Reprod. 1999;14:1791–1793. doi: 10.1093/humrep/14.7.1791. [DOI] [PubMed] [Google Scholar]
- 30.Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. 2012;10:1. doi: 10.1186/1477-7827-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Said TM, Agarwal A, Sharma RK, Mascha E, Sikka SC, Thomas AJ. Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil Steril. 2004;82:871–877. doi: 10.1016/j.fertnstert.2004.02.132. [DOI] [PubMed] [Google Scholar]
- 32.Jafari A, Zahmatkesh M, Sadeghipour H-R, Kajbafzadeh A, Sarrafnejd A, Shahrestany T, et al. Flow cytometric evaluation of sperm superoxide anion production in rats with experimental varicocele. Urology. 2010;75:217–222. doi: 10.1016/j.urology.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 33.El-Tohamy MM. The mechanisms by which oxidative stress and free radical damage produces male infertility. Life Sci J. 2012;9:674–688. [Google Scholar]
- 34.Ghasemzadeh J, Talebi AR, Khalili MA, Fesahat F, Halvaei I, Nabi A, et al. Sperm parameters, protamine deficiency, and apoptosis in total globozoospermia. Iran J Reprod Med. 2015;13:495–502. [PMC free article] [PubMed] [Google Scholar]
- 35.Ni K, Spiess AN, Schuppe HC, Steger K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: a systematic review and meta‐analysis. Andrology. 2016;4:789–799. doi: 10.1111/andr.12216. [DOI] [PubMed] [Google Scholar]
- 36.Simon L, Castillo J, Oliva R, Lewis SE. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod Biomed Online 2011; 23: 724-734. doi: 10.1016/j.rbmo.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Utsuno H, Miyamoto T, Oka K, Shiozawa T. Morphological alterations in protamine-deficient spermatozoa. Hum Reprod. 2014;29:2374–2381. doi: 10.1093/humrep/deu225. [DOI] [PubMed] [Google Scholar]
- 38.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talebi AR, Khalili MA, Vahidi S, Ghasemzadeh J, Tabibnejad N. Sperm chromatin condensation, DNA integrity, and apoptosis in men with spinal cord injury. J Spinal Cord Med. 2013;36:140–146. doi: 10.1179/2045772312Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]