Abstract
Ano-genital warts are considered one of the commonest and highly infectious sexually transmitted infections. These warts are primarily caused by the human papillomavirus (HPV) of the family Papillomaviridae, genus alpha-papillomavirus, species 10 and types 6 and 11. However the high recurrence rate of warts is a matter of serious concern to the patients and a challenge for the treating physician. The conventional treatment options are targeted only to the local site of warts. There is no systemic treatment modality as there is limited understanding of the disease immune-pathogenesis. The role of cell-mediated immunity in combating HPV infection is not clearly defined. Hence the present study is aimed at investigating the CD4+ T helper (Th1 and Th2) and CD8+ T cell responses among wart patients. In this study, we compared HPV6 and HPV11 antigen-specific T cell responses among venereal wart patients relative to healthy controls. Significant decrease in percent frequencies of IFN-γ producing CD4+ and CD8+ T cells were observed in HPV infected wart patients. On the other hand, the frequency of CD4+ T cells expressing IL-4 was significantly increased in these patients as compared to healthy controls. The observed functional skewing of HPV specific T cells from Th1 to Th2 response in patients indicated suppressed immunity against the HPV. Moreover, decrease in CD8 T cell function correlated with poor wart clearance. Our findings open future avenues for exploring potential immunomodulation strategies as an adjunct to standard treatment for better management of these patients and prevention of recurrence.
Keywords: Human papillomavirus, Venereal warts, CD4 T cell, CD8 T cell, IFN-γ, IL-4
Introduction
Ano-genital warts are considered one of the commonest sexually transmitted infections (STIs) [32] with rising incidence worldwide [35]. Human papillomavirus HPV types 6 and 11 of the family Papillomaviridae, genus alpha-papillomavirus account for the majority (90%) of anogenital warts (AGWs) [8, 14, 22, 24, 25, 42]. These warts are highly infectious. Ano-genital warts manifest within 3 weeks to 8 months of exposure in approximately 65% of individuals [29]. Although the condition is benign and not associated with mortality, there are significant associated psychosocial issues associated [23, 31]. There are other common sub-types of HPV which are known to cause cervical cancer and dysplasia in some of the cases [30].
Management options for AGWs are solely local ablation of warts, either pharmacologically with podophyllin, trichloracetic acid, imiquimod cream, interferon, [43] or surgically with cryotherapy, laser or surgical excision [5, 28, 38, 44]. The main drawback of local ablation is high recurrence rate even after apparently complete clearance [9, 44, 45]. This is a major concern in treatment of ano-genital warts hence there is a need to explore alternate treatment modalities.
The role of the immune system in combating (HPV) is believed to be critical but still not completely understood. There are anecdotal reports regarding the role of the host immunity in treatment outcomes of ano-genital warts [1, 2, 7, 15, 19, 34, 37, 41]. The involvement of cell-mediated immune response has been demonstrated in venereal wart patients responding to treatment [17, 26]. Various agents like immunovir [33], interferon [16, 20] have been used to modulate the patients immunity based on poor immune response against the wart virus. Thus, an improved understanding of the immune-pathogenesis of HPV-induced warts might lead to better clinical management in future.
Here, we investigated the functional profile of HPV6 and HPV11 antigen-specific CD4+ and CD8+ T cell responses from peripheral blood of patients with genital warts in this study. Our findings demonstrated functional skewing of T cell responses in HPV wart patients.
Materials and methods
Study population
A total of 65 patients (55 males, 10 females between 18 and 64 years) with ano-genital warts were recruited from the STD OPD of the Department of Dermatology and Venereology at the Dr. Ram Manohar Lohia Hospital, New Delhi. The ano-genital wart patients had an average disease period of 5 months (minimum 1 week, maximum 12 months), were HIV negative and had no history of immunosuppressive disease, immunosuppressive therapy or skin warts. We also included age and sex matched twenty-six healthy individuals (Negative for HIV-1 and 2, HBV and HCV) as healthy controls (with no clinical evidence or history of any viral warts). These controls were examined for any illness. Patients with other immune-compromised conditions and pregnant females were excluded from the study.
The Institutional Ethics Committee approval was obtained for the study. All the patients were given the patient information sheet and informed written consent was obtained prior to recruitment.
Sample collection and peripheral blood mononuclear cells (PBMCs) isolation
Approximately, 8–10 ml of peripheral blood was collected in heparinised tubes, from all the recruited subjects by vene-punctures. The subjects other than controls under study were provided the standard treatment after sample collection. Peripheral Blood Mononuclear cells (PBMCs) were isolated from heparinised blood within 3 h of collection by Ficoll Hypaque Gradient Centrifugation (Lymphoprep, Oslo, Norway) and suspended in RPMI-1640 and 10% heat inactivated fetal calf serum (FCS) supplemented with 2 mM Glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin. After proper washing with RPMI supplemented with FCS, these cells were used for subsequent immunophenotyping. The viability of cells was measured by trypan blue dye exclusion test.
Synthetic HPV 6 and 11 antigens
The HPV 6 and HPV 11 recombinant antigens for in vitro cell culture were synthesized commercially and procured (ProSpec, USA). Recombinant HPV6 antigen (Catalogue No. HPV 003) and HPV11 antigen (Catalogue No. HPV-004) are sequences of immunodominant antigens that are expressed in E. coli. Lyophilized antigens were dissolved in PBS at a concentration of 100 mg/ml (stock) and the aliquots were stored at −70 °C for use. The titrated dose of 2.5 µg/ml of culture was used for both the antigens.
In vitro stimulation of PBMCs
For each patient sample, the PBMCs were isolated, suspended in complete T cell medium (2 × 106 cells/ml) and were cultured in 96-well plates (round-bottomed). Recombinant HPV6 or HPV11 antigen and 10 µg/ml brefeldin A/Golgi transport blocker (Sigma) were added to the culture at 37 °C in 5% CO2 incubator for 24 h. For each set of stimulation one set of unstimulated PBMC and one negative control of Fluorescent minus one (FMO) were set up. All the samples were further processed for cell surface and intracellular staining with titrated antibody dose.
Staining for cell surface markers and intracellular cytokines
Cells were stained with fluorescently conjugated antibodies for cell surface markers including anti-CD3 PE-cy5 (eBiosciences, CA, USA) and anti-CD8 ECD (Beckman Coulter, USA). Thereafter intracellular staining with anti-IFN-γ-FITC and anti-IL-4 PE (eBiosciences, CA, USA) was performed by standard protocol. Briefly, cells were given EDTA (2 mM) treatment for 15 min followed by FACS lysing solution (BD Biosciences) for 10 min at room temperature. The cells were then washed once (400×g for 5 min) using FACS buffer (PBS, 0.5% bovine serum albumin, 0.1% sodium azide) and permeabilizing solution II (1×) was added (BD Biosciences) and incubated at room temperature for 10 min. After washing twice in FACS buffer (400×g for 5 min), the cells were stained with a mixture of fluorochrome conjugated antibodies specific for IFN-γ and IL-4 for 30–45 min. After washing again, the cells were resuspended in PBS and all samples were fixed with 2% paraformaldehyde before acquisition.
Flow cytomtery and data analysis
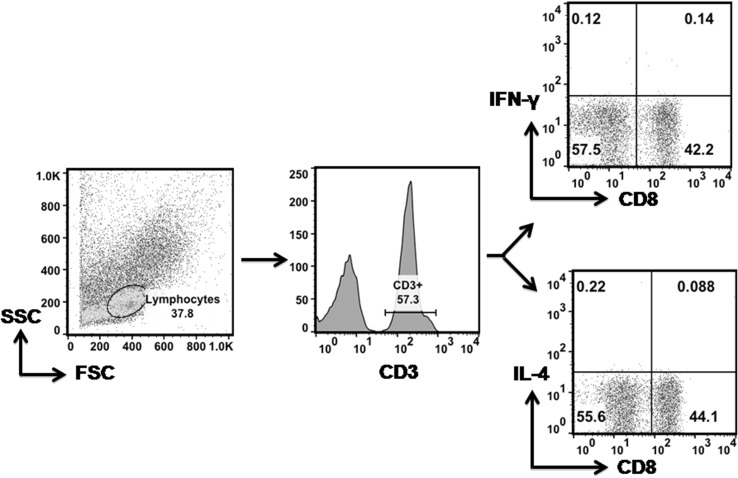
Stained cells were acquired on FC500 (Beckman Coulter) and Listmode raw cytometer data files were analyzed by the in-built CXP analysis software. For each analysis 500,000 events were acquired. The lymphocytes gate was defined on Forward and Side scatter. CD3+ T cells were further gated on CD4+ and CD8+ T cells and evaluated for IFN-γ or IL-4 positivity (Fig. 1). Percent frequency of CD4+ IFN-γ+, CD4+ IL-4+ and CD8+ IFN-γ+ were determined relative to un-stimulated control.
Fig. 1.
Gating strategy for defining IFN-γ and IL-4 producing CD4+ and CD8+ T cells. Left panel represents scatter profile of PBMCs gated on lymphocytes with forward scatter (FSC) on x-axis and side scatter (SSC) on y-axis. Lymphocytes stained with fluorescently conjugated anti-CD3 PECy5 and CD8 ECD were gated on CD3+ T cells as shown in a histogram (middle panel). The CD3+ T cells were further gated on CD8+ T cell subset and CD8- population was taken as CD4+ (right panel). Dot plots show CD4+ IFN-γ+ and CD8+ IFN-γ+ population (top right panel) and CD4+ IL-4+ subset (bottom right panel). The CD8 T cell subset producing IL-4 was negligible
Statistical analysis
SPSS version 18 was used to perform the statistical analysis by independent t test. P value of <0.05 was considered statistically significant.
Results
Patient characteristics
The study participants included 65 sexually active HPV-infected individuals and 26 healthy controls. The age ranged from 18 to 65 years. Male and female ratio was 5.5:1. The number of warts in HPV-infected patients at presentation varied from 2 to 50. The size of the wart varied from 1 to 30 mm. The warts were mainly seen on penile shaft, prepuce, glans penis and perianal, scrotum, gluteal region and buttocks in males and on labia majora, labia minora, vulva, perineal area and perianal region in females.
Th1 response to HPV6 and HPV11 antigens among wart patients
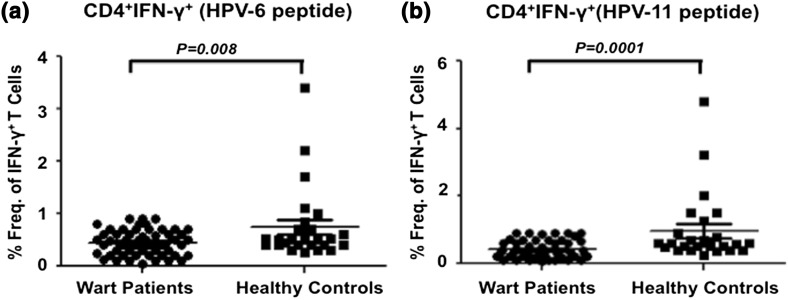
We compared the HPV6 and HPV11 antigen-specific Th1 (CD4+ IFN-γ+) responses of ano-genital wart patients with healthy controls. Analysis of intracellular IFN-γ expression in in vitro antigen stimulated PBMCs from all the patients demonstrated a significantly reduced frequency of HPV6-specific IFN-γ+ CD4+ T cells (Fig. 2a). The mean value of HPV6-specific CD4+ T cells expressing IFN-γ was 0.44 (SD ± 0.24) in patients with wart as opposed to 0.74 (SD ± 0.70) in HCs (Fig. 3a). The P value of 0.003 between the two groups was highly statistically significant. Furthermore, similar pattern of HPV antigen specific T cell immune response was observed after stimulation with HPV11 antigen. The mean percent frequency of HPV11 antigen stimulated T cells expressing IFN-γ was decreased with a value of 0.41 (SD ± 0.26) in subjects with warts in contrast to 0.96 (SD ± 1.0) in HCs with a significance of P < 0.001 (Fig. 3b).
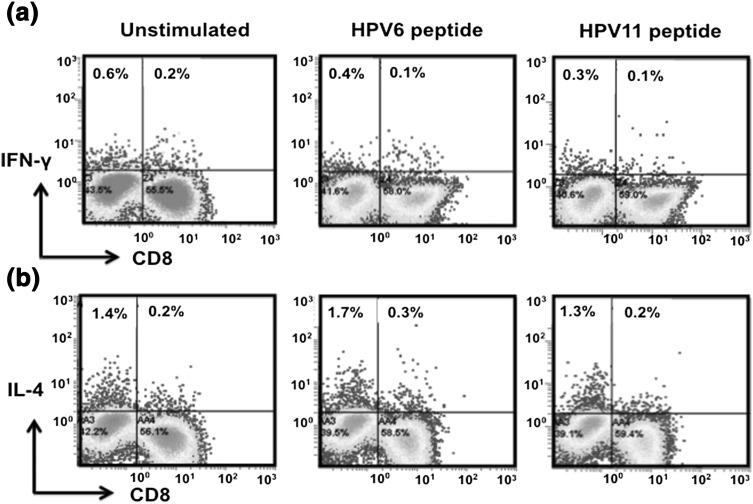
Fig. 2.
HPV antigen-specific CD4 and CD8 T cell immune responses in wart patients. PBMCs obtained from the patient were stimulated with recombinant HPV-6 or HPV-11 antigen for 24 h in presence of Brefeldin A to block secretion of cytokines. Intracellular cytokine staining was performed to determine IFN-γ+ and IL-4+ producing CD4 and CD8 T cell subsets. Unstimulated PBMCs served as a negative control. a Representative dot plots showing IFN-γ producing CD4 and CD8 T cell population under conditions as indicated. b Representative dot plots showing CD4+ IL-4+ T cell subset under the indicated conditions
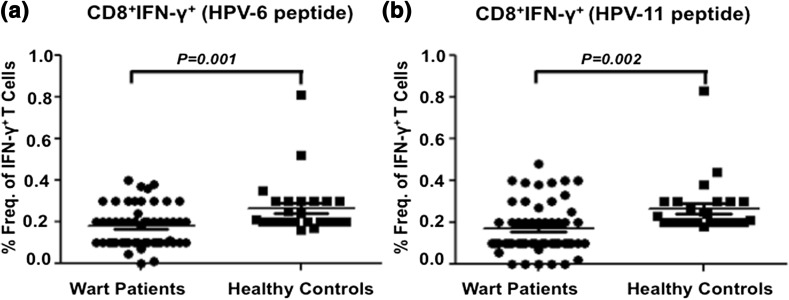
Fig. 3.
Th1 response to HPV6 and HPV11 antigens among wart patients and healthy controls. PBMCs were stimulated with either HPV-6 or HPV-11 recombinant antigen in vitro for 24 h and percent frequency of CD4+ T cell subset producing IFN-γ+ was determined relative to unstimulated control. a Cumulative scatter plots showing percent frequency of HPV-6 antigen-specific CD4+ T cells producing IFN-γ+ in wart patients (n = 65) and healthy controls (n = 25); b percent frequency of CD8+ T cells producing IFN-γ+ in response to HPV-11 in wart patients (n = 65) and healthy controls (n = 25) is shown as a cumulative scatter plot. P value < 0.05 was considered significant and <0.001 as highly significant
Th2 response to HPV6 and HPV 11 antigens
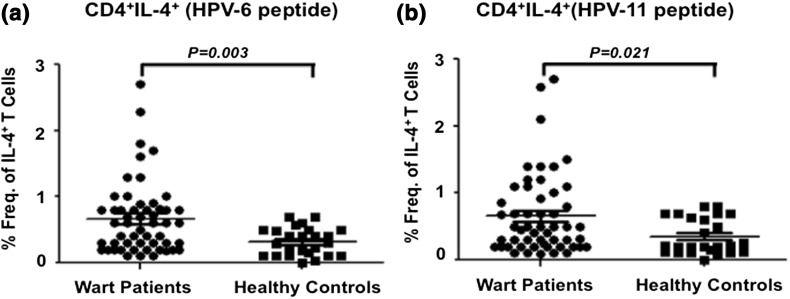
In contrast to Th1 response in wart patients, analysis of intracellular IL-4 expression in in vitro antigen stimulated PBMCs from all the patients demonstrated a significantly reduced frequency of HPV6-specific IL-4+ CD4+ T cells (Fig. 2b). The mean percent frequency of HPV6 antigen stimulated T cells expressing IL-4 was 0.66 (SD ± 0.55) in wart patients and was significantly higher (p = 0.003) than HCs (mean ± SD = 0.32 ± 0.21) (Fig. 4a). Consistently, the mean percent frequency of HPV11 antigen stimulated T cells expressing IL-4 was significantly increased (p < 0.001) in subjects with wart with mean value of 0.64 (SD ± 0.60) than with mean value of 0.35 (SD ± 0.26) in HCs (Fig. 4b).
Fig. 4.
Th2 response to HPV6 and HPV11 antigens among wart patients and healthy controls. PBMCs were stimulated with either HPV-6 or HPV-11 recombinant antigen in vitro and percent frequency of CD4+ T cell subset producing IL-4 was determined relative to unstimulated control. a Cumulative scatter plots showing percent frequency of CD4+ T cells producing IL-4+ in response to HPV-6 antigen in wart patients (n = 65) and healthy controls (n = 25); b HPV-11 specific percent frequency of CD4+ T cells producing IL-4 in wart patients and healthy controls is shown as a cumulative scatter plot. P value <0.05 was considered significant and <0.001 as highly significant
CD8+ T cell response to HPV6 and HPV 11 antigens
Since role of CD8+ T cells in clearing the viral infection is pivotal, therefore we determined CD8+ T cells responses against HPV6 and HPV11 antigens in wart patients and compared to healthy controls. Significantly reduced HPV6 antigen stimulated CD8+ IFN-γ+ T cell response was observed in wart patients (mean ± SD = 0.17 ± 0.1) as compared to healthy controls (mean ± SD = 0.26 ± 0.13) (Fig. 5a). The P value of 0.001 between the two groups was statistically significant. Similar pattern was observed for HPV11 antigen stimulated T cells with low frequency of IFN-γ+ CD8+ T cells (mean ± SD = 0.17 ± 0.12) as compared to healthy controls (mean ± SD = 0.26 ± 0.13) with a statistical significant P value of 0.001 between the groups (Fig. 5b). Negligible CD8+ IL-4+ T cell subset was observed in the study subjects.
Fig. 5.
CD8 T cell response to HPV6 and HPV11 antigens among wart patients and healthy controls. PBMCs were stimulated with either HPV-6 or HPV-11 recombinant antigen in vitro and percent frequency of CD8+ T cell subset producing IFN-γ was determined relative to unstimulated control. a Cumulative scatter plots showing percent frequency of CD8+ T cells producing IFN-γ+ in response to HPV-6 antigen in wart patients (n = 65) and healthy controls (n = 25); b percent frequency of CD8+ T cells producing IFN-γ+ in response to HPV-11 antigen in wart patients and healthy controls is shown as a cumulative scatter plot. P value <0.05 was considered significant and <0.001 as highly significant
Discussion
Active cell-mediated immune response is paramount in clearing HPV infection from the genital tract [4]. Prolonged course of genital warts can be attributed defective T-cell mediated immune response [12, 13]. Individuals with increase in size and/or number of genital warts are primarily in a state of immune suppression such as HIV/AIDS, pregnancy, immune-suppressive therapy. Although the host immune players including monocyte-macrophage system and lymphocytes of various sub-types play major role against HPV infection but understanding of their involvement in clearance of HPV is limited. Here, we dissected the HPV antigen-specific CD4+ and CD8+ T cell responses among HPV-infected patients with ano-genital warts.
Our findings revealed reduced percent frequency of both CD4+ IFN-γ+ and CD8+ IFN-γ+ cells and increased frequency of Th2 (CD4+ IL4+) cells in wart patients as compared to healthy controls. The magnitude of immune response against HPV6 antigen was observed to be more robust than the corresponding HPV11 antigen-specific response in anogenital wart patients. Only few studies so far have reported the role of localized immunity with diminished Th1 and increased Th2 response in patients infected with HPV6 or/and 11 at the site of the warts [10, 36]. To the best of our knowledge, till date no study has reported the role of HPV antigen-specific immune response in subjects with ano-genital warts due to highly localized nature, lack of significant systemic manifestations of the infection. Regression of genital warts in humans and animal models provide an indirect evidence suggesting that HPV might trigger a Th1 type cell-mediated immune response [11]. However, there is a paucity of available data regarding the functional characterization of cell-mediated immune responses specific to HPV 6 and 11 antigens.
We demonstrated HPV antigen-specific functional skewing of protective (Th1) versus suppressive (Th2) immune response in peripheral blood from subjects with warts. Immune-inhibitory Th2-type cytokines (IL-4), which predominantly induce humoral immunity that eliminates the extracellular infections and toxins, which unlike intracellular pathogens are exposed to antibodies in blood and other body fluids, these cytokines act as B-cells activating factors. This indicates that predominantly Th2- helper cells response will induce B cells proliferation that can generate specific neutralizing antibodies [6].
Down-regulation of cellular response may suppress clearance of virus-infected and tumor cells [4]. Patients with defective cell mediated immune response (HIV/AIDS, transplant recipients) have higher incidence of HPV infection indicating that the cell mediated immunity may have an important role in control of HPV infections [18, 40]. Our results also indicate weakened Th1 and T cytotoxic cell-mediated immunity against HPV infections that might be responsible for persistent genital warts. This reduced cell-mediated immunity in wart patients correlated with elevated levels of IL-4 producers, which might inhibit the development of a cellular response by inhibition of IFN-γ+ production. These results are in agreement with other studies, wherein reduced Th1 response and increased Th2 response have been correlated with lesion progression [1, 10, 36]. These results are also in agreement with other studies, which observed decreased levels of IFN-γ and TGF-β in HPV16 and CIN compared to normal HPV-negative tissue [46]. Low concentration of interferon gamma (IFN-γ) revealed that there is a significant decrease (P < 0.05) in GW patients as compared to healthy control. Our observation of incidence of reduced frequency of HPV specific IFN-γ+ CD4 and CD8 T cell responders in peripheral blood is consistent with the earlier studies conducted in localized warts. In contrast, increased frequency of HPV antigen-specific IL-4 producing CD4 T cells observed in peripheral blood of wart patients suggested that genital warts induced a systemic immune suppression in the host. Our results strengthen the evidences pointing to a weakened and Th2 skewed HPV specific host T cell immune response in warts thus strongly supporting a need for an adjunct immunomodulatory therapy in this condition. Therefore the desired strategies for HPV associated lesions should target boosting T-cell mediated immune responses not ignoring the viral mediated immune evasion [3, 21, 27, 39].
There is a need to design therapeutic strategies for genital warts that would target boosting the virus specific immune response using adjunct immunomodulatory therapy for treatment and/or for prevention. Defining the role played by HPV6 and 11 antigen-specific T cells in the pathogenesis of venereal wart, can pave the way in developing a prognostic immunological assays. The data reported in our study also provides convincing evidence that ano-genital warts in humans are strongly associated with Th2 skewed peripheral T cell response. Our study findings may be helpful in designing future immunomodulation strategies which may be used as adjunct immunotherapy to boost host immune response in patients of ano-genital warts. This might help in prevention of recurrence of warts after treatment. Thus our findings warrant future investigations to design specifically targeted, effective immune-prophylactic and immune-therapeutic strategies against HPV-induced lesions of the genital tract, which might lead to early clearance and prevention of relapse.
Acknowledgements
The authors hereby acknowledge the valuable contribution of Junior and Senior Residents of Department of Dermatology, Dr. Ram Manohar Lohia Hospital, New Delhi for patient recruitment, Mr. N.P. Singh and Mr. Pankaj, Lab. technician, Abhinav Saurabh, Prabin and Girija, SRF, Department of Transplant Immunology and Immunogenetics, AIIMS for their laboratory support and Dr. Abha Agrawal, Former Scientist ‘F’, National Institute of Medical Statistics, New Delhi for statistical analysis.
Footnotes
Hemanta Kumar Kar formerly affiliated at Dr. Ram Manohar Lohia Hospital, Baba Kharak Singh Marg, New Delhi 110001, India.
References
- 1.Al-Saeed IAM, Al-Saadi MAK, Ewadh WAA. Immunological study on genital wart patients in Babylon Province-Iraq. Med J Babylon. 2015;12(1):233–239. [Google Scholar]
- 2.Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5:2624–2642. doi: 10.3390/v5112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard P, McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-alpha. Virology. 1999;259:305–313. doi: 10.1006/viro.1999.9771. [DOI] [PubMed] [Google Scholar]
- 4.Benton C, Shahdullah H, Hunter JAA. Human papillomavirus in the immunocompromised. Papillomavirus Rep. 1992;3:23–26. [Google Scholar]
- 5.Brodell LA, Mercurio MG, Brodell RT. The diagnosis and treatment of human papillomavirus-mediated genital lesions. Cutis. 2007;79:5–10. [PubMed] [Google Scholar]
- 6.Broomall EM, Reynolds SM, Jacobson RM. Epidemiology, clinical manifestations, and recent advances in vaccination against human papillomavirus. Postgrad Med. 2010;122(2):121–129. doi: 10.3810/pgm.2010.03.2129. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Zhao J, Lei Z, Shen S, Liu C, Li D, Liu J, Shen GX, Zhang GM, Feng ZH, et al. Local accumulation of FOXP3+ regulatory T cells: evidence for an immune evasion mechanism in patients with large condylomata acuminate. J Immunol. 2008;180:7681–7686. doi: 10.4049/jimmunol.180.11.7681. [DOI] [PubMed] [Google Scholar]
- 8.Castellsague X, Ghaffari A, Daniel RW, Bosch FX, Munoz N, Shah KV. Prevalence of penile human papillomavirus DNA in husbands of women with and without cervical neoplasia: a study in Spain and Colombia. J Infect Dis. 1997;176:353–361. doi: 10.1086/514052. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines. MMWR. 2006;55(No.RR-11):62–67. [PubMed] [Google Scholar]
- 10.Clerici M, Merola M, Ferrario E. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J Natl Cancer Inst. 1997;89(3):245–250. doi: 10.1093/jnci/89.3.245. [DOI] [PubMed] [Google Scholar]
- 11.Coleman N, Birley HD, Renton AM, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 12.Critchlow CW, Holmes KK, Wood R, Krueger L, Dunphy C, Vernon DA, Daling JR, Kiviat NB. Association of human immunodeficiency virus and anal human papillomavirus infection among homosexual men. Arch Intern Med. 1992;152:1673–1676. doi: 10.1001/archinte.1992.00400200105019. [DOI] [PubMed] [Google Scholar]
- 13.Critchlow CW, Hawes SE, Kuypers JM, Goldbaum GM, Holmes KK, Surawicz CM, Kiviat NB. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12:1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 14.De Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Devoti J, Hatam L, Lucs A, Afzal A, Abramson A, Steinberg B, Bonagura V. Decreased langerhans cell responses to IL-36γ: altered innate immunity in patients with recurrent respiratory papillomatosis. Mol Med. 2014;20:372–380. doi: 10.2119/molmed.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einhorn N, Ling P, Strander H. Systemic interferon alpha treatment of human condylomata acuminata. Acta Obstet Gynecol Scand. 1983;62:285–287. doi: 10.3109/00016348309155812. [DOI] [PubMed] [Google Scholar]
- 17.Elliot J, Androphy DRL. Warts. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s dermatology in general medicine. 7. New York: McGraw-Hill; 2008. pp. 1914–1922. [Google Scholar]
- 18.Fairley CK, Chen S, Tabrizi SN, McNeil J, Becker G, Walker R, Atkins RC, Thomson N, Allan P, Woodburn C, et al. Prevalence of HPV DNA in cervical specimens in women with renal transplants: a comparison with dialysis-dependent patients and patients with renal impairment. Nephrol Dial Transplant. 1994;9:416–420. [PubMed] [Google Scholar]
- 19.Feng JY, Peng ZH, Tang XP, Geng SM, Liu YP. Immunohistochemical and ultrastructural features of langerhans cells in condyloma acuminatum. J Cutan Pathol. 2008;35:15–20. doi: 10.1111/j.1600-0560.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 20.Gall SA, Hughes CE, Trofatter K. Interferon for the therapy of condyloma acuminatum. Am J Obstet Gynecol. 1985;153:157–163. doi: 10.1016/0002-9378(85)90103-6. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195(3):346–355. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 22.Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, Munoz N, Schiffman M, Bosch FX. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(Suppl 10):K17–K28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graziottin A, Serafini A. HPV infection in women: psychosexual impact of genital warts and intraepithelial lesions. J Sex Med. 2009;6:633–645. doi: 10.1111/j.1743-6109.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 24.Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995;33(8):2058–2063. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med MicrobiolImmunol (Berl) 2004;193:35–44. doi: 10.1007/s00430-003-0181-2. [DOI] [PubMed] [Google Scholar]
- 26.Iwatsuki K, Tagami H, Takigawa M, Yamada M. Plane warts under spontaneous regression. Immunopathologic study on cellular constituents leading to the inflammatory reaction. Arch Dermatol. 1986;122:655–659. doi: 10.1001/archderm.1986.01660180061015. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Modlin RL, Moy RL, et al. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T-cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 28.Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician. 2004;70:2335–2342. [PubMed] [Google Scholar]
- 29.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(Issue 3):35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Lombard I, et al. Human papillomavirus genotype as a major determinant of the course of cervical cancer. J Clin Oncol. 1998;16(8):2613–2619. doi: 10.1200/JCO.1998.16.8.2613. [DOI] [PubMed] [Google Scholar]
- 31.Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS. 1998;9:571–578. doi: 10.1258/0956462981921143. [DOI] [PubMed] [Google Scholar]
- 32.Milner DA. Diagnostic pathology: infectious diseases. Amsterdam: Elsevier Health Sciences; 2015. p. 40. [Google Scholar]
- 33.Mohanty KC, Scott CS. Immunotherapy of genital warts with inosine pranobex (Imunovir): preliminary study. Genitourin Med. 1986;62:352–355. doi: 10.1136/sti.62.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morelli AE, Belardi G, DiPaola G, Paredes A, Fainboim L. Cellular subsets and epithelial ICAM-1 and HLA-DR expression in human papillomavirus infection of the vulva. Acta Derm Venereol. 1994;74:45–50. doi: 10.2340/00015555744550. [DOI] [PubMed] [Google Scholar]
- 35.Narayanan B. A retrospective study of the pattern of sexually transmitted diseases during a ten-year period. Indian J Dermatol Venereol Leprol. 2005;71:333–337. doi: 10.4103/0378-6323.16784. [DOI] [PubMed] [Google Scholar]
- 36.Peghini BC, Abdalla DR, Barcelos AC, Teodoro L, Murta EF, Michelin MA. Local cytokine profiles of patients with cervical intraepithelial and invasive neoplasia. Hum Immunol. 2012;73(9):920–926. doi: 10.1016/j.humimm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Sasagawa T, Takagi H, Makinoda S. Immune response against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012;18:807–815. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 38.Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5. [PubMed] [Google Scholar]
- 39.Schneider A, Papendick U, Gissmann L, De Villiers EM. Interferon treatment of human genital papillomavirus infection: importance of viral type. Int J Cancer. 1987;40:610–614. doi: 10.1002/ijc.2910400506. [DOI] [PubMed] [Google Scholar]
- 40.Sillman F, Stanek A, Sedlis A, Rosenthal J, Lanks KW, Buchhagen D, Nicastri A, Boyce J. The relationship between human papillomavirus and lower genital intraepithelial neoplasia in immunosuppressed women. Am J Obstet Gynecol. 1984;150:300–308. doi: 10.1016/S0002-9378(84)90369-7. [DOI] [PubMed] [Google Scholar]
- 41.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(Suppl. S2):15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Tay EH, Garland S, Tang G, Nolan T, Huang LM, Orloski L, Lu S, Barr E. Clinical trial experience with prophylactic HPV 6/11/16/18 VLP vaccine in young women from the Asia-Pacific region. Int J Gynaecol Obstet. 2008;102:275–283. doi: 10.1016/j.ijgo.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Tzellos TG, Sardeli C, Lallas A, Papazisis G, Chourdakis M, Kouvelas D. Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2011;25:345–353. doi: 10.1111/j.1468-3083.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 44.von Krogh G, Lacey CJ, Gross G, Barrasso R, Schneider A. European guideline for the management of anogenital warts. Int J STD AIDS. 2001;12(Suppl 3):40–47. doi: 10.1258/0956462011924100. [DOI] [PubMed] [Google Scholar]
- 45.Wiley DJ, Douglas J, Beutner K, Cox T, Fife K, Moscicki AB, Fukumoto L. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35(Suppl 2):S210–S224. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Zhu K-J, Zhu N, Jiang D-H, Chen X-Z, Cheng H. Expression of Foxp3+ CD4+ CD25+ regulatory T cells and Th1/Th2, Tc1/TC2 profiles in the peripheral blood of patients with candyloma acuminatum. Clin Exp Dermatol. 2009;34(2):229–235. doi: 10.1111/j.1365-2230.2008.03001.x. [DOI] [PubMed] [Google Scholar]