Abstract
Inspired by the ferromagnetic coupling in the cubane model CaMnIV3O4 of the oxygen-evolving complex of photosystem II, 3d–4f mixed-metal DyMn3O4 clusters were prepared for investigation of the magnetic properties. For comparison, YMnIV3O4 and YMnIV2MnIIIO4 clusters were investigated as well and showed ferromagnetic interactions, like the calcium analogue. DyMnIV3O4 displays single-molecule-magnet properties, while the one-electron-reduced species (DyMnIV2MnIIIO4) does not, despite the presence of a MnIII center with higher spin and single-ion anisotropy.
Graphical abstract
INTRODUCTION
Transition-metal lanthanide (3d–4f) clusters have shown promise as single-molecule-magnet (SMM) candidates.1 The combination of high single-ion anisotropy of particular lanthanides and the high spin of multinuclear complexes provides a strategy for the synthesis of SMMs with large relaxation barriers.2 Although the largest energy barriers of SMMs are found in mono- or dinuclear lanthanide complexes because of the weak exchange interactions of lanthanide ions,3 coupling with transition metals has also been investigated to increase the relaxation barrier.4 Despite the discovery of numerous 3d–4f SMMs, the rational design of molecules with improved SMM properties remains challenging, and the study toward a detailed understanding of the structural factors that control the SMM behavior is an active area of research.
We have reported the preparation of tetranuclear heterometallic MMn3O4 cubanes5 as structural models of the oxygen-evolving complex of photosystem II that displays a CaMn4O5 cluster.6 The CaMnIV3O4 cubane, with a diamagnetic Ca2+, displays predominantly ferromagnetic coupling between the transition-metal ions, with the higher-symmetry cubane showing smaller coupling constants.7 The magnetic behavior of these clusters was studied computationally, and a high-spin ground state was concluded to be an intrinsic characteristic of the CaMnIV3O4 heterometallic cubane motif because of its structural parameters, in particular the acute Mn–O–Mn angles.8 The ferromagnetic coupling of the Mn3 motif was envisioned to be a useful design element for the study of the magnetism of 3d–4f clusters.9 A CaMnIV3O4 precursor provides facile synthetic access by metal substitution to lanthanide-containing cubanes of interest related to SMMs.5b Access to two oxidation states, LnMnIV3O4 and LnMnIV2MnIIIO4, allows for evaluation of the impact of a single electron transfer on the SMM properties of the clusters. This is of particular interest given the potential for facile modulation of the magnetic properties via redox chemistry, but its effects on the 3d–4f SMM properties are poorly understood. Although manganese-only SMM studies of isostructural clusters with different Mn oxidation states have been performed,10 similar investigations of 3d–4f mixed-metal complexes are rare. We report herein on the magnetic properties of the DyIII and YIII versions of heterometallic cubanes.
RESULTS AND DISCUSSION
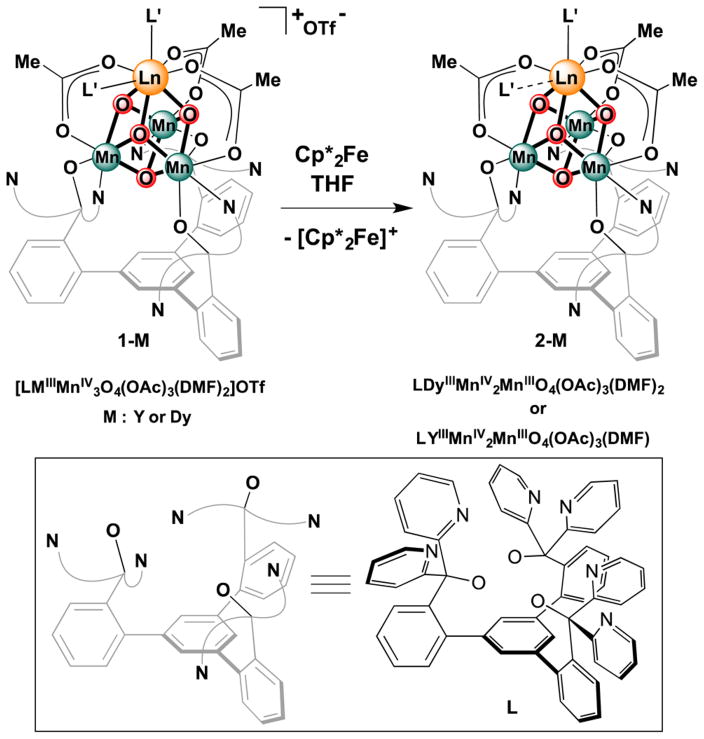
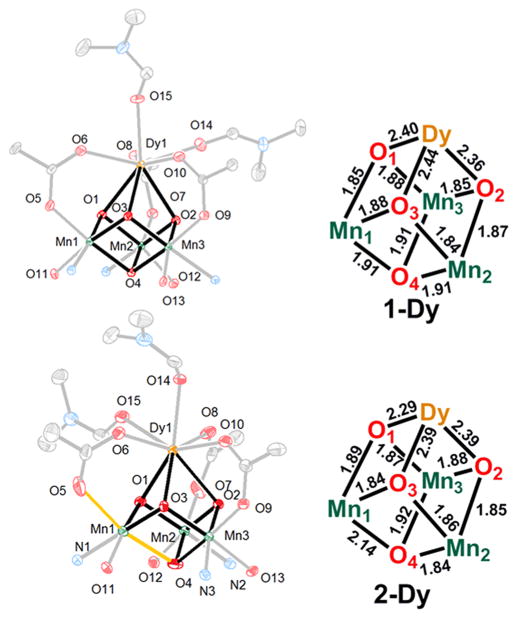
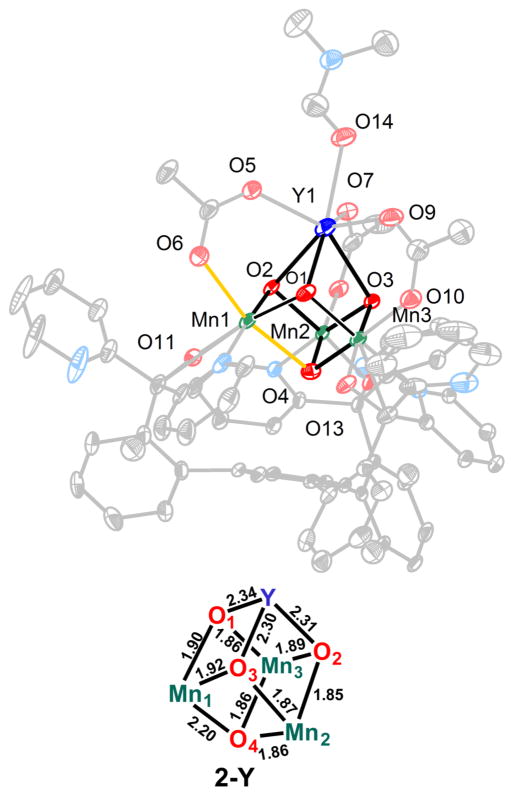
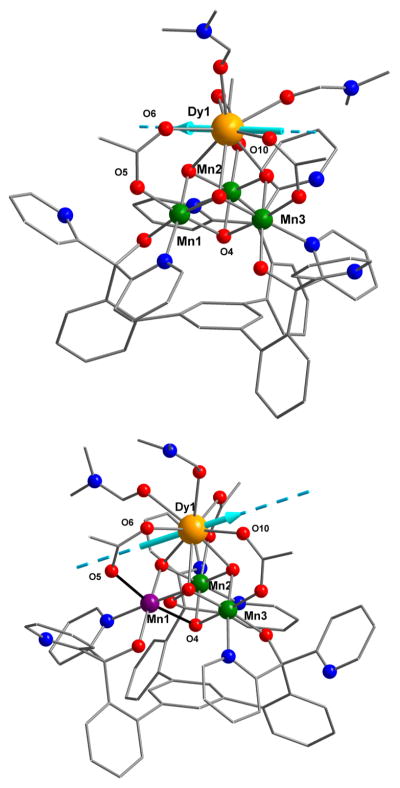
The heteronuclear complexes [LYIIIMnIV3O4(OAc)3(DMF)2]-[OTf] (1-Y) and [LDyIIIMnIV3O4(OAc)3(DMF)2][OTf] (1-Dy), where DMF = N,N-dimethylformamide and −OTf = trifluoromethanesulfonate, have been reported by us, including structural characterization for 1-Y.5b,c The reduced species ([LYIIIMnIV2MnIIIO4(OAc)3(DMF)] (2-Y) and [LDyIIIMnIV2MnIIIO4(OAc)3(DMF)2] (2-Dy)) were prepared by the treatment of a tetrahydrofuran (THF) suspension of a cationic precursor (1-Y and 1-Dy, respectively) with 1 equiv of decamethylferrocene (Figure 1). Single-crystal X-ray diffraction studies were performed for compounds 1-Dy, 2-Y, and 2-Dy. The top metal is eight-coordinate in compounds 1-Y, 1-Dy, and 2-Dy with three acetates, three oxidos, and two DMF molecules completing the coordination sphere (Figure 2). 2-Y displays a single DMF ligand, resulting in a seven-coordinate Y center (Figure 3). The Mn–O(oxido) distances are short and similar (1.84–1.91 Å) in 1-Y and 1-Dy, consistent with the all-MnIV assignment. Compounds 2-Y and 2-Dy (Figures 2 and 3) show elongation of the O5–Mn1 and O4–Mn1 distances (to >2.10 Å), consistent with a localized MnIII site with population of a σ-antibonding orbital along this axis.
Figure 1.
Synthesis of reduced cubane complexes.
Figure 2.
Left: Cluster thermal ellipsoid plot for 1-Dy (top) and 2-Dy (bottom). H atoms, solvent molecules, counteranions, and parts of the ligand are not shown for clarity. The elongated Mn–O bonds in 2-Dy are highlighted in orange. Right: Bond distances (Å) in the cubane core.
Figure 3.
Top: Thermal ellipsoid plot for 2-Y. H atoms, solvent molecules, and counteranions are not shown for clarity. The elongated Mn–O bonds in 2-Y are highlighted in orange. Bottom: Bond distances (Å) in the cubane core.
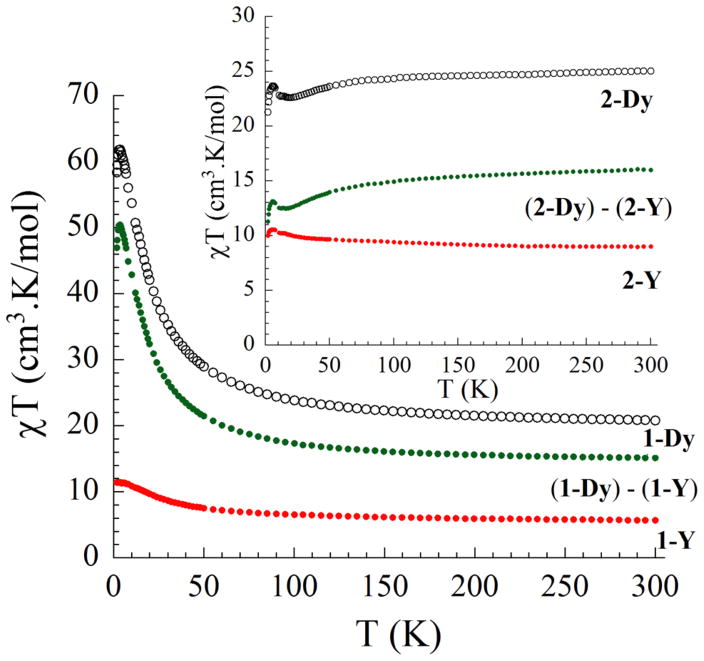
Direct-current (dc) magnetic susceptibility measurements were performed on 1-Y, 2-Y, 1-Dy, and 2-Dy between 1.8 and 300 K under an external field of 1000 Oe (Figure 4). The observed paramagnetic behavior of all four complexes arises from the 3d MnIII and MnIV ions and/or 4f LnIII ions. The experimentally obtained χT values are 5.67, 9.02, 20.78, and 25.02 cm3·K·mol−1 for complexes 1-Y, 2-Y, 1-Dy, and 2-Dy, respectively. According to the free-ion approximation of each metal ion [DyIII (6H15/2, S = 5/2, L = 5, g = 4/3, χT = 14.17 cm3·K·mol−1), MnIII (S = 2; g = 2.00, χT = 3.00 cm3·K·mol−1), and MnIV (S = 3/2; g = 2.00, χT = 1.875 cm3·K·mol−1)], the theoretical values for four noninteracting metal ions are calculated to be 5.63, 6.75, 19.80, and 20.92 cm3·K·mol−1 for complexes 1-Y, 2-Y, 1-Dy, and 2-Dy, respectively. The different χT values between the experimental and theoretical values for 2-Y and 2-Dy may be due to residual solvents in the samples that were also observed by combustion analysis. The χT values observed for 2-Y and 2-Dy are higher than those for 1-Y and 1-Dy, respectively, confirming that one MnIV ion in the cubane was reduced. Upon a decrease in the temperature, the χT products remain fairly constant down to ~70 K for all complexes. The χT values then increase to a maximum value for 1-Dy and 1-Y around 6 K because of intramolecular ferromagnetic interactions and then slightly decrease at lower temperature likely because of intermolecular antiferromagnetic interactions. The χT values of 2-Dy decreasing gradually below 70 K are presumably due to the presence of large magnetoanisotropy in the DyIII system, which was not observed in 2-Y. Both χT values of 2-Dy and 2-Y increase at lower temperatures to maximum values of 23.70 cm3·K·mol−1 (at 6 K) and 10.57 cm3·K·mol−1 (at 5.5 K) for 2-Dy and 2-Y, respectively, which are indicative of ferromagnetic interactions between the metal ions within the LnMn3 unit. A slight decrease at lower temperatures (with values of 21.30 and 10.02 cm3·K·mol−1 at 2 K for 2-Dy and 2-Y, respectively) indicates intermolecular antiferromagnetic interactions.
Figure 4.
Temperature dependence of the χT product at 1000 Oe for 1-Dy (black), 2-Dy (black inset), 1-Y (red), and 2-Y (red inset). The green dots represent the values of 1-Y and 2-Y subtracted from 1-Dy and 2-Dy, respectively. χ is the molar susceptibility per cubane complex defined as M/H.
The amplitude of the increase of the χT values suggests weak coupling between metal ions in the reduced compounds. Considering the number of magnetic exchange pathways between metal centers as well as the presence of the highly anisotropic DyIII ion renders modeling of 1-Dy and 2-Dy difficult. Therefore, complexes 1-Y and 2-Y containing the diamagnetic YIII ion become ideal models for probing the overall exchange interactions between the Mn ions.
The magnetic susceptibility data can be fit to obtain the nature and magnitude of the magnetic interaction between MnIII and/or MnIV. By using the isotropic spin Hamiltonian Ĥ = −2J(Ŝ1·Ŝ2 + Ŝ1·Ŝ3) − 2J′(Ŝ2·Ŝ3) with S1 = S2 = S3 = 3/2 for 1-Y and S1 = 2, S2 = S3 = 3/2 for 2-Y (Scheme S1), the best-fit parameters obtained are J = 5.35 cm−1, J′ = −0.81 cm−1, g = 1.93, and S = 9/2 (1-Y) and J = 0.78 cm−1, J′ = −0.98 cm−1, g = 2.31, and S = 5 (2-Y) (Figure S1 and Scheme S1), showing ferromagnetic contributions, similar to experimental and computed CaMn3O4 cubane systems, albeit with small coupling constants.7,8 Although the fit parameters did not include the anisotropy of the MnIII ions and despite the fitting being slightly different from that of the experimental data, it is estimated that the ferromagnetic interaction between MnIV ions in 1-Y is slightly larger than the interaction between MnIII and MnIV in 2-Y, which agrees well with the values reported for other MnIII/MnIV isostructural systems.11 We can further assess the interactions between DyIII and MnIII or MnIV by subtracting χT values of 1-Y and 2-Y from 1-Dy and 2-Dy to give suitably adjusted plots (green circles, Figure 4 and inset).
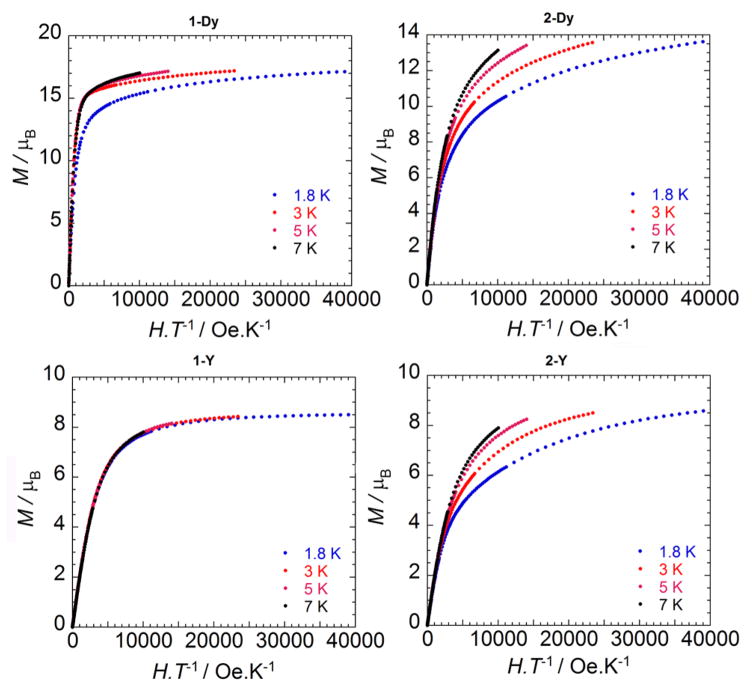
The field dependences (up to 7 T) of the magnetization of all four complexes in the temperature range of 1.8–7 K were also obtained (Figure 5). The saturation of the magnetization suggests the absence of significant magnetic anisotropy, consistent with three MnIV ions in compound 1-Y. The magnetization at 1.8 K saturates above 5.5 T at 8.5 μB, which is lower than those of the other trinuclear MnIV complexes. The Dy–Mn interactions of 1-Dy are much stronger than those of 2-Dy based on the larger maximum χT value and the sharper increase at low temperature. This magnetic effect corresponds to shorter Dy–O(oxido) distances in 2-Dy [2.294(2), 2.385(2), and 2.389(2) Å] compared to those in 1-Dy [2.355(1), 2.400(1), and 2.443(1) Å]. The reasons behind this structural change and its influence on magnetism are not understood but could include an increase in the electron density on the Mn centers due to reduction, leading to weaker Mn–O interactions and stronger Dy–O interactions; however, this proposal is not substantiated by a lengthening of the Mn–O(oxido Dy) distances in 2-Dy.
Figure 5.
Field dependences of the magnetization at variable temperatures for the reported complexes.
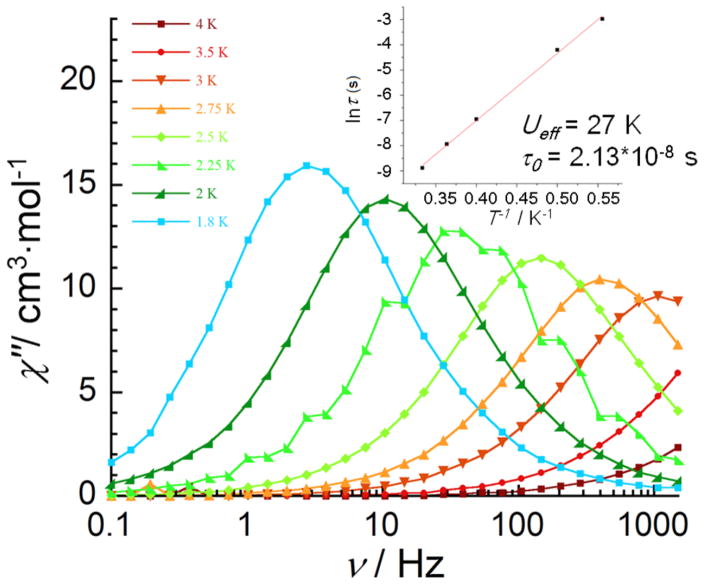
In order to probe the possible SMM behavior in 1-Dy, 2-Dy, and 2-Y, the temperature dependences of the in-phase (χ′) and out-of-phase (χ″) magnetic susceptibilities were measured in the temperature range of 2–5 K. Only 1-Dy exhibited a temperature-and frequency-dependent signal indicating slow relaxation of the magnetization under a zero dc field and a 3 Oe oscillating field at frequencies between 1 and 1500 Hz as expected for an SMM (Figure 6). For 1-Dy, the maxima can be observed for a range of temperatures between 3 and 1.8 K. The relaxation time deduced from this data is consistent with an activated behavior (Figure 6, inset), with an anisotropic energy barrier (Ueff) of 27 K and a preexponential factor (τ0) of 2.13 × 10−8 s. The peaks of 1-Dy in χ″) versus ν are shifted under the various dc fields, but the alternating-current (ac) measurements under the applied optimum field of 800 Oe only reveal a slightly higher energy barrier (Figures S2–S4). In contrast to 1-Dy, there is no evidence that the one-electron-reduced species 2-Dy is an SMM (Figures S5 and S6). The observed frequency-independent χ″ signal with a large applied dc field is likely due to an intermolecularly driven relaxation process.12 We also measured the ac susceptibility for 2-Y because of the anisotropic nature of the MnIII ion, but no out-of-phase signals were observed, indicating that the Mn centers alone are not sufficient for SMM properties in this geometry.
Figure 6.
Temperature dependence of the out-of-phase (χ″) ac susceptibility of 1-Dy from 0.1 to 1500 Hz without an applied dc field. Inset: Arrhenius plot, ln(τ) versus 1/T. The red line indicates the fit yielding the energy barrier for spin reversal.
On the basis of a comparison of the dc and ac data of 1-Dy and 2-Dy, the interaction between the Dy and Mn ions is key to the SMM properties in these compounds. Even though a MnIII ion has magnetoanisotropy due to Jahn–Teller distortion and one more unpaired electron than MnIV, the SMM properties of our 3d–4f cubane system were not improved when one MnIV was reduced to MnIII. A significantly higher χT value for 1-Dy compared to that of 2-Dy at 6 K indicates a stronger 3d–4f ferromagnetic interaction, with a larger spin ground state being likely. Because of the presence of significant spin–orbit coupling of the DyIII ion, no fits have been made to date. It is reasonable to assume that, in addition to a larger spin ground state, significantly stronger coupling will likely provide a well-defined ground state with the first excited state higher in energy, leading to enhanced SMM behavior for 1-Dy.
Additionally, the different Dy–ligand interactions and change in the coordination environment induced by the reduction could affect the Dy-ion anisotropy. To validate this, Magellan magnetic software11 was employed to probe the anisotropy axis direction on the DyIII ions. The modeled anisotropy axes of DyIII (Figure 7) give an indication that, in the case of 2-Dy, the anisotropy on the Dy center is nearly perpendicular to the anisotropy of the MnIII ion (the Jahn–Teller elongation axis highlighted in black); this reduces the overall anisotropy of the system. In this case, the addition of anisotropic MnIII ions can have an effect on the weaker interactions between DyIII and MnIV/MnIII, which may decrease the spin ground state (ST) as well as lower the overall anisotropy of the complex, which reduced the Ising-type magnetoanisotropy (D) and thus decreased the SMM performance.
Figure 7.
Pictures of 1-Dy (top) and 2-Dy (bottom) containing the anisotropy axis direction for the Dy ions. The axes were modeled using Magellan magnetic software.11
CONCLUSION
In summary, the heterometallic 3d–4f Mn/Dy and Mn/Y clusters LnMnIV3O4 were reduced by one electron to form LnMnIV2MnIIIO4 species that maintain the cubane motif and show a localized MnIII site with corresponding metal–ligand distortions. The YMnIV3O4 cubane (1-Y) displays ferromagnetism, supporting the notion that this is a characteristic property of MMnIV3O4 cubanes, as was previously computed for the biologically relevant CaMnIV3O4 cluster. The related DyMnIV3O4 (1-Dy) and DyMnIV2MnIIIO4 (2-Dy) clusters offer suitable models for studying the effect of the oxidation state of Mn in the SMM behavior of 3d–4f clusters. 2-Dy is not an SMM despite the presence of an additional electron and of a MnIII center with magnetoanisotropy. The ferromagnetic interactions within the Mn3IV core and the nonnegligible Dy–Mn interactions result in a large spin ground state, contributing to the SMM properties of 1-Dy.
EXPERIMENTAL SECTION
Reactions performed under an inert atmosphere were carried out in oven-dried glassware in a glovebox under a nitrogen atmosphere. Anhydrous dichloromethane and diethyl ether were purified by sparging with nitrogen for 15 min and then passing under nitrogen pressure through a column of activated A2 alumina (Zapp’s). CD2Cl2 was purchased from Cambridge Isotope Laboratories, dried over calcium hydride, then degassed by three freeze–pump–thaw cycles, and vacuum-transferred prior to use. 1H NMR spectra were recorded on a Varian 300 MHz instrument, with shifts reported relative to the residual solvent peak. 19F NMR spectra were recorded on a Varian 300 MHz instrument, with shifts reported relative to the internal lock signal. Elemental analyses were performed by Robertson Microlit Laboratories. All commercial chemicals were used as received. Dysprosium trifluoromethanesulfonate (Dy(OTf)3) and decamethylferrocene were purchased from Strem, and yttrium trifluoromethanesulfonate (Y-(OTf)3) was purchased from Aldrich. [LDyMn3O4(OAc)3(DMF)2]-[OTf]5b and [LYMn3O4(OAc)3(DMF)2][OTf]5c were prepared according to previously published procedures.
Synthesis of [LDyMn3O4(OAc)3(DMF)2] (2-Dy)
A solution of decamethylferrocene (0.007 g, 0.02 mmol, 1 equiv) in THF (2 mL) was added to a solution of 1-Dy (0.034 g, 0.02 mmol, 1 equiv) in THF (2 mL). The dark-brown solution was stirred overnight. The dark-brown precipitate generated was collected on a fritted glass funnel, washed with acetonitrile (4 mL) to remove the remaining decamethylferrocenium triflate, and further washed with dichloromethane. The resulting brown powder was dissolved in benzene/THF and concentrated in vacuo. Recrystallization from DMF/benzene/ether yields the product as dark-brown crystals (0.009 g, 29%). 1H NMR (C6D6, 300 MHz): δ 7.2, 3.4, 1.6, 1.2, −2.4. Anal. Calcd for C73H63Cl2DyMn3N7O14([2-Dy]·CH2Cl2·C6H6): C, 52.65; H, 4.07; N, 6.46. Found: C, 52.49; H, 3.72; N, 5.96.
Synthesis of [LYMn3O4(OAc)3(DMF)] (2-Y)
A solution of decamethylferrocene (0.017 g, 0.0527 mmol) in THF (2 mL) was added to a solution of 1-Y (0.079 g, 0.0527 mmol, 1 equiv) in THF (8 mL). The mixture was stirred at room temperature for 30 min and then filtered through Celite. The filtrate was dried in vacuo. Benzene was added, and the mixture was filtered through Celite to remove decamethylferrocenium triflate. The benzene filtrate was dried in vacuo to yield the product as a red-brown solid (0.063 g, 94%). X-ray-quality crystals were grown by vapor diffusion of diethyl ether into a DMF solution of 2-Y. 1H NMR (C6D6, 300 MHz): δ 25.1, 13.1, 11.3, 10.2, 9.3, 7.8, 5.5, 4.5, −25.3. Anal. Calcd for C66H55Mn3N7O14Y: C, 55.67; H, 3.89; N, 6.89. Anal. Calcd for C69H62Mn3N8O15Y (one DMF solvate): C, 55.36; H, 4.17; N, 7.49. Found: C, 53.96; H, 4.88; N, 7.26.
Magnetic Measurements
The magnetic susceptibility measurements were obtained using a Quantum Design SQUID MPMS-XL7 magnetometer operating between 1.8 and 300 K for dc applied fields ranging from −7 to +7 T. dc analyses were performed on polycrystalline samples of 7.3, 13.0, 18.0, and 25.0 mg for 1-Dy, 2-Dy, 1-Y, and 2-Y, respectively, restrained in a polyethylene membrane and under a field ranging from 0 to 7 T between 1.8 and 300 K. ac susceptibility measurements were carried out under an oscillating ac field of 3 Oe and ac frequencies ranging from 1 to 1500 Hz. The magnetization data were collected at 100 K to check for ferromagnetic impurities that were absent in all samples. A diamagnetic correction was applied for the sample holder and the sample.
Supplementary Material
Acknowledgments
We thank Caltech, the NIH (Grant R01 GM102687A), and a Sandia Campus Executive Fellowship (to E.Y.T.) for financial support. T.A. is grateful for Sloan, Cottrell, and Dreyfus scholarships. We also thank R. Holmberg for Magellan analysis.
Footnotes
Notes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.inorgchem.6b00630.
Crystallographic data in CIF format (CIF)
Crystallographic data in CIF format (CIF)
Crystallographic data in CIF format (CIF)
Experimental procedures and spectroscopic characterization (PDF)
References
- 1.(a) Sharples JW, Collison D. Coord Chem Rev. 2014;260:1–20. doi: 10.1016/j.ccr.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Winpenny REP. Chem Soc Rev. 1998;27:447–452. [Google Scholar]; (c) Rosado Piquer L, Sanudo EC. Dalton Trans. 2015;44:8771–8780. doi: 10.1039/c5dt00549c. [DOI] [PubMed] [Google Scholar]; (d) Liu K, Shi W, Cheng P. Coord Chem Rev. 2015;289–290:74–122. [Google Scholar]; (e) Andruh M, Costes JP, Diaz C, Gao S. Inorg Chem. 2009;48:3342–3359. doi: 10.1021/ic801027q. [DOI] [PubMed] [Google Scholar]
- 2.(a) Funes AV, Carrella L, Rentschler E, Albores P. Dalton Trans. 2014;43:2361–2364. doi: 10.1039/c3dt52765d. [DOI] [PubMed] [Google Scholar]; (b) Langley SK, Ungur L, Chilton NF, Moubaraki B, Chibotaru LF, Murray KS. Inorg Chem. 2014;53:4303–4315. doi: 10.1021/ic4029645. [DOI] [PubMed] [Google Scholar]; (c) Li XL, Min FY, Wang C, Lin SY, Liu Z, Tang J. Dalton Trans. 2015;44:3430–3438. doi: 10.1039/c4dt03713h. [DOI] [PubMed] [Google Scholar]
- 3.(a) Fatila EM, Clerac R, Rouzieres M, Soldatov DV, Jennings M, Preuss KE. J Am Chem Soc. 2013;135:13298–13301. doi: 10.1021/ja4067783. [DOI] [PubMed] [Google Scholar]; (b) Le Roy JJ, Jeletic M, Gorelsky SI, Korobkov I, Ungur L, Chibotaru LF, Murugesu M. J Am Chem Soc. 2013;135:3502–3510. doi: 10.1021/ja310642h. [DOI] [PubMed] [Google Scholar]; (c) Pointillart F, Le Guennic B, Golhen S, Cador O, Ouahab L. Chem Commun. 2013;49:11632–11634. doi: 10.1039/c3cc47087c. [DOI] [PubMed] [Google Scholar]; (d) Rinehart JD, Fang M, Evans WJ, Long JR. Nat Chem. 2011;3:538–542. doi: 10.1038/nchem.1063. [DOI] [PubMed] [Google Scholar]
- 4.(a) Liu JL, Chen YC, Zheng YZ, Lin WQ, Ungur L, Wernsdorfer W, Chibotaru LF, Tong ML. Chem Sci. 2013;4:3310–3316. [Google Scholar]; (b) Sun W-B, Yan P-F, Jiang S-D, Wang B-W, Zhang Y-Q, Li H-F, Chen P, Wang Z-M, Gao S. Chem Sci. 2016;7:684–691. doi: 10.1039/c5sc02986d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Kanady JS, Tsui EY, Day MW, Agapie T. Science. 2011;333:733–736. doi: 10.1126/science.1206036. [DOI] [PubMed] [Google Scholar]; (b) Lin PH, Takase MK, Agapie T. Inorg Chem. 2015;54:59–64. doi: 10.1021/ic5015219. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tsui EY, Agapie T. Proc Natl Acad Sci U S A. 2013;110:10084–10088. doi: 10.1073/pnas.1302677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suga M, Akita F, Hirata K, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen JR. Nature. 2014;517:99–103. doi: 10.1038/nature13991. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kanady JS, Mendoza-Cortes JL, Tsui EY, Nielsen RJ, Goddard WA, Agapie T. J Am Chem Soc. 2013;135:1073–1082. doi: 10.1021/ja310022p. [DOI] [PubMed] [Google Scholar]; (b) Mukherjee S, Stull JA, Yano J, Stamatatos TC, Pringouri K, Stich TA, Abboud KA, Britt RD, Yachandra VK, Christou G. Proc Natl Acad Sci U S A. 2012;109:2257–2262. doi: 10.1073/pnas.1115290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krewald V, Neese F, Pantazis D. A J Am Chem Soc. 2013;135:5726–5739. doi: 10.1021/ja312552f. [DOI] [PubMed] [Google Scholar]
- 9.Lampropoulos C, Stamatatos TC, Abboud KA, Christou G. Inorg Chem. 2009;48:429–431. doi: 10.1021/ic802005a. [DOI] [PubMed] [Google Scholar]
- 10.(a) Habib F, Brunet G, Loiseau F, Pathmalingam T, Burchell TJ, Beauchemin AM, Wernsdorfer W, Clerac R, Murugesu M. Inorg Chem. 2013;52:1296–303. doi: 10.1021/ic301820w. [DOI] [PubMed] [Google Scholar]; (b) Soler M, Wernsdorfer W, Abboud KA, Huffman JC, Davidson ER, Hendrickson DN, Christou G. J Am Chem Soc. 2003;125:3576–3588. doi: 10.1021/ja021066s. [DOI] [PubMed] [Google Scholar]; (c) Jones LF, Inglis R, Cochrane ME, Mason K, Collins A, Parsons S, Perlepes SP, Brechin EK. Dalton Trans. 2008:6205–6210. doi: 10.1039/b811143j. [DOI] [PubMed] [Google Scholar]
- 11.Pathmalingam T, Gorelsky SI, Burchell TJ, Bedard AC, Beauchemin AM, Clerac R, Murugesu M. Chem Commun. 2008:2782–2784. doi: 10.1039/b802279h. [DOI] [PubMed] [Google Scholar]
- 12.Habib F, Korobkov I, Murugesu M. Dalton Trans. 2015;44:6368–6373. doi: 10.1039/c5dt00258c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.