Abstract
Purpose
Cholangiocarcinoma (CCA) is a malignancy of the biliary epithelium that is associated with low five-year survival. The apelin receptor (APLNR), which is activated by the apelin peptide, has not been studied in CCA. The purpose of this study is to determine if inhibition of the apelin/APLNR axis can inhibit CCA growth.
Methods
Immunohistochemistry, rtPCR, immunofluorescence, flow cytometry, and ELISA was used to measure APLNR expression in human CCA cells and tissues. Mz-ChA-1 cells were treated with increasing concentrations of apelin and ML221, an APLNR antagonist. Expression of proliferative and angiogenic genes were measured via rtPCR. In vivo, Mz-ChA-1 cells were injected into the flanks of nu/nu mice, which were treated with ML221 (150 μg/kg) via tail vein injection.
Results
Expression of the apelin/APLNR axis was increased in CCA. In vitro, CCA proliferation and angiogenesis was inhibited by ML221 treatment. ML221 treatment significantly decreased tumor growth in nu/nu mice.
Conclusion
The apelin/APLNR axis regulates CCA proliferation and angiogenesis. Inhibition of the apelin/APLNR axis decreases tumor growth in our xenograft model. Targeting APLNR signaling has the potential to serve as a novel, tumor directed therapy for CCA.
Keywords: Apelin, Apelin receptor, Cholangiocarcinoma, Biliary epithelium, Proliferation
Introduction
Cholangiocarcinoma (CCA) is a malignancy that arises from the intrahepatic or extrahepatic biliary epithelium. It is the second most common primary liver cancer and accounts for approximately 15% of liver cancers worldwide [1]. In the United States, the incidence of CCA is approximately 1.6 cases per 100,000 people; however, the incidence in some Asian countries is much higher [2]. Risk factors strongly associated with cholangiocarcinoma development include Primary Sclerosing Cholangitis (PSC), choledochal cysts, hepatolithiasis, liver cirrhosis, thorotrast, and Opisthorcosis viverrini infection [3]. Despite multidisciplinary treatment strategies, overall 5-year survival rates for resectable intrahepatic CCA tumors remains between 30 and 35% [4]. Developing novel, tumor specific therapies for cholangiocarcinoma could broaden the scope of current treatment strategies and provide the necessary adjuncts to improve long-term survival.
Apelin is a bioactive peptide and endogenous ligand for the APJ receptor (APLNR), a member of the G protein coupled receptor family that shares a similar sequence as the angiotensin type-1 receptor (AT1) [5]. Early studies demonstrated that the apelin/APLNR receptor axis plays a significant role in blood pressure regulation and cardiovascular disease by regulating angiogenesis and the response of endothelial cells to hypoxic injury [6–9]. APLNR signaling is also essential for embryonic angiogenesis and has been shown to regulate blood vessel diameter [8,10].
More recent studies have shown that the apelin/APLNR axis also regulates angiogenesis and growth of certain malignancies, potentially serving as a novel therapeutic target [5,11]. Additionally, apelin has been shown to promote lymphangiogenesis and lymph node metastasis, further emphasizing its importance in cancer physiology [12]. Berta et al. demonstrated that apelin expression is up regulated in human non-small cell lung cancer, expression is associated with poor overall survival, and apelin stimulates tumor growth and microvessel densities in their in vivo model [13]. Additionally, an apelin/APLNR autocrine loop has been identified in colon adenocarcinoma tumors that regulates tumor growth, but is inhibited by administration of an APLNR antagonist [14]. Within the liver, Muto et al. demonstrated that the apelin-APLNR system induces hepatocellular carcinoma angiogenesis [15].
In this study we present the novel findings that the apelin/APLNR axis is up regulated in CCA cells lines and human CCA tissues. We demonstrate that apelin promotes CCA proliferation and angiogenesis, a process that is inhibited by co-treatment with an APLNR antagonist. Additionally, we show that CCA growth is inhibited in vivo by intravenous administration of ML221, an apelin receptor antagonist, in our nu/nu mouse xenograft model.
Materials and methods
Materials
Reagents were purchased from Sigma (St. Louis, MO) unless otherwise indicated. The rabbit polyclonal apelin receptor (APLNR) antibody was purchased from Thermo Fisher Scientific (Waltham, MA). Anti-rabbit secondary antibodies used for immunoblots were purchased from Li-cor (Lincoln, NE). Alexa Fluor® 488 secondary antibodies used in flow cytometry were purchased from Jackson ImmunoResearch (West Grove, PA). The apelin receptor ligand, Pyr-Apelin-13 (2420), and the receptor antagonist, ML 221 (4748), were purchased from Tocris Pharmaceuticals (Avon-mouth, Bristol, UK) [16]. Antibodies for p-ERK (4370) and t-ERK (4695) were purchased from Cell Signaling (Danvers, MA).
Expression of apelin and APLNR in human CCA tissues
Immunohistochemistry (IHC) was used to evaluate the expression of APLNR in human non-malignant and CCA tissue samples. CCA tissue arrays and normal human liver slides were purchased from abcam® (Cambridge, MA). Expression of APLNR in CCA tissues was compared with tissues biopsied from adjacent non-malignant liver. The tissues were stained with rabbit polyclonal APLNR antibody using a 1:200 dilution. The rabbit IgG Vectastain® ABC Kit from Vector Laboratories, INC. (Burlingame, CA) was used for secondary staining. Light microscopy and IHC observations were taken with a BX-40 light microscope (Olympus) (Tokyo, Japan).
Apelin and APLNR gene expression was also measured by real-time PCR (rtPCR) from mRNA isolated from several human CCA tumors compared to non-malignant liver tissue. The human samples were obtained from Dr. Pietro Invernizzi (Humanitas Research Hospital, Rozzano, Italy) under a protocol approved by the Ethics Committee of the Humanitas Research Hospital; the protocol was reviewed by the Veterans’ Administration IRB and R&D Committee. The use of human tissue was also approved by the Texas A&M HSC Institutional Review Board.
Cell lines
The study was performed in six human CCA cell lines of different origin: Mz-ChA-1, TFK-1, SG231, CCLP-1, HuCC-T1, and HuH-28. The human intrahepatic CCA cell lines CCLP-1, HuCC-T1 and SG231 were a gift of Dr. A. J. Demetris of University of Pittsburgh (Pittsburgh, PA) [17–19]. The human extrahepatic CCA line, Mz-ChA-1, was a gift from Dr. G. Fitz (UT Southwestern Medical Center, Dallas, TX) [20]. The human intrahepatic biliary cell line, HuH-28 and the human extrahepatic biliary TFK-1 cells were obtained from the Cancer Cell Repository (Tohoku University, Japan); the cell lines were maintained as described [21–23]. The human immortalized, nonmalignant, cholangiocyte cell line, H69 [24,25], was obtained from Dr. G. J. Gores, Mayo Clinic, MN. Human hepatocytes were purchased from ScienceCell (Carlsbad, CA).
Expression of APLNR in non-malignant and CCA cell lines
APLNR expression was evaluated by immunofluorescence in H69 control chol-angiocytes and Mz-ChA-1 cells. Approximately 200,000 cells were plated on coverslips in a 6-well plate and grown 24–48 h until ~75% confluent. Mounted cells were fixed, washed, and incubated with primary antibody diluted 1:200 in 1% donkey serum overnight at 4 °C. Cells were incubated with AlexaFluor488 species appropriate secondary antibody (Jackson Immuno) diluted 1:100 in 1% donkey serum. Finally, coverslips mounted on slides with DAPI (Invitrogen) and imaged with a confocal microscope (Olympus FluoView 500 laser scan microscope with DP70 digital camera, Tokyo, Japan).
Measurement of APLNR expression was also performed using flow cytometry as described [26]. H69 and selected CCA cells were isolated, resuspended and incubated with slow agitation for 15 min at room temperature with anti-APLNR antibody at a dilution of 1:100. Then Alexa Fluor® 488 conjugated secondary antibody was added to suspension at a dilution of 1:50 and cells were incubated with slow agitation for 15 min at room temperature in the dark. Cells incubated without antibody or with only Alexa Fluor® 488 conjugated secondary antibody were used as negative controls. Cells were analyzed using (FACSCalibur, Becton Dickinson, San Jose, CA), with CellQuest Pro 5.2 software. At least 10,000 events in the light scatter (SSC/FSC) were acquired. The expression of apelin receptor was identified and gated on FL1-A/Count plots. The relative quantity of the selected protein (mean selected protein fluorescence intensity) was expressed as mean FL1-A (samples)/mean FL1-A (secondary antibodies only).
Expression of apelin in supernatant of non-malignant and CCA cell lines
Apelin levels measured from supernatant (incubated for 48 h at 37 °C) from H69 and selected CCA cell lines using the Apelin-36 (human) EIA Kit according to the manufacturer’s instructions (Phoenix Pharmaceuticals, INC.). Undiluted samples (50 mL) were prepared in triplicates according to the protocol. Absorbance O.D. was measured at 450 nm on a microplate spectrophotometer (VersaMax, Molecular Devices, Sunnyvale, CA). The PRISM® software (GraphPad) was used to prepare the standard curve and to calculate the concentration of apelin in each sample. Data is expressed as an average concentration ± SEM.
Treatment of CCA with apelin and APLNR antagonist
Mz-ChA-1 cells were cultured in 250 mL flasks until 90% confluent and transferred to 6 well plates with an equal number of cells in each well. Cells were cultured for 24 h under normal conditions with 5% serum media and then grown in serum free media for an additional 24 h. Cells were then treated with increasing concentrations of apelin (5, 10, 15 μM) and ML221 APLNR antagonist (7.5, 10, 15 μM) over various time points using standard solutions of 1 mM and 100 μM, respectively. To confirm our findings in Mz-ChA-1 cells, H69 cholangiocytes and additional CCA cell lines (HuH-28 and SG231) were treated with 10 μM of ML221 over 24 h. Human hepatocytes were also cultured as previously described and treated with 10 μM of apelin for 24 h. Cells were collected following treatment using TrypLE solution (Gibco®) and used for RNA isolation.
Expression of angiogenic and proliferative markers
Total RNA was isolated from treated CCA cells using the RNeasy Plus Micro Kit (Qiagen) (74034) according to protocol’s instructions. rtPCR analysis [27] was used to determine the effects of apelin and ML221 treatment on CCA cells. cDNA was created from 1200 μg of total RNA using iScript™ Reverse Transcription Supermix for rtPCR (Bio Rad). RtPCR was performed using human apelin primers (Qiagen) [28] and SYBR Green PCR Master Mix (SABiosciences) on the Agilent Technologies Mx3005P rtPCR system. Proliferation was evaluated by rtPCR using human primers for PCNA and Ki-67 (Qiagen). Markers of angiogenesis were measured using human primers (Qiagen) for vascular endothelial growth factor-A (VEGF-A), vascular endothelial growth factor-C (VEGF-C), angiopoietin 1 (Ang-1), and angiopoietin 2 (Ang-2). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, the housekeeping) primers (Qiagen) [29] were used for relative control. A ΔΔCT (delta delta of the threshold cycle) analysis was performed using H69 as the control sample [30]. Data is expressed as relative mRNA levels ± SEM.
Wound-healing assay
H69, HuccT, and Mz-ChA-1 cells were grown in a 6-well flask until 100% confluence was achieved. Cells were incubated at 37 °C in 5% albumin media and treated with 10 μM of ML221 or left untreated as a control. Using a 19G needle and 20-μL pipette tip, a wound was made through the cell monolayer. Serial images were obtained at time 0, 3, 6, 12, 24 and 48 h under light microscopy. Wound-healing was evaluated by measuring the total surface area of the image covered by the cells. Thus, as the cells began to migrate, the percentage of cell surface area increased. Measurements of control and ML221 treated cells were recorded as triplicates.
Invasion assay
H69, HuccT, and Mz-ChA-1 cells were grown in a 6-well flask until 60% confluence was achieved. Cells were incubated at 37 °C in 5% albumin media and treated with 10 μM of ML221 for 24 h or left untreated as a control. Cells were collect and transferred to the QCM ECMatrix Cell Invasion Assay chamber purchased from EMD Millipore (Billerica, MA). Invasion assay was conducted in aforementioned cell lines according to the assay’s protocol.
In vivo studies
Male BALB/c eight week old nude (nu/nu) mice were kept in a temperature and light controlled environment with free access to drinking water and rodent chow [31]. Three million Mz-ChA-1 cells were suspended in extracellular matrix gel and subcutaneously injected into the rear flanks of these nude mice. Mice were treated with ML221 (150 μg/kg) [32] 3× weekly via tail vein injection for 4 weeks. Tumor growth was measured three times a week using an electronic caliper, and volume was determined as follows: tumor volume (mm3) = length (mm) × width (mm) × height (mm). Tumors were allowed to grow until the maximum allowable tumor burden was reached, as set forth by the Baylor Scott & White Healthcare IACUC tumor burden policy. After 4 weeks of treatment, mice were euthanized with sodium pentobarbital (50 mg/kg i.p.). Hematoxylin and eosin (H&E) staining was performed using an H&E stain kit purchased from ScyTek Laboratories, INC. Tumors were confirmed to be primarily CCA cells by positive IHC staining and immunoblots for cytokeratin-19 (CK-19), a cholangiocyte specific marker [33]. IHC and immunoblots were used to demonstrate expression of APLNR, p-ERK and t-ERK. Alpha tubulin was used as a relative control using a mouse monoclonal anti-alpha tubulin antibody purchased from abcam. Markers of proliferation (PCNA, Ki-67), angiogenesis (VEGF-A, VEGF-C, Ang-1, and Ang-2) and tumor progression (Vimentin, MMP-9, MMP-3) (Qiagen) were measured via rtPCR using the aforementioned protocol.
Statistical analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by Student’s unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test. P < 0.05 was considered to be statistically significant.
Results
Expression of apelin and APLNR is increased in human CCA tissues
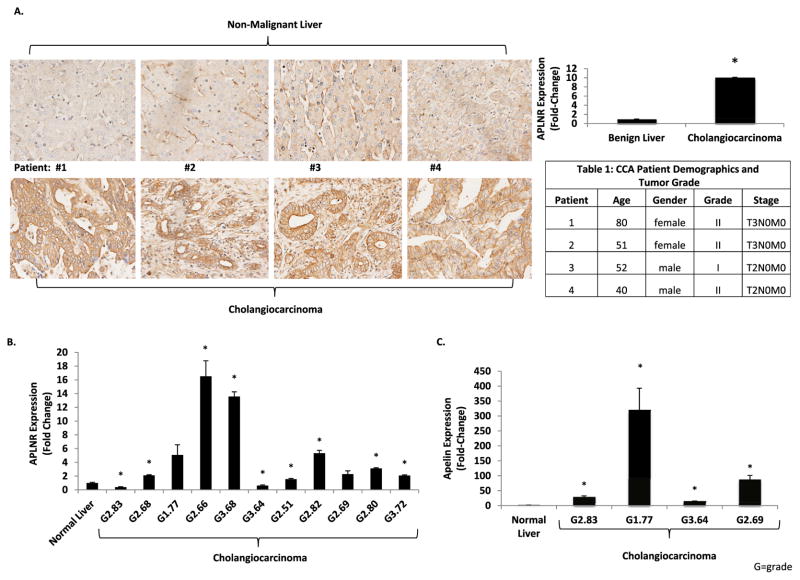
IHC images show positive staining and up-regulation of APLNR in CCA tissue compared to non-malignant liver tissue (Fig. 1A). Semi-quantitative analysis of CCA tissues in the tissue array shows significantly increased expression of APLNR in CCA tumors compared to non-malignant liver sections (Fig. 1A). In liver sections from benign samples, IHC demonstrated positive APLNR staining in cholangiocytes but minimal staining in hepatocytes (Supplementary Fig. 1A). RtPCR for APLNR (Fig. 1B) in human CCA tumors shows a significant up-regulation of gene expression in seven of eleven human CCA tumors. APLNR expression was up regulated in two other CCA tumors but failed to reach statistical significance (Fig.1B). Apelin expression was quantified by rtPCR in four of the same CCA tumor samples as previously shown in Fig. 1B. Apelin gene expression was significantly up regulated in all four CCA tumors (Fig. 1C).
Fig. 1.
A: Positive APLNR staining by IHC in four human CCA tissues (bottom row) compared to adjacent non-malignant liver tissues (top row). Semiquantitative analysis of IHC images shows significantly increased expression of APLNR in CCA tissues compare to non-malignant liver tissues (n = 18). Table 1: Corresponding patient demographics and tumor grade from patients included in IHC images. B: APLNR gene expression is increased by rtPCR in human CCA tissues (n = 3). Patient samples are labeled as G = tumor grade, followed by the patient age. C: Apelin gene expression is increased by rtPCR in human CCA tissues (n = 3). Patient samples are labeled as G = tumor grade, followed by the patient age (* = P < 0.05).
Expression of APLNR and apelin is increased in CCA cell lines
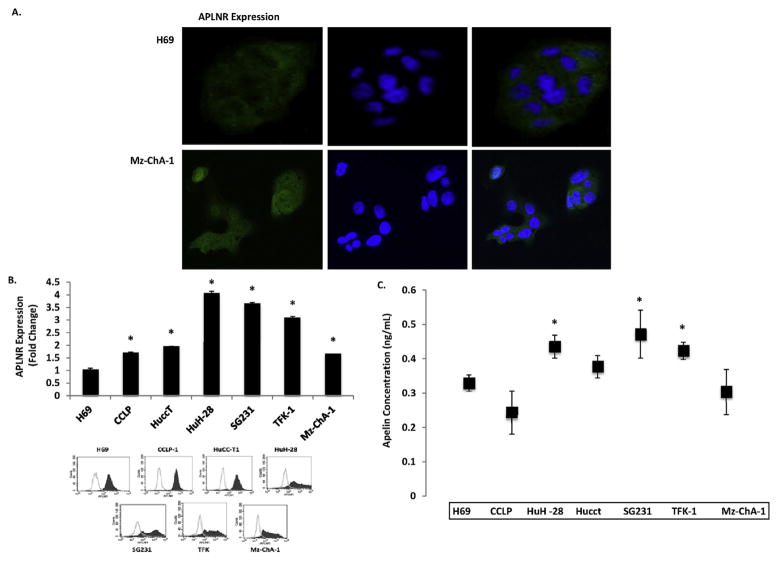
Immunofluorescence demonstrated that H69 cholangiocytes Mz-ChA-1 CCA cells express APLNR (Fig. 2A). Flow cytometry confirmed that APLNR expression is increased in CCA cells compared to H69 cells (Fig. 2B). Apelin secretion from CCA cells was identified by ELISA and found to be up regulated compared to non-malignant H69 cells (Fig. 2C).
Fig. 2.
A: APLNR is expressed in H69 control cholangiocytes (top row) and Mz-ChA-1 cholangiocarcinoma cells (bottom row) shown by immunofluorescence. B: APLNR expression is significantly increased in CCA cell lines compared to benign cholangiocytes (H69) shown by flow cytometry (n = 3). C: ELISA results show that Apelin is secreted from CCA cells in higher concentrations than benign cholangiocytes (H69) (n = 3) (* = P < 0.05).
Apelin promotes CCA proliferation and angiogenesis in vitro
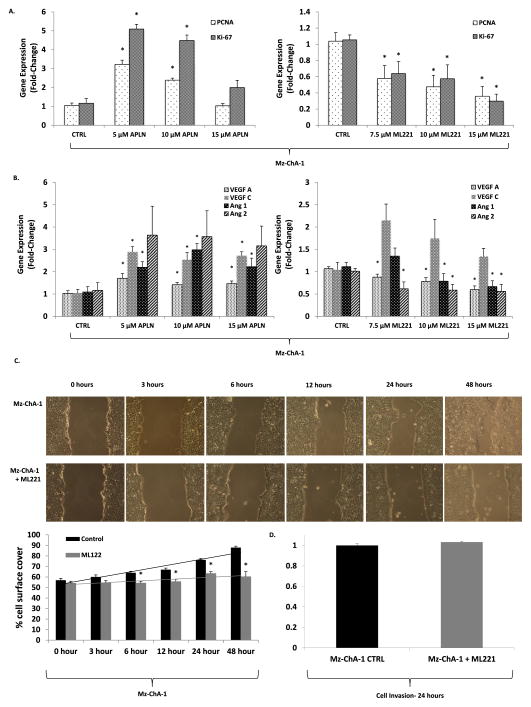
Proliferation (PCNA, Ki-67) and angiogenesis (VEGF A, Ang 1, Ang 2) markers in MzChA-1 CCA cells demonstrated a dose dependent response to treatment with apelin and APLNR antagonist (Fig. 3A–B). Apelin significantly increased expression of PCNA and Ki-67 compared to untreated cells at 5 and 10 μM, but this was not seen with a 15 μM treatment. ML221 treatment of 7.5, 10 and 15 μM significantly decreased PCNA and Ki-67 expression (Fig. 3A). Treatment of Mz-ChA-1 cells with 5, 10 and 15 μM of apelin for 24 h resulted in increased expression of angiogenesis factors (VEGF-A, VEGF-C, Ang-1, and Ang-2). Whereas, treatment of Mz-ChA-1 cells with 7.5, 10 and 15 μM ML221 for 24 h significantly decreased expression of VEGF-A, Ang-1 and Ang-2 (Fig. 3B). VEGF-C expression was increased in Mz-ChA-1 cells following ML221 treatment, but these results were not statistically significant. Treatment of human hepatocytes with apelin did not significantly alter expression of Ki-67 or PCNA (Supplementary Fig. 1B).
Fig. 3.
A: Apelin treatment promotes Mz-ChA-1 gene expression of Ki-67 and PCNA (left), whereas, ML221 treatment decreases Mz-ChA-1 gene expression of Ki-67 and PCNA in a dose dependent manner (n = 5) via rtPCR. B: Gene expression of angiogenic factors (VEGF-A, Ang-1, and Ang-2) is increased when Mz-ChA-1 cells are treated with increasing concentrations of apelin (left), whereas, gene expression is decreased with increasing concentrations of ML221, an APLNR antagonist (right) (n = 5) via rtPCR. C: 10 μM of ML221 treatment significantly decreases cell proliferation and migration at 6, 12, 24, and 48 h during wound-healing assay (* = P < 0.05). D: Mz-ChA-1 cell invasiveness did not significantly change following treatment with 10 μM of ML221 for 24 h compared to untreated control cells.
ML221 decreases Mz-ChA-1 cell migration
Results of the wound-healing assay in control and ML221 treated Mz-ChA-1 cells is shown in Fig. 3C. The percentage of cell surface coverage significantly increased in untreated cells at 6 h compared to ML221 treated cells. This difference became more pronounced at 12, 24, and 48 h. Near complete healing of the wound was seen at 48 h in the untreated Mz-ChA-1 cells, whereas, the ML221 treated cells showed minimal change in the percentage of cell surface coverage. Treatment of Mz-ChA-1 cells with ML221 did not significantly change cell invasion compared to untreated controls (Fig. 3D).
Wound-healing and cell invasion assays were repeated in H69 and HuccTcells treated with ML221. In H69 cells, ML221 significantly decreased wound-healing over 24 h, but statistical significance was lost at 48 h (Supplementary Fig. 2A). There was a trend towards decreased cell invasion in H69 cells using the cell invasion assay (P = 0.07) (Supplementary Fig. 2B). In HuccT cells, ML221 significantly inhibited wound-healing over 48 h compared to untreated cells (Supplementary Fig. 2C). HuccT cell invasion did not significantly change with ML221 treatment (Supplementary Fig. 2D).
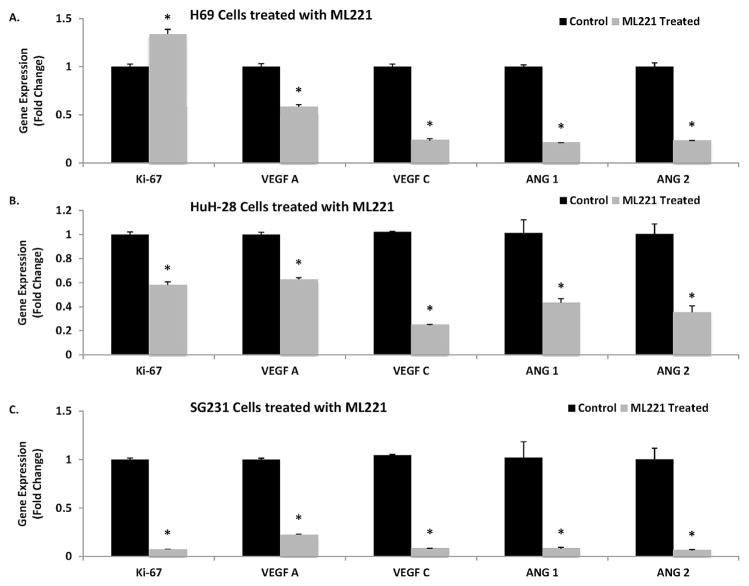
APLNR antagonist inhibits proliferation and angiogenesis in HuH-28 and SG231 cells
Control H69 human cholangiocytes and additional CCA cell lines (HuH-28 and SG231) were treated with 10 μM of ML221 for 24 h. H69 cells demonstrated increased expression of Ki-67, but significantly decreased expression of angiogenic factors (VEGF-A, VEGF-C, Ang-1 and Ang-2) (Fig. 4A). HuH28 cells treated with ML221 showed significantly decreased expression of Ki-67, as well as VEGF-A, VEGF-C, Ang-1 and Ang-2 (Fig. 4B). ML221 treatment also decreased expression of these factors in SG231 cells (Fig. 4C).
Fig. 4.
A: Treatment of benign human cholangiocytes (H69) with 10 μM of ML221 over 24 h increased Ki-67 gene expression, but significantly decreased expression of angiogenic factors (VEGF-A, VEGF-C, Ang-1, and Ang-2) via rtPCR. B. Treatment of HuH-28 cholangiocarcinoma cells with 10 μM of ML221 over 24 h significantly decreased Ki-67 gene expression, as well as angiogenic factors (VEGF-A, VEGF-C, Ang-1, and Ang-2) via rtPCR. C. Treatment of SG231 cholangiocarcinoma cells with 10 μM of ML221 over 24 h significantly decreased Ki-67 gene expression, as well as angiogenic factors (VEGF-A, VEGF-C, Ang-1, and Ang-2) via rtPCR.
APLNR antagonist inhibits CCA tumor growth in vivo
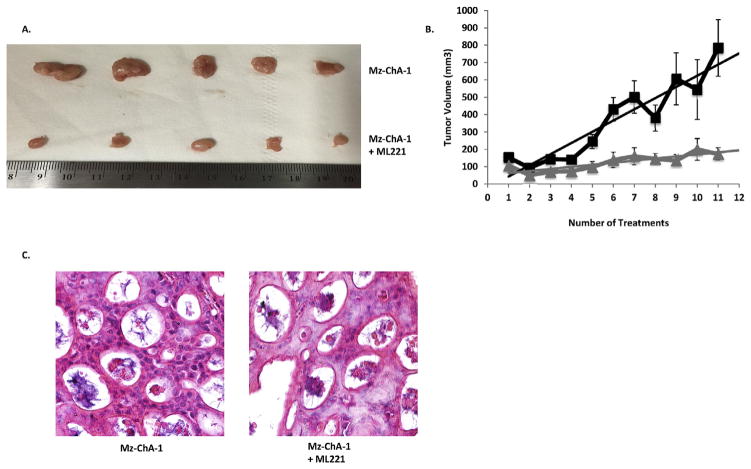
Tumor growth was significantly decreased in mice treated with APLNR antagonist compared to untreated control mice (Fig. 5A). Average tumor volumes in the treatment and control groups were recorded prior to each ML221 treatment and results are shown in Fig. 5B. Tumors in mice treated with ML221 were significantly smaller compared to the tumors in the untreated control mice. H&E staining was performed on paraffin embedded tumors that were collected from the control and ML221 treated mice. H&E staining confirmed that the xenograft tumors histologically resembled CCA (Fig. 5C). We did not identify any significant side effects of the ML221 treatments, but one mouse in the control group did have to be placed on medical watch due to excessive weight loss (15% of original body weight).
Fig. 5.
A: Parenteral administration of ML221 in nu/nu mice decreases Mz-ChA-1 tumor size (bottom row) compared to untreated control tumors (top row). B: Mz-ChA-1 tumor volume significantly increases in untreated control tumors (n = 9) compared to ML221 treated tumors (n = 12). C: H&E staining of control (left) and ML221 treated (right) Mz-ChA-1 tumors isolated from nu/nu mice.
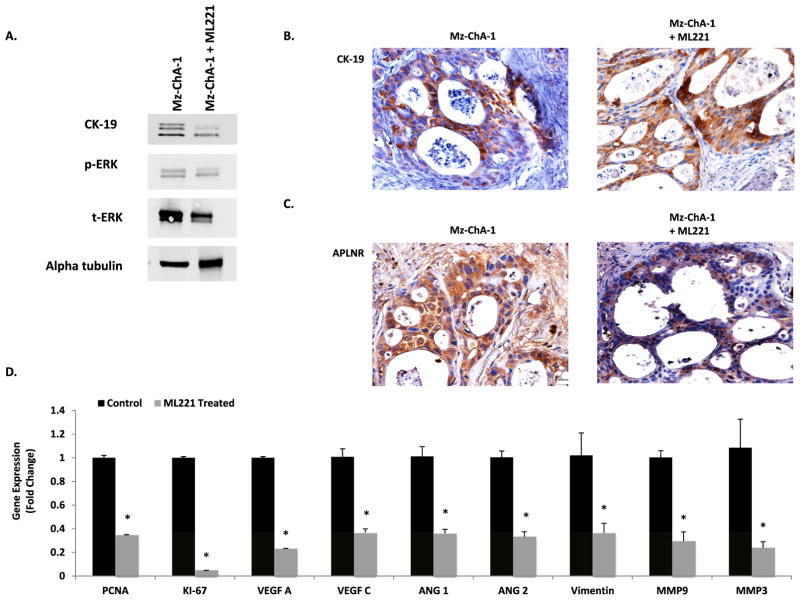
Immunoblots were performed on protein isolated form control and ML221 treated Mz-ChA-1 tumors (Fig. 6A). Control and ML221 treated tumors showed similar expression of CK-19. There was decreased expression of p-ERK and t-ERK in ML221 treated tumors. Control and ML221 treated Mz-ChA-1 tumors demonstrated positive CK-19 (Fig. 6B) and APLNR staining (Fig. 6C). RtPCR confirmed decreased gene expression of proliferative markers (PCNA, Ki-67), angiogenic factors (VEGF-A, VEGF-C, Ang-1, Ang-2), and markers of tumor progression (Vimentin, MMP-9, MMP-3) in tumors treated with ML221 compare to untreated controls (Fig. 6D).
Fig. 6.
A: Immunoblots of protein isolated from control and ML221 treated Mz-ChA-1 tumors shows expression of CK-19. p-ERK and t-ERK expression is decreased in ML221 treated Mz-ChA-1 tumors compared to untreated Mz-ChA-1 tumors. B: Control and ML221 treated Mz-ChA-1 tumors demonstrate positive staining for CK-19, a cholangiocyte specific marker, shown by IHC. C: IHC shows positive staining for APLNR in control and ML221 treated Mz-ChA-1 tumors. D. Mz-ChA-1 tumors treated with ML221 demonstrated decreased gene expression of proliferative markers (PCNA, Ki-67), angiogenic factors (VEGF-A, VEGF-C, Ang-1, and Ang-2), and markers of tumor progression (Vimentin, MMP-9, MMP-3) via rtPCR.
Discussion
Our results demonstrate the novel finding that the apelin/APLNR receptor axis participates in an autocrine/paracrine feedback loop to regulate cholangiocarcinoma growth and angiogenesis. Inhibition of APLNR signaling with an APLNR antagonist (ML221) significantly inhibited tumor growth in our xenograft model using human Mz-ChA-1 CCA cells. These results suggest that targeting the apelin/APLNR axis may provide new, tumor directed therapies to enhance CCA treatment strategies by inhibiting CCA tumor growth.
These results further show that the apelin receptor and its cognate peptide ligand, apelin, are important for tumor growth and angiogenesis. Sorli et al. demonstrated that apelin is a potent activator of neoangiogenesis, which in turn regulates tumor growth, using mouse mammary carcinoma cell clones (TS/A-apelin) [11]. Their data from a human cancer-profiling array shows that the apelin gene is expressed in a variety of benign and malignant tissues, however, the frequency of gene up regulation was high in carcinomas of the colon, skin and pancreas [11]. They did not see an increase of apelin gene expression in tumors of liver origin, however, carcinomas of the pancreatic head and extrahepatic CCA share similar features, including embryologic origin and several phenotypic characteristics [34].
Our in vitro data showed significant up regulation of apelin and APLNR in intrahepatic and extrahepatic cell lines. Not all tumors in our CCA tissue array showed increased APLNR expression and the array did not make a distinction between intrahepatic or extrahepatic tumors, so we are unable to determine whether or not expression of APLNR is dependent on tumor location. More research about the heterogeneity of apelin and APLNR expression in CCA is needed to determine if anatomical location changes expression of this axis, which would further impact potential therapeutic strategies. Furthermore, in normal liver samples APLNR expression was primarily located in cholangiocytes. Our CCA tissue array staining suggests that hepatocyte APLNR expression also increases in the presence of an adjacent CCA tumor. It is possible that the tumor microenvironment promotes up regulation of the apelin/APLNR axis; however, additional studies are needed to investigate these findings. These results parallel other studies in which hepatic APLNR expression is minimal in normal conditions but significantly up regulated in the setting of liver fibrosis and cirrhosis [35].
The physiologic conditions and signaling mechanisms that regulate apelin secretion and APLNR expression in malignant tissues appear to be multifactorial. Previous studies have shown that hyperplastic and neoplastic cholangiocytes secrete a variety of hormones, peptides, and growth factors that help regulate cell proliferation [36]. Hypoxia appears to be a major factor in tumor angiogenesis by increasing expression of VEGF and hypoxia-inducible factor (HIF) [5,37]. Similarly, Heo et al. demonstrated that apelin expression was significantly up regulated under hypoxic conditions in oral squamous cell carcinoma, which correlated with increased cell proliferation and migration [38]. Additionally, high levels of serum apelin in gastric and esophageal cancer patients correlates with high levels of C-reactive protein, indicating that apelin may be involved in the systemic inflammatory response of certain malignancies [39]. Studies in chronic liver diseases have also shown that hypoxia and inflammatory conditions are capable of inducing apelin expression, which creates an angiogenic and fibroproliferative response [40]. Additionally, Wan et al. demonstrated that apelin is a target gene for microRNA-224 (miR-224) and that low miR-224 levels correlates with elevated apelin levels in prostate cancer tissues, which is associated with increased cancer progression, advanced stage, and decreased disease-free survival [41].
Benign and malignant cholangiocytes proliferate in response to numerous peptides, hormones, and growth factors during normal physiologic conditions and in response to biliary injury [2,36]. Activation of the PKA/Src/MEK/ERK1/2 phosphorylation cascade is a common pathway that promotes cholangiocyte proliferation [42]. ERK1/2 signaling has also been implicated in the proliferation and autophagy of lung adenocarcinoma cells when stimulated with apelin [43]. Additionally, apelin-mediated ERK signaling has also been shown to regulate cardiomyocyte hypertrophy and to activate the expression of inflammatory cytokines in microglial cells [44,45]. Our results show that ML221 treatment decreased Mz-ChA-1 tumor expression of p-ERK and t-ERK, indicating that this pathway may become less active with ML221 treatment.
Previous studies have demonstrated that apelin’s involvement in cell proliferation is not limited to the ERK1/2 phosphorylation cascade. Masri et al. demonstrated that apelin induces a time-dependent phosphorylation of p70S6K, which is associated with transduction of PI3K and ERK phosphorylation cascades [46]. More recent studies have shown that apelin-mediated activation of PI3K/Akt is associated with proliferative and anti-apoptotic properties [47]. Zeng et al. demonstrated that apelin is neuroprotective by inhibiting apoptosis in cortical neurons via phosphorylation of Akt and ERK1/2 [48]. Additionally, apelin has been shown to stimulate proliferation and inhibit apoptosis in mouse osteoblasts via activation of JNK and PI3K/AKT signaling pathways [49]. APLNR signaling has also been shown to induce nitric oxide synthase in endothelial cells and decrease intracellular reactive oxygen species, however, it is unclear if these signaling properties also help regulate cell proliferation [47].
Our data shows that ML221 treatment decreased expression of angiogenic factors in a dose-dependent response. Angiogenesis has been considered critical to for the development and progression of CCA [50]. In recent studies, tumor-associated angiogenesis and lymphangiogenesis has been implicated in intrahepatic and hilar CCA disease progression, lymph node metastases, and overall prognosis [51–53]. Furthermore, microvascular density has been shown to significantly decrease 5-year survival rates [51]. Additionally, angiogenesis was linked to a poor prognosis in patients with node-negative intrahepatic cholangiocarcinoma [54]. These studies emphasize why targeting the mechanisms of angiogenesis and neovascularization in CCA, such as the apelin/APLNR axis, may help improve long-term survival.
The results of our in vivo experiments provide promising evidence that the apelin/APLNR axis is implicated in CCA growth and that targeting this axis with a receptor specific antagonist may help develop effective, tumor directed therapies. Not only do we show decreased proliferation and angiogenesis in ML221 treated tumors, but we also demonstrate decreased expression of vimentin, MMP-9 and MMP-3. Previous studies in CCA have shown that vimentin expression is induced by epithelial-mesenchymal transition (EMT) and is associated with progressive tumor growth and a poor prognosis [55]. MMP-9 and MMP-3 have also been implicated in cancer proliferation, angiogenesis and the induction of EMT [56].
These results are similar to previously mentioned studies in lung and colon cancer [14,43]. We did not identify any side effects to ML221 treatment in our xenograft model, however, since apelin signaling also regulated blood pressure and cardiac activity, it is possible significant side effects could develop in more advanced therapeutic trials. Furthermore, apelin signaling has been shown to be organ protective in specific circumstances such as cardiac ischemia/reperfusion injury and hemorrhagic shock [57,58]. Additionally, Chen et al. demonstrated that intranasal apelin treatment following an ischemic stroke was neuroprotective and induced angiogenesis in mice [59]. Additional studies focusing on dose optimization and potential systemic side effects are necessary to determine if the therapeutic benefits of an APLNR antagonist outweigh the potential risks.
This study does have some limitations to address. The amount of human data in this study is limited due to the availability of human tissues in our laboratory. Our data suggests that not all CCA tumors over-express apelin and its receptor. We are unable to make accurate predictions into the percentage of CCA tumors that over-express components of the apelin signaling pathway. The potential therapeutic benefit of an APLNR antagonist is tumor specific and may not be applicable to all patients with CCA. Additionally, our in vivo studies in immunocompromised mice provide a useful model, however, there is a degree of variability in tumor measurements and drug administration due to technical error. We attempted to minimize this error by having one person perform all measurements and treatments throughout the study period. Also, the design of our xenograft model allowed for frequent tumor measurements and ease of tumor collection, however, ML221 dosing, administration frequency, and treatment efficacy have to be considered in other models. Additionally, we only used one cell line to conduct our in vivo experiments. Our experience has shown that Mz-ChA-1 cells produce the most reliable tumors in our xenograft model and we have not been able to consistently grow tumors using other cell lines [27,60–63].
In conclusion, the apelin/APLNR axis regulates CCA growth and angiogenesis. Inhibition of apelin signaling with ML221, an APLNR antagonist, significantly inhibited tumor growth in our xenograft model using nu/nu mice. An APLNR antagonist may serve as a novel, tumor-directed, treatment strategy to limit tumor neo-vascularization and subsequent disease progression, however, additional studies are needed to determine its therapeutic potential.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit award to Dr. Alpini (5I01BX000574), a VA Merit Award (5I01BX002192) to Dr. Glaser, and the NIH grants DK58411, DK07698, and DK062975 to Drs. Alpini, and Glaser. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations
- APLNR
apelin receptor
- Ang 1
angiopoietin 1
- Ang 2
angiopoietin 2
- AT1
angiotensin-type 1
- CCA
cholangiocarcinoma
- CK-19
cytokeratin-19
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HIF
hypoxia-inducible factor
- IHC
immunohistochemistry
- PBS
phosphate buffered saline
- PSC
primary sclerosing cholangitis
- VEGF-A
vascular endothelial growth factor-A
- VEGF-C
vascular endothelial growth factor-C
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.canlet.2016.11.025.
Footnotes
Conflict of interest
The authors of this manuscript do not have any conflicts of interest in relation to the preparation and submission of this manuscript to Cancer Letters.
References
- 1.Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. J Gastroenterol Hepatol. 2002;17:1049–1055. doi: 10.1046/j.1440-1746.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 2.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 3.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 4.Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Lv SY, Ye W, Zhang L. Apelin/APJ system and cancer. Clin Chim Acta. 2016;457:112–116. doi: 10.1016/j.cca.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Quazi R, Palaniswamy C, Frishman WH. The emerging role of apelin in cardiovascular disease and health. Cardiol Rev. 2009;17:283–286. doi: 10.1097/CRD.0b013e3181b3fe0d. [DOI] [PubMed] [Google Scholar]
- 7.Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103:432–440. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 8.Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T, et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27:522–534. doi: 10.1038/sj.emboj.7601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidoya H, Takakura N. Biology of the apelin-APJ axis in vascular formation. J Biochem. 2012;152:125–131. doi: 10.1093/jb/mvs071. [DOI] [PubMed] [Google Scholar]
- 10.Kalin RE, Kretz MP, Meyer AM, Kispert A, Heppner FL, Brandli AW. Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumor angiogenesis. Dev Biol. 2007;305:599–614. doi: 10.1016/j.ydbio.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Sorli SC, Le Gonidec S, Knibiehler B, Audigier Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene. 2007;26:7692–7699. doi: 10.1038/sj.onc.1210573. [DOI] [PubMed] [Google Scholar]
- 12.Berta J, Hoda MA, Laszlo V, Rozsas A, Garay T, Torok S, et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget. 2014;5:4426–4437. doi: 10.18632/oncotarget.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berta J, Kenessey I, Dobos J, Tovari J, Klepetko W, Jan Ankersmit H, et al. Apelin expression in human non-small cell lung cancer: role in angiogenesis and prognosis. J Thorac Oncol. 2010;5:1120–1129. doi: 10.1097/JTO.0b013e3181e2c1ff. [DOI] [PubMed] [Google Scholar]
- 14.Picault FX, Chaves-Almagro C, Projetti F, Prats H, Masri B, Audigier Y. Tumour co-expression of apelin and its receptor is the basis of an autocrine loop involved in the growth of colon adenocarcinomas. Eur J Cancer. 2014;50:663–674. doi: 10.1016/j.ejca.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Muto J, Shirabe K, Yoshizumi T, Ikegami T, Aishima S, Ishigami K, et al. The apelin-APJ system induces tumor arteriogenesis in hepatocellular carcinoma. Anticancer Res. 2014;34:5313–5320. [PubMed] [Google Scholar]
- 16.Maloney PR, Khan P, Hedrick M, Gosalia P, Milewski M, Li L, et al. Functional antagonists of the Apelin (APJ) receptor, Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. [Google Scholar]
- 17.Shimizu Y, Demetris AJ, Gollin SM, Storto PD, Bedford HM, Altarac S, et al. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer J Int du cancer. 1992;52:252–260. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- 18.Miyagiwa M, Ichida T, Tokiwa T, Sato J, Sasaki H. A new human chol-angiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In vitro Cell Dev Biol J Tissue Cult Assoc. 1989;25:503–510. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- 19.Storto PD, Saidman SL, Demetris AJ, Letessier E, Whiteside TL, Gollin SM. Chromosomal breakpoints in cholangiocarcinoma cell lines, Genes, Chromosom. Cancer. 1990;2:300–310. doi: 10.1002/gcc.2870020408. [DOI] [PubMed] [Google Scholar]
- 20.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, et al. Biliary adenocarcinoma: characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–596. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 21.Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, et al. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–291. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 22.Kusaka Y, Tokiwa T, Sato J. Establishment and characterization of a cell line from a human cholangiocellular carcinoma, Research in experimental medicine. Z fur gesamte Exp Med Einschl Exp Chir. 1988;188:367–375. doi: 10.1007/BF01851205. [DOI] [PubMed] [Google Scholar]
- 23.Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, et al. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 24.Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, et al. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060–G1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- 25.Francis H, Demorrow S, Venter J, Onori P, White M, Gaudio E, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut. 2012;61:753–764. doi: 10.1136/gutjnl-2011-300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Kachapati K, Adams D, Wu Y, Leung PS, Yang GX, et al. Murine autoimmune cholangitis requires two hits: cytotoxic KLRG1(+) CD8 effector cells and defective T regulatory cells. J Autoimmun. 2014;50:123–134. doi: 10.1016/j.jaut.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Y, Demorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A, et al. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2011;301:G623–G633. doi: 10.1152/ajpgi.00118.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal SK, Kennedy PA, Scacheri PC, Novotny EA, Hickman AB, Cerrato A, et al. Menin molecular interactions: insights into normal functions and tumorigenesis. Hormone Metabol Res = Hormon- und Stoffwechselforschung = Hormones metabolisme. 2005;37:369–374. doi: 10.1055/s-2005-870139. [DOI] [PubMed] [Google Scholar]
- 29.Nowak K, Kuczek M, Ostropolska L, Malarska A, Wolny M, Baranowski T. The covalent structure of glyceraldehyde-phosphate dehydrogenase from human muscles. Isolation and amino acid sequences of peptides from tryptic digest. Hoppe-Seyler’s Z fur Physiol Chem. 1975;356:1181–1183. [PubMed] [Google Scholar]
- 30.DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, et al. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–13113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- 31.Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184–9193. doi: 10.1158/0008-5472.CAN-08-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birsen I, Gemici B, Acar N, Ustunel I, NIzgut-Uysal V. The role of apelin in the healing of water-immersion and restraint stress-induced gastric damage. J Physiol Sci. 2016 doi: 10.1007/s12576-014-0317-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onori P, Franchitto A, Alvaro D, Gaudio E. Immunohistochemical features of bile duct epithelial cells in normal and experimental liver conditions. Ital J Anat Embryol. 2001;106:371–378. [PubMed] [Google Scholar]
- 34.Schmuck RB, de Carvalho-Fischer CV, Neumann C, Pratschke J, Bahra M. Distal bile duct carcinomas and pancreatic ductal adenocarcinomas: postulating a common tumor entity. Cancer Med. 2016;5:88–99. doi: 10.1002/cam4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Principe A, Melgar-Lesmes P, Fernandez-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, et al. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology. 2008;48:1193–1201. doi: 10.1002/hep.22467. [DOI] [PubMed] [Google Scholar]
- 36.Hall C, Sato K, Wu N, Zhou T, Kyritsi T, Meng F, et al. Regulators of chol-angiocyte proliferation. Gene Expr. 2016 doi: 10.3727/105221616X692568. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heo K, Kim YH, Sung HJ, Li HY, Yoo CW, Kim JY, et al. Hypoxia-induced up-regulation of apelin is associated with a poor prognosis in oral squamous cell carcinoma patients. Oral Oncol. 2012;48:500–506. doi: 10.1016/j.oraloncology.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Diakowska D, Markocka-Maczka K, Szelachowski P, Grabowski K. Serum levels of resistin, adiponectin, and apelin in gastroesophageal cancer patients. Dis Markers. 2014;2014:619649. doi: 10.1155/2014/619649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melgar-Lesmes P, Pauta M, Reichenbach V, Casals G, Ros J, Bataller R, et al. Hypoxia and proinflammatory factors upregulate apelin receptor expression in human stellate cells and hepatocytes. Gut. 2011;60:1404–1411. doi: 10.1136/gut.2010.234690. [DOI] [PubMed] [Google Scholar]
- 41.Wan Y, Zeng ZC, Xi M, Wan S, Hua W, Liu YL, et al. Dysregulated microRNA-224/apelin axis associated with aggressive progression and poor prognosis in patients with prostate cancer. Hum Pathol. 2015;46:295–303. doi: 10.1016/j.humpath.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 42.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Su T, Lv D, Xie F, Liu W, Cao J, et al. ERK1/2 mediates lung adenocarcinoma cell proliferation and autophagy induced by apelin-13. Acta Biochim Biophys Sin (Shanghai) 2014;46:100–111. doi: 10.1093/abbs/gmt140. [DOI] [PubMed] [Google Scholar]
- 44.Xie F, Liu W, Feng F, Li X, He L, Lv D, et al. Apelin-13 promotes cardiomyocyte hypertrophy via PI3K-Akt-ERK1/2-p70S6K and PI3K-induced autophagy. Acta Biochim Biophys Sin (Shanghai) 2015;47:969–980. doi: 10.1093/abbs/gmv111. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Tao Y, Jiang Y. Apelin activates the expression of inflammatory cytokines in microglial BV2 cells via PI-3K/Akt and MEK/Erk pathways. Sci China Life Sci. 2015;58:531–540. doi: 10.1007/s11427-015-4861-0. [DOI] [PubMed] [Google Scholar]
- 46.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65-77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004;18:1909–1911. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 47.O’Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219:R13–R35. doi: 10.1530/JOE-13-0227. [DOI] [PubMed] [Google Scholar]
- 48.Zeng XJ, Yu SP, Zhang L, Wei L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res. 2010;316:1773–1783. doi: 10.1016/j.yexcr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang SY, Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, et al. Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides. 2007;28:708–718. doi: 10.1016/j.peptides.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Tang D, Nagano H, Yamamoto H, Wada H, Nakamura M, Kondo M, et al. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol Rep. 2006;15:525–532. [PubMed] [Google Scholar]
- 51.Thelen A, Scholz A, Benckert C, Schroder M, Weichert W, Wiedenmann B, et al. Microvessel density correlates with lymph node metastases and prognosis in hilar cholangiocarcinoma. J Gastroenterol. 2008;43:959–966. doi: 10.1007/s00535-008-2255-9. [DOI] [PubMed] [Google Scholar]
- 52.Thelen A, Scholz A, Benckert C, Weichert W, Dietz E, Wiedenmann B, et al. Tumor-associated lymphangiogenesis correlates with lymph node metastases and prognosis in hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15:791–799. doi: 10.1245/s10434-007-9774-0. [DOI] [PubMed] [Google Scholar]
- 53.Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gessner R, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105:1123–1132. doi: 10.1038/ajg.2009.674. [DOI] [PubMed] [Google Scholar]
- 54.Shirabe K, Shimada M, Tsujita E, Aishima S, Maehara S, Tanaka S, et al. Prognostic factors in node-negative intrahepatic cholangiocarcinoma with special reference to angiogenesis. Am J Surg. 2004;187:538–542. doi: 10.1016/j.amjsurg.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 55.Sato Y, Harada K, Itatsu K, Ikeda H, Kakuda Y, Shimomura S, et al. Epithelial-mesenchymal transition induced by transforming growth factor-{beta}1/Snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol. 2010;177:141–152. doi: 10.2353/ajpath.2010.090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y, Lv SY, Lyu SK, Wu D, Chen Q. The protective effect of apelin on ischemia/reperfusion injury. Peptides. 2015;63:43–46. doi: 10.1016/j.peptides.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Soliman M, Arafah M. Apelin protect against multiple organ injury following hemorrhagic shock and decrease the inflammatory response. Int J Appl Basic Med Res. 2015;5:195–199. doi: 10.4103/2229-516X.165377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen D, Lee J, Gu X, Wei L, Yu SP. Intranasal delivery of Apelin-13 is neuroprotective and promotes angiogenesis after ischemic stroke in mice. ASN Neuro. 2015;7 doi: 10.1177/1759091415605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeMorrow S, Onori P, Venter J, Invernizzi P, Frampton G, White M, et al. Neuropeptide Y inhibits cholangiocarcinoma cell growth and invasion. Am J Physiol Cell Physiol. 2011;300:C1078–C1089. doi: 10.1152/ajpcell.00358.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller T, Yang F, Wise CE, Meng F, Priester S, Munshi MK, et al. Simvastatin stimulates apoptosis in cholangiocarcinoma by inhibition of Rac1 activity. Dig Liver Dis. 2011;43:395–403. doi: 10.1016/j.dld.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han Y, Meng F, Venter J, Wu N, Wan Y, Standeford H, et al. miR-34a-dependent overexpression of Per1 decreases cholangiocarcinoma growth. J Hepatol. 2016;64:1295–1304. doi: 10.1016/j.jhep.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng F, Francis H, Glaser S, Han Y, DeMorrow S, Stokes A, et al. Role of stem cell factor and granulocyte colony-stimulating factor in remodeling during liver regeneration. Hepatology. 2012;55:209–221. doi: 10.1002/hep.24673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.