Abstract
Infectious bronchitis virus (IBV) is one of the major respiratory viral threats for chickens. Despite the intensive application of IBV vaccines, several outbreaks have been reported worldwide. Here, we report several IBV outbreaks in thirteen poultry farms in Eastern Saudi Arabia (ESA) from 2013 to 2014. The main goals of the current study were as follows: (1) isolation and molecular characterization of the currently circulating strains in ESA (Al-Hasa, Dammam, and Buqayq) and (2) evaluation of the immune status of these birds to IBV. To achieve our goals, tissue specimens (trachea, lungs, liver, kidney and cecal tonsils) and sera were collected. High morbidity up to 100% and mortality ranging from 18 to 90% were reported. Severe infection was observed in the trachea, bronchi, and kidneys of the infected birds. IBV strains were isolated using embryonated chicken eggs. The isolated viruses induced hemorrhage, dwarfing and death of the inoculated embryos 3–5 days post-infection. The circulating IBV strains were identified by sequencing the partial IBV-N and IBV-S1 genes. These viruses showed 95% sequence identity to Indian, Italian, Egyptian and Chinese strains and were quite distinct from the locally used vaccines on the genomic level. Interestingly, high antibody titers against IBV were reported in some of these farms, suggesting the presence of new virulent strains in ESA. The seroconversion of infected birds was reported among the affected flocks. In conclusion, very virulent IBV strains are currently circulating in ESA. Further studies are currently in progress to molecularly characterize these IBV strains.
Electronic supplementary material
The online version of this article (doi:10.1007/s13337-017-0375-7) contains supplementary material, which is available to authorized users.
Keywords: ELISA, Eastern Saudi Arabia, Infectious bronchitis virus, PCR
Introduction
Avian infectious bronchitis virus (IBV) is a highly contagious virus of chickens worldwide. IBV belongs to the family Coronaviridae and genus Gammacoronavirus. IBV is classified as a group 3 coronavirus [22]. The IBV genome is single-strand positive sense RNA. The viral genome organization is as follows: 5′UTR-Replicase gene (ORF1a and ORF1b)-S-M-E-N-3′UTR [22]. The nucleocapsid (N) gene is the most conserved among different coronaviruses, whereas the spike (S) glycoprotein (especially S1) is highly variable [22]. IBV was first identified in 1931 in the USA and occasional outbreaks are still reported throughout the world [2]. IBV has a wide tissue tropism, including the upper respiratory tract, urinary tract and reproductive system of chickens. IBV infection is manifested clinically in chickens in the form of respiratory distress, kidney failure, a drop in egg production and the production of malformed eggs [21]. IBV replicates in the tissues and membranes of embryonated chicken eggs, resulting in hemorrhage, dwarfing of the inoculated embryos and death in a short time after inoculation [36]. Monitoring of the immune response of different poultry flocks has been practiced using several serological techniques, such as hemagglutination inhibition (HI) and enzyme-linked immunosorbent assay (ELISA) [40]. Several molecular techniques were recently developed to monitor the prevalence of IBV, including gel-based PCR and real-time PCR [14]. IBV frequently undergoes changes through mutation and recombination. Mutation within the IBV-S1 gene may change viral virulence and tropism. These mutations may also affect the physicochemical and biological properties of the virus [33], resulting in the continuous emergence of new IBV strains. Conventional techniques do not cope with the rapid and frequent dynamic changes of the virus [27]. Several molecular surveys have been conducted to verify the identity of IBV strains in many regions worldwide [7, 26]. Additionally, several vaccines have been developed to protect chickens against IBV worldwide [38]. Live attenuated vaccines such as the Massachusetts strain produced good protection against several IBV strains; however, these results may be limited by the introduction of this strain to a specific area [32]. Moreover, these vaccines do not guarantee the protection of different poultry flocks against newly emergent IBV strains [32]. Currently, there is high demand for studies of different coronaviruses on the molecular level, especially in the Middle East due to the emergence of the Middle East Respiratory syndrome coronavirus (MERSCoV) [39]. We recently reported the absence of any neutralizing antibodies against MERSCoV in chicken sera from Eastern Saudi Arabia [20]. However, very few studies have been performed in Saudi Arabia to assess the prevalence of IBV, and none of these studies reported the clinical and molecular characterization of these viruses [1]. The main goal of the current study was to isolate and perform a molecular characterization of the new emergent strains of IBV in Eastern Saudi Arabia. Based on our obtained results, we report the isolation and molecular characterization of several virulent strains of IBV in ESA. Further ongoing studies are in progress in our laboratory to do full characterization of these strains. We believe this study will have great impact on our understanding of coronaviruses in the Arabian Peninsula.
Materials and methods
Area of study
Samples were collected from thirteen poultry farms in Eastern Saudi Arabia that reported clinical IBV outbreaks from 2013 to 2014 (AL-Hasa (Al-Hofuf), Dammam and Buqayq). We denote these farms as follows: AL-Hasa (Al-Hofof) (H), Dammam (D) and Buqayq (A). Supplementary Figure shows a Saudi map illustrating the geographic distribution of the poultry farms under study that experienced IBV outbreaks from 2013 to 2014.
Sampling
Tissue specimens
Tissues were collected from birds showing clinical IBV signs after the birds were euthanized in a carbon dioxide (10%) chamber. Licensed veterinarians collected samples from the trachea, lungs, kidney, spleen, intestine, gizzard, proventriculus, cecal tonsil and ovaries. These tissue samples were pooled into groups of five like tissue samples in one tube. The samples were collected in the Dulbecco’s modified eagle medium (DMEM) transport medium supplemented with 10% fetal bovine serum and then transported to the laboratory on ice. Tissue suspensions (10%) were prepared as previously described [29]. All animal experiments and sample collection were performed as per The King Abdul-Aziz City of Science and Technology, Royal Decree No. M/59, (http://www.kfsh.med.sa/KFSH_WebSite/usersuploadedfiles%5CNCBE%20Regulations%20ENGLISH.pdf). This animal utilization protocol was amended by the King Faisal’s Animal Ethics and the National Committee of Bio-Ethics (NCBE).
Serum samples
A total of 370 serum samples were collected via venipuncture of the wing vein from the farms under study (Supplementary Table). Sera were separated as previously described [30]. The collected sera were heat inactivated at 56 °C for 30 min then stored at −20 °C.
Virus isolation
Virus was isolated from the tissue suspensions using 9–11-day-old embryonated chicken eggs from native breeds (non-IBV vaccinated). The source of these eggs was free from IBV antibodies based on analysis with a commercial ELISA kit (IDEXX IBV Ab, USA). The IBV strains was isolated as previously described [10].
Pathogenicity index of the circulating IBV strains in ESA
Tissue suspensions of pooled organs from each farm were used to assess the pathogenicity indices. Approximately 100 µl of each suspension representing one farm was used to inoculate five 9-day-old embryonated chicken eggs. Daily observation of the inoculated Embryonated chicken eggs (ECE) was performed by candling for up to 5 dpi. Sham PBS-inoculated eggs were also included in this experiment. Allantoic fluid and embryo processing was performed at 5 dpi as previously described [12]. These experiments were independently repeated three times.
RNA extraction
Viral RNA was extracted from the tissue suspensions, embryonated egg tissues and their fluids. The extraction was performed following the kit’s instructions (QIAamp Viral RNA Mini Kit, Qiagen, Inc., Valencia, CA, USA). The viral RNA was stored at −80 °C.
Amplification
The oligonucleotides used to amplify the partial IBV-N, full length IBV-N and partial IBV-S1 genes are listed in Table 1.
Table 1.
List of oligonucleotides used in the current study and their information
| Primer name | Sequence 5′——3′ | Target gene | Referencs |
|---|---|---|---|
| IBV-NF1 | AATTTTGGTGATGACAAGATGA | PN | Jones et al. (2011) |
| IBV-NR1 | CATTGTTCCTCTCCTCATCTG | PN | Jones et al. (2011) |
| IBV-NF2 | ATGGCGAGCGGTAAAGGATCTG | FLN | Jones et al. (2011) |
| IBV-NR2 | CAGCTGAGGTCAATGCTTTATC | FLN | Jones et al. (2011) |
| IBV-S1F1 | TAATACTGGC/TAATTTTTCAGA | PS1 | Jones et al. (2011) |
| IBV-S1R1 | AATACAGATTGCTTACAACCACC | PS1 | Jones et al. (2011) |
PN Partial IBV-N gene, PS1 partial S1, FLN Full length IBV-N gene
The RT-PCR reactions were performed using the antisense strands of the oligonucleotides (Table 1) as previously described [23]. The PCR reactions were performed as previously described [1, 23].
Sequence analysis
The purified PCR products of the partial IBV-N and IBV-S1 genes were sequenced in both directions. The obtained sequences were assembled into one contig. The sequences was initially analysed using the NCBI/BLAST/blastn suite (https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome).
The accession numbers of the sequences used to develop the IBV-N and IBV-S1-based phylograms are as follows: HQ916669.1, HQ916668.1, KF663559.1, EF185916.1, FN182283.1, EU350552.1, JQ977698.1, M95169.1, FJ904722.1, X58002.1, EU812232.1, M21515.1, AY363968.1, FN182283.1, KF696629.1, KF377577.1, AF352310.1, FJ807652.1, GU393335.1, AY942745.1, GQ504721.2, AJ311362.1, GU393333.1, GU393334.1, and GU393336.1.
The phylograms were constructed using the obtained truncated IBV-N and IBV-S1 sequences. A multiple alignment was performed with the Mega-6 package using the neighbor-joining method with 1000 bootstrap replicates as previously described [35].
Enzyme-linked immunosorbent assay (ELISA)
Testing of the collected sera was performed with a commercial enzyme-linked immunosorbent assay (The IDEXX IBV Ab Test, IDEXX, USA). The ELISA procedures were performed according to the kit’s instructions.
Results
Clinical features of very virulent IBV outbreaks in Eastern Saudi Arabia
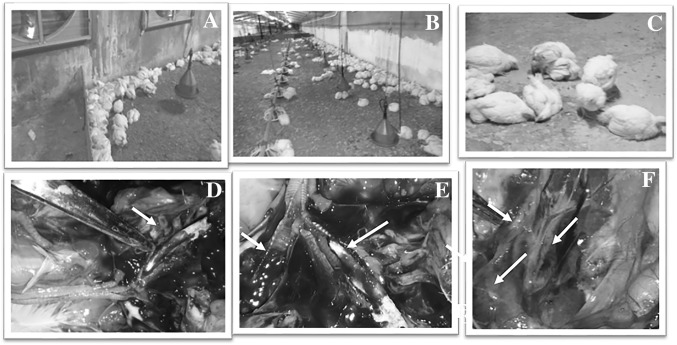
Thirteen poultry farms in Eastern Saudi Arabia (12 broilers and 1 layer) experienced typical IBV infections from 2013 to 2014. Eight farms were located in Al-Hasa province (Al-Hofuf city, H1–H8), four farms (D1–D4) were in Dammam city and one farm was in Buqayq city (A1) (Supplementary Figure). All farms received regular commercial vaccines against IBV, NDV and IBDV, with the exception that D4 did not receive any vaccines. Chickens in some farms started to show clinical symptoms as early as 11 days of age, whereas birds in other farms showed clinical signs at 28 days. In contrast, the layer farm reported the onset of the outbreak at the 33rd week of age. High morbidity rates were reported in most of the affected farms in up to 100% of the birds. The mortality rates varied from 18% (H8 farm) to 90% (A1 farm) (Supplementary Table). The general health conditions of the birds were affected; the diseased birds exhibited ruffled feather and sometimes diarrhea (Fig. 1a). The affected birds showed typical IBV signs of respiratory distress, such as mouth breathing, gasping, rales, and nasal discharges (Fig. 1a; Supplementary movie S1). Birds within the affected farms were huddled together and concentrated around the heaters (Fig. 1b, c). Supplementary movies S1–S5 describe the progress of the IBV infection in one big commercial broiler farm in ESA. The IBV infection started from a small group of birds and then progressed quickly to include other parts of the farm, as shown in movies S1–S5. Within 6–10 days, almost the entire flock became infected. Necropsy examinations of the diseased birds showing typical signs revealed congestion and hemorrhage of the upper respiratory tracts, especially the trachea and bronchi (Fig. 1d). The presence of prominent white cheesy caseous plugs in the trachea of the affected birds was observed in most of the birds during the gross pathological examination (Fig. 1d, e). In the most severely affected birds, the trachea was fully occluded with cheesy caseous plugs (Fig. 1b, c). Hemorrhage in the trachea at the bifurcation was also reported. Figure 2e shows one tracheal bifurcation of an affected bird in which one side is occluded by cheesy material and the other side is occluded by a blood clot. Kidney involvement was clearly observed in the carcasses after examination (Fig. 1f). Enlargement of the kidneys and the loss of their globular appearance was obvious (Fig. 1f).
Fig. 1.
Clinical profile of IBV infection in chicken farms in Eastern Saudi Arabia. An overview of the clinical picture of virulent IBV infection in poultry farms in ESA. a Birds are huddled together and collected against the source of heat beside the walls in the farm. b Very few birds are left after high mortality among the affected birds. c Close view of a group of IBV-infected birds at the morbid stage. The birds are recumbent, showing respiratory distress and soiled vents. d Congestion and hemorrhage of the trachea in a bird showing a prominent caseous plug occluding the trachea of bird during the necropsy examination. e Tracheal bifurcation of one bird showing that one side of the trachea is occluded with a white cheesy caseous plug while the other side is showing a hemorrhage. f Kidneys of birds are enlarged in size and lose their globular appearance and the ureter is impacted with urates
Fig. 2.
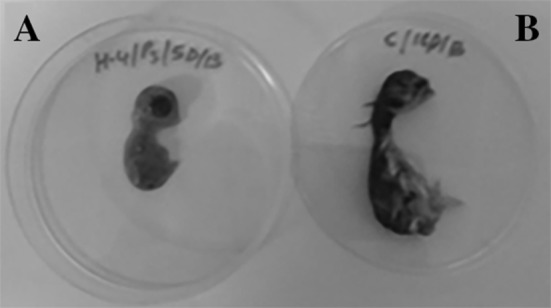
Isolation of IBV using embryonated chicken eggs. Embryonated chicken eggs (9–11 days old) inoculated with IBV tissue suspensions isolated from field outbreaks in ESA. a The morphology of the IBV inoculated embryo at 5 dpi. The embryo shows dwarfism, malformation and a lack of feathers. b The PBS inoculated (sham) embryo showing the normal size and morphology with well-developed feather
Isolation and pathogenicity index of isolated IBV strains
The circulating IBV strains were isolated using 9–11 non-IBV vaccinated native breed embryonated chicken eggs. Briefly, the ECE were inoculated with the corresponding IBV isolates from each farm, and the allantoic fluids and embryonated tissues were harvested at 1, 3, and 5 days post-inoculation. Embryos inoculated with field specimens collected from an IBV outbreak showed congestion, hemorrhage, dwarfing and death at various intervals (Fig. 2). Table 2 summarizes the pathogenicity indices of different IBV isolates using the ECE system. Most IBV-suspected samples caused embryonic death of the inoculated embryos as early as 2 days post-inoculation (dpi). By 5 dpi, most inoculated embryos had died. This result is in contrast to the PBS sham-inoculated eggs, where no death was reported during this observation period (Table 2). Some IBV isolates were highly pathogenic, such as H1, H3, H7, and A. These isolates were responsible for 100% of the mortality of the inoculated ECE by the 5th dpi. Other IBV isolates were moderately pathogenic, such as those isolated from farms H5, H8, and D2 that induced approximately 30–50% mortality and/or pathology in the inoculated ECE. The remaining IBV isolates were mildly pathogenic (H4, H6, D1, and D3) and induced mortality ranging from 0 to 20%. None of the sham-inoculated eggs showed any pathology or mortality by the 5th dpi (Fig. 2). Table 3 summarizes the IBV detection in ECE by RT-PCR (Fig. 3). This study was conducted on IBV isolated from farms H3–H8 and D1-D3. Samples from farms H4, H6, H7 and D2 showed positive PCR signals at 1 dpi. Only samples from farm H3 showed positive PCR signals at 3 dpi, whereas IBV isolated from farms H3, H5, D1 and D3 showed positive PCR signals at 5 dpi (Table 3). These pathogenicity experiments were repeated three times to ensure that the obtained results were solid. All data from the three independent experiments were quite consistent (data not shown).
Table 2.
Pathogenecity index of the isolated IBVs from farms in Eastern Saudi Arabia
| No | Farm | 1 dpi | 2 dpi | 3 dpi | 4 dpi | 5 dpi | Live 5 dpi | Total dead by 5 dpi |
|---|---|---|---|---|---|---|---|---|
| 1 | H1 | 0 | 0 | 2 | 2 | 1 | 0 | 5 |
| 2 | H3 | 0 | 0 | 2 | 3 | 0 | 5 | |
| 3 | H4 | 0 | 0 | 5 | 0 | |||
| 4 | H5 | 0 | 0 | 1 | 4 | 1 | ||
| 5 | H6 | 0 | 0 | 5 | 0 | |||
| 6 | H7 | 0 | 0 | 1 | 2 | 2 | 4 | |
| 7 | H8 | 0 | 0 | 1 | 1 | 1 | 2 | 3 |
| 8 | D1 | 0 | 0 | 1 | 2 | 2 | 3 | |
| 9 | D2 | 0 | 0 | 2 | 1 | 1 | 1 | 5 |
| 10 | D3 | 0 | 0 | 2 | 3 | 3 | ||
| 11 | A1 | 0 | 0 | 3 | 2 | 5 | ||
| 12 | Sham | 0 | 0 | 5 | 0 |
Table 3.
Summary of the RT-PCR results on the passage 1 of different IBV isolates from Eastern Saudi Arabia
| Farm/day | H-3 | H-4 | H-5 | H-6 | H-7 | H-8 | D-1 | D-2 | D-3 |
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | +Ve | +Ve | +Ve | +Ve | |||||
| Day 3 | +Ve | ||||||||
| Day 5 | +Ve | +Ve | +Ve | +Ve |
Fig. 3.
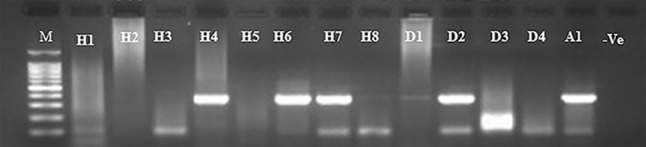
Detection of IBV in embryonated chicken egg tissues and fluids. Agarose gel picture showing the amplified PCR products of the partial IBV-N gene from samples collected from the pathogenicity index studies. Samples from embryos and their fluids were tested for the presence of IBV at different intervals after egg inoculation. Lane (M) DNA marker, 1 kb ladder, Lanes (H1–H8) represent RT-PCR results of samples collected from farms in Al-Hasa (Farm-1–Farm 8), Lanes (D1–D4) showing results of samples collected from farms in Dammam (D1–D4), Lane (A1) showing result of samples collected from farm in Abqaia region. Lane (−Ve) showing non template negative control
Molecular-based prevalence of IBV in Eastern Saudi Arabia
We conducted molecular-based surveillance of IBV among the 13 farms in ESA. We used two primers to conduct this molecular surveillance. The truncated IBV-N primers amplified 260 nt and the truncated IBV-S1 primer amplified 313 nt. IBV was detected in the pooled organs from each farm (Fig. 3). Both primers showed consistent results for the detection of the IBV isolated in this survey; however, the IBV-S1 primers showed higher specificity than the IBV-N primers (Fig. 4). To verify the tropism of the isolated IBV strains, IBV amplicons were detected in different body organs (trachea, lung, kidney, spleen, gizzard, proventriculus, intestine, cecal tonsils and pancreas) by RT-PCR as previously described (Fig. 5).
Fig. 4.
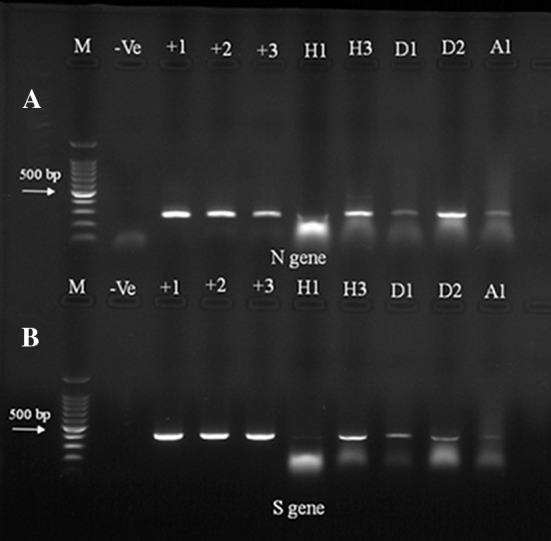
Molecular-based prevalence of IBV in ESA by gel-based RT-PCR. Agarose gel electrophoresis picture showing the RT-PCR results of tested specimens from farms in Alhasa (H1and H3), Dammam (D1and D2) and one farm from Abqaiq (A1). The size of the amplified amplicons by using the partial IBV-N primer (220 nt, upper panel) and the partial IBV-S1 primer (330 nt, lower panel). Lane (M) 100 bp DNA marker, Lanes 1–3 (IBV vaccines). Lane (−Ve) non DNA template negative control
Fig. 5.
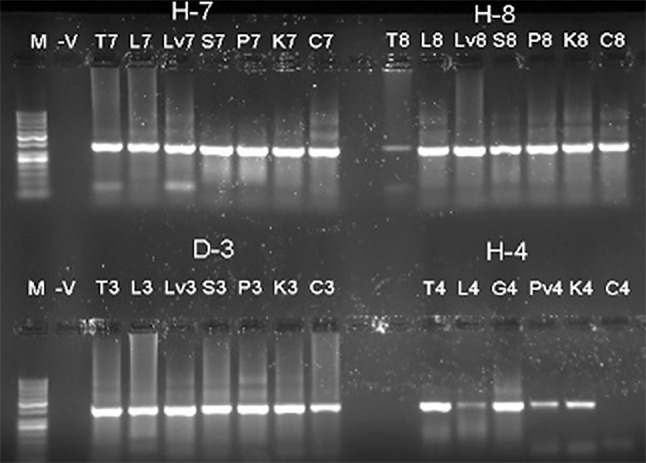
Detection of IBV in different body organs. Agarose gel picture showing the amplified PCR products of the partial IBV-N from samples collected from IBV-infected chickens. Lane (M): DNA ladder 1 kb. Results of tested organs from (T7, L7, S7, P7, K7, and C7) are showing pooled samples from trachea (T), lung (L), spleen (S), proventriculus (P), kidney (K), and cecal tonsils (C). Lane (−V) representing non-template DNA was included as a negative control
The currently circulating IBV strains in Eastern Saudi Arabia are different from the locally used vaccine strains
Our results showed that the isolated IBV strains did not exhibit positive amplification when tested using the IBV-N full-length gene oligonucleotides based on the currently used vaccine in Eastern Saudi Arabia (Fig. 6).
Fig. 6.
IBV isolated from ESA is different from common vaccine strains. Agarose gel picture showing full length IBV-N gene amplification. Lane M: DNA marker, 1 kb, lanes 1–11 show IBV confirmed samples by partial IBV-N gene primers showing no amplicons using the full length IBV gene. Lanes 12–14 showing positive amplification in the the full length IBV-N gene for three common commercial IBV vaccines (H120 and IBV-4-91, respectively)
Phylogenetic analysis of isolated IBV from Eastern Saudi Arabia
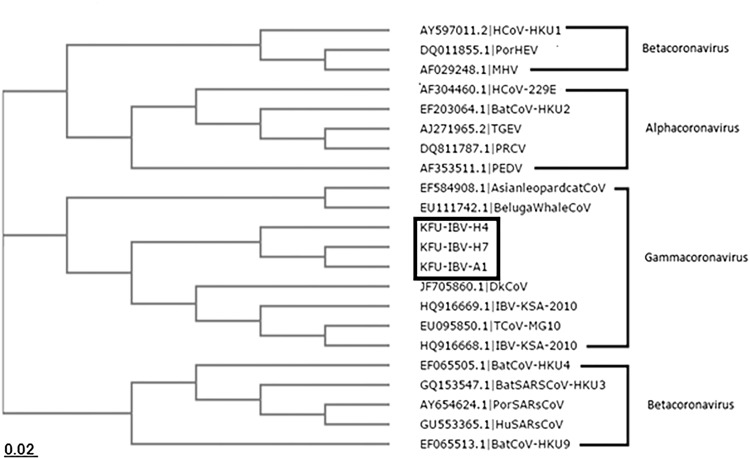
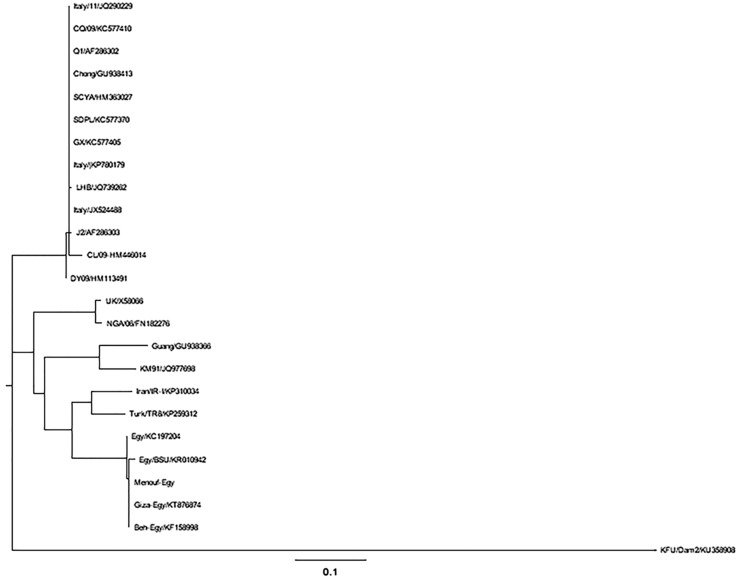
The partial IBV-N and IBV-S1 gene sequences reported in this study were deposited in Genbank and assigned accession numbers KT762154, and KU358908, respectively. Blasting these sequences against the Genbank revealed high nucleotide identity to other IBV and TCoV strains from Israel, South Africa, and South America (95, 94, and 93%, respectively). Both the IBV-N and IBV-S1-based phylograms showed close relationships to other IBV strains from India, China, Egypt, and Israel (Figs. 7 and 8) (Table 4).
Fig. 7.
Phylogenetic analysis based on the partial IBV-N sequences of the isolated IBV from Eastern Saudi Arabia. Phylogenetic tree based on the obtained IBV-N partial sequences from IBV-infected farms in Eastern Saudi Arabia. The neighbor-joining tree was constructed from the truncated IBV-N gene alignment with the other IBV-V sequences in Genbank. IBV isolated from Eastern Saudi Arabia clustered with other IBV strains from Egypt, India, and China as well as other members of group 3 coronaviruses such as the beluga whale coronavirus and duck coronavirus
Fig. 8.
Phylogenetic analysis based on the partial IBV-S1 sequences of the isolated IBV from Eastern Saudi Arabia. Phylogenetic tree based on the obtained IBV-S1 partial sequences from IBV-infected farms in Eastern Saudi Arabia. The neighbor-joining tree was constructed from the truncated IBV-S1 gene alignment with the other listed IBV-S1 sequences in Genbank. IBV isolated from Eastern Saudi Arabia clustered with other IBV strains from Iran, Korea, China, and Egypt
Table 4.
Summary of molecular based detection of IBV in different body organs by RT-PCR
| No | Farm | Trachea | Lung | Liver | Gizzard | Proventriculus | Intestine | Spleen | Pancrease | Kidney | C.T |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H-1 | +Ve | +Ve | – | – | +Ve | – | – | – | +Ve | |
| 2 | H-2 | +Ve | – | – | – | – | – | – | – | – | |
| 3 | H-3 | +Ve | +Ve | +Ve | |||||||
| 4 | H-4 | +Ve | +Ve | +Ve | +Ve | 0 | +Ve | ||||
| 5 | H-5 | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve | ||||
| 6 | H-6 | ||||||||||
| 7 | H-7 | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve | |||
| 8 | H-8 | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve | ||||
| 9 | D-1 | +Ve | +Ve | +Ve | |||||||
| 10 | D-2 | +Ve | +Ve | +Ve | |||||||
| 11 | D-3 | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve | |||
| 12 | D-4 | ||||||||||
| 13 | A-1 | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve | +Ve |
Seroprevalence of IBV in the Eastern region of KSA
A total of 370 serum samples were tested for the presence of IBV antibodies using the commercially available IBV ELISA kits. According to the obtained results, 298 (80%) samples were positive and 72 samples (20%) were negative (Table 5). We developed a scoring system for the positive samples based on the concentration of IBV antibodies in the sera of tested birds. For example, out of the 19 samples tested from farm H3, five were negative and 14 (73%) were positive (+). The details of the serosurveillance of the selected farms are listed in Table 5.
Table 5.
Seroprevalence of IBV in some chicken farms in Eastern Saudi Arabia
| Farm | Total tested | (−Ve) | (+Ve) | % (+Ve) | (+Ve) scores | ||||
|---|---|---|---|---|---|---|---|---|---|
| + | ++ | +++ | ++++ | +++++ | |||||
| H-3 | 19 | 5 | 14 | 73 | 14 | ||||
| H-5 | 74 | 25 | 49 | 66 | 40 | 9 | |||
| H-7 | 47 | 8 | 39 | 82 | 25 | 10 | 4 | ||
| H-8 | 77 | 7 | 70 | 90 | 60 | 10 | |||
| D-1 | 24 | 15 | 9 | 37 | 9 | ||||
| D-2 | 26 | 5 | 21 | 80 | 20 | 1 | |||
| D-3 | 63 | 7 | 56 | 88 | 46 | 7 | 3 | ||
| D-4* | 40 | 0 | 40 | 100 | 1 | 8 | 11 | 9 | 11 |
| Total | 370 | 72 | 298 | 80 | 215 | 45 | 18 | 9 | 11 |
* D4 farm did not received any IBV vaccines
Discussion
IBV is an endemic viral disease of poultry in many regions of the world [11], including the. Middle East [2]. Little is known about the molecular prevalence of IBV in the Gulf area, especially Saudi Arabia. Although intensive IBV vaccination is currently practiced in most poultry populations throughout the Kingdom, many outbreaks are still reported [1]. These outbreaks may be due to the massive and random use of IBV vaccines. Another possibility is the emergence of new IBV strains resulting from the frequent mutations and recombination between several IBV strains [1]. The main goals of the current study were to isolate the currently circulating IBV strains in ESA, to use molecular methods to assess the prevalence among the affected farms and to evaluate the immune status of the chickens in those farms against IBV. From 2013 to 2014, several chicken farms (12 broilers and 1 layer) reported IBV infections in ESA. The affected farms showed variable mortality rates that ranged from 15% (H8) to 90% (A1) (Supplementary Table). High mortality rates due to IBV infection were reported in many parts in the world, especially with the emergence of new strains of the virus [11, 15, 16, 34]. Moreover, experimental detection of IBV infections in vaccinated chicken flocks has recently been reported [37]. In the current study, 12 out of 13 farms received regular IBV vaccines and other recommended vaccines for Newcastle disease virus (NDV) and infectious bursal disease virus (IBDV) ( Supplementary Table). Only farm D4 did not receive any vaccines to IBV or any other viruses; the owner of this farm believed that vaccination was not of great help in protecting his birds against IBV. In response to this outbreak, we collected tissue samples (trachea, lung, kidney, intestine, gizzards, proventriculus, and pancreas) and sera from these farms. The affected birds showed clinical symptoms that included coughing, sneezing, rales, nasal discharge, white diarrhea, dullness, huddling together, and sudden death in acute cases. Here, we report the progression of IBV infections in some chicken farms in ESA. First, a few birds became infected and showed signs of rapid breathing, loss of appetite, and loss of body weight gain (Supplementary movie 1; Fig. 1a–c). The infection spread throughout the flock as more birds were affected. An increase in the reported daily mortality was also observed (Supplementary movies 2 and 3). Within 6–10 days, most of the birds in certain farms became infected. The mortality reports reached their peak approximately 5–6 days post-infection. By the 10th day from the onset of the infection, most of the birds were dead. The chicken pen was found to be almost empty with the exception of a very few birds scattered in the moribund stage (Supplementary movie 4, Fig. 1a–c).
Necropsy examination of birds in the morbid stage revealed congested trachea, occluded bronchi with white cheesy caseous plugs, congested lungs, swollen kidneys with a loss of lobulation, impacted ureters, and vent pasting with white diarrhea (Fig. 1d–f).
ECE is a good system for IBV isolation [18]. Tissue suspensions from each farm were inoculated into 9–11-day-old embryonated (non-vaccinated native breed) eggs from IBV-free Baladi birds. The source of these eggs was demonstrated to be IBV-free using a commercial IBV ELISA kit (data not shown). The isolated IBV induced hemorrhage, dwarfing, curling and death of the ECE 3–5 dpi (Fig. 2). The inoculated materials, including embryos and their fluids, were isolated 5 dpi. Then, the ECE was used to determine the pathogenicity indices of the IBV strains as previously described [8]. IBV isolates from farms H1, H3, H7 and A1 were highly pathogenic and produced 100% mortality among the inoculated ECE by 5 dpi (Table 3). In contrast, the IBV isolates from farms H5, H8, and D2 were moderately pathogenic and induced approximately 50% mortality among the inoculated eggs, and isolates from farms H4, H6, D1 and D3 exhibited low pathogenicity and did not induce any deaths or marked effects on the inoculated eggs. All embryos survived until the end of the experiment. These experiments were independently repeated three times to ensure that our data were solid. We also tested for the presence of IBV in the allantoic fluid and tissues of the inoculated ECE by RT-PCR using the IBV-N primers (Table 3). We were able to detect IBV nucleic acids in samples from farms H4, H6, H7 and D2 at 1 dpi. Only H3 was positive for IBV by RT-PCR at 3 dpi. However, samples from the eggs inoculated with isolates from farms H3 H5, D1 and D3 were positive up to 5 dpi (Table 3; Fig. 3). Both gel-based PCR and real-time PCR were previously used to test different samples for IBV [4, 14]. We used gel-based RT-PCR to conduct molecular-based prevalence testing among the poultry farms under study. We used this approach to detect the tropism of different IBV isolates by detecting the presence of IBV nucleic acids in different tissues collected from the diseased birds. Our results showed positive IBV signals from all 13 tested farms (Fig. 4a, b). For instance, the trachea, lung, intestine, and cecal tonsil samples from farm H1 were positive, as shown in Table 4 and Fig. 4a, b. However, only samples from farm H4 showed a positive reaction in the gizzard and proventriculus of the tested birds. Interestingly, samples from several farms showed positive reactions in the pancreas, such as H5, H7, H8, D3 and A1. The most prevalent positive reactions among most farms were in the trachea, lung, spleen kidneys and cecal tonsils (Table 4; Fig. 4). These data suggested that the isolated IBV strains were pantropic viruses because they had the potential to replicate in many organs of several body systems. Interestingly, the clinical picture of these IBV isolates in chickens was very similar to that of the Middle East respiratory syndrome coronavirus (MERSCoV). MERSCoV was recently demonstrated to induce lower respiratory tract infections in the lungs as well as kidney failure [41]. This phenomenon may be due to the adoption of mechanisms by the coronavirus to induce pathology in the affected host [41]. Interestingly, we recently reported the absence of MERSCoV antibodies in chicken sera in Eastern Saudi Arabia [20]. IBV vaccination failure has been previously reported [31]. There are many reasons for the vaccination failures, such as immunosuppressive agents (IBDV infection, Marek’s disease virus, Cryptosporidium or Eimeria infections), inadequate vaccine dosage, and the use of vaccines prepared from strains different from the circulating strains [5, 9, 13, 25]. Our results showed that the currently circulating IBV strains in ESA were quite distinct from the commonly used vaccine in this area. Figure 7 shows the absence of any amplicons of the IBV strains isolated from ESA in an assay based on three different vaccines. These results suggest the presence of new virulent IBV strains circulating in ESA. The continuous reports of IBV outbreaks may be at least partially due to the use of non-homologous vaccine strains. Indeed, frequent recombination was previously reported among different IBV strains in cases of multiple infections [17]. The obtained IBV-N sequences were used to construct the neighborhood-joining phylogram (Fig. 7). Our results showed that the isolated IBV strains belonged to the avian coronaviruses group (gamma coronaviruses) along with the turkey coronavirus, duck coronavirus and beluga whale coronavirus [19]. Similar results were obtained using a partial IBV-S1 gene from the isolated strains (Fig. 8). These isolates were closely related to other IBV strains from Italy, the Middle East and Asia [1, 3, 9, 28]. Most local farms are currently using the Mas-H120 vaccine for regular IBV vaccination. Based on the obtained partial IBV-N and IBV-S1 sequences, the local isolated strains show 79% nucleotide identity to the Mass-H120 vaccine strains (Figs. 7, 8). This result implies that these poultry producers need to revise their vaccination programs. Vaccines from the currently circulating strains should be prepared and used for their vaccination programs.
Several approaches have been used to detect antibodies against IBV in the sera of different birds [6]. We used a commercial ELISA kit to evaluate the immune status of chickens in poultry farms under investigation for IBV. Although all farms in this study were vaccinated against IBV except D4, we were able to detect antibodies in the sera from all 13 farms including D4 (Table 5). The presence of antibodies in the sera of the test birds in farm D4 in the absence of any vaccine against IBV suggested that these birds seroconverted in response to IBV infection. According to our data, 298 (80%) birds showed positive antibodies against IBV, whereas 72 birds (20%) were antibody negative (Table 5). Furthermore, we designed a scoring system based on the antibody concentration in a given sample. Briefly, serum samples with S/P ratios less than 0.2 were considered negative, from 0.2 to 0.9 (+), from 1 to 1.9 (++), from 2 to 2.9 (+++), from 3 to 3.9 (++++) and more than 4 (+++++). Interestingly, we found 11 birds from farm D4 that were (+++++) although this farm did not administer an IBV vaccine. This result suggested that active field infection triggered very high antibody production among the infected birds (Table 5). Farm H3 (showing 60% mortality) had 14 birds with only a (+) antibody concentration, which might be due to the partial neutralization of the effect of active field infection. Perhaps the presence of antibodies against IBV in the sera of chickens in association with field infection due to the emergence of new IBV strains does not affect the action of the vaccine administered to the birds. To the best of our knowledge, this is the first study reporting the isolation and molecular characterization of very virulent IBV strains in Eastern Saudi Arabia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 6 Map showing the geographic coordinates of the farms and bird sampling. A map showing the geographical locations of the farms under study in Eastern Saudi Arabia. The farms are located around three major cities in ESA (Al-Ahsa (Al-Hofuf), Dammam and Buqayq). A circle highlighted the location of these farms in ESA (TIFF 3088 kb)
Acknowledgements
This work was supported by the King Faisal University Deanship of research Grant No. (142017). We also than Mr. Ahmed Alkhars with his laboratory technical helps during the virus isolation and pathogenicity index.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13337-017-0375-7) contains supplementary material, which is available to authorized users.
References
- 1.Ababneh M, Dalab AE, Alsaad S, Al-Zghoul M. Presence of infectious bronchitis virus strain CK/CH/LDL/97I in the Middle East. ISRN Vet Sci. 2012;2012:201721. doi: 10.5402/2012/201721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Moneim AS, El-Kady MF, Ladman BS, Gelb J. S1 gene sequence analysis of a nephropathogenic strain of avian infectiousbronchitis virus in Egypt. Virology. 2006;3:78. doi: 10.1186/1743-422X-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Moneim AS, Zlotowski P, Veits J, Keil GM, Teifke JP. Immunohistochemistry for detection of avian infectious bronchitis virus strain M41 in the proventriculus and nervous system of experimentally infected chicken embryos. Virol J. 2009;6:15. doi: 10.1186/1743-422X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acevedo AM, Perera CL, Vega A, Rios L, Coronado L, Relova D, et al. A duplex SYBR Green I-based real-time RT-PCR assay for the simultaneous detection and differentiation of Massachusetts and non-Massachusetts serotypes of infectious bronchitis virus. Mol Cell Probes. 2013;27(5–6):184–192. doi: 10.1016/j.mcp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Atkins KE, Read AF, Walkden-Brown SW, Savill NJ, Woolhouse ME. The effectiveness of mass vaccination on Marek’s disease virus (MDV) outbreaks and detection within a broiler barn: a modeling study. Epidemics. 2013;5(4):208–217. doi: 10.1016/j.epidem.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronzoni RV, Fatima M, Montassier S, Pereira GT, Gama NM, Sakai V, et al. Detection of infectious bronchitis virus and specific anti-viral antibodies using a Concanavalin A-Sandwich-ELISA. Viral Immunol. 2005;18(3):569–578. doi: 10.1089/vim.2005.18.569. [DOI] [PubMed] [Google Scholar]
- 7.Chacon JL, Rodrigues JN, Assayag Junior MS, Peloso C, Pedroso AC, Ferreira AJ. Epidemiological survey and molecular characterization of avian infectious bronchitis virus in Brazil between 2003 and 2009. Avian Pathol. 2011;40(2):153–162. doi: 10.1080/03079457.2010.544641. [DOI] [PubMed] [Google Scholar]
- 8.Choi KS, Lee EK, Jeon WJ, Park MJ, Kim JW, Kwon JH. Pathogenicity and antigenicity of a new variant of Korean nephropathogenic infectious bronchitis virus. J Vet Sci. 2009;10(4):357–359. doi: 10.4142/jvs.2009.10.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Geus ED, van Haarlem DA, Poetri ON, de Wit JJ, Vervelde L. A lack of antibody formation against inactivated influenza virus after aerosol vaccination in presence or absence of adjuvantia. Vet Immunol Immunopathol. 2011;143(1–2):143–147. doi: 10.1016/j.vetimm.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 10.De Wit JJ. Detection of infectious bronchitis virus. Avian Pathol. 2000;29(2):71–93. doi: 10.1080/03079450094108. [DOI] [PubMed] [Google Scholar]
- 11.Dhama K, Singh SD, Barathidasan R, Desingu PA, Chakraborty S, Tiwari R, et al. Emergence of Avian Infectious Bronchitis Virus and its variants need better diagnosis, prevention and control strategies: a global perspective. Pak J Biol Sci. 2014;17(6):751–767. doi: 10.3923/pjbs.2014.751.767. [DOI] [PubMed] [Google Scholar]
- 12.Dhinakar Raj G, Suresh Kumar K, Nainar AM, Nachimuthu K. Egg: embryo weight ratio as an indicator of dwarfism induced by infectious bronchitis virus. Avian Pathol. 2004;33(3):307–309. doi: 10.1080/0307945042000205883. [DOI] [PubMed] [Google Scholar]
- 13.Eladl AH, Hamed HR, Khalil MR. Consequence of Cryptosporidiosis on the immune response of vaccinated broiler chickens against Newcastle disease and/or avian influenza. Vet Res Commun. 2014;38(3):237–247. doi: 10.1007/s11259-014-9610-5. [DOI] [PubMed] [Google Scholar]
- 14.Fan WQ, Wang HN, Zhang Y, Guan ZB, Wang T, Xu CW, et al. Comparative dynamic distribution of avian infectious bronchitis virus M41, H120, and SAIBK strains by quantitative real-time RT-PCR in SPF chickens. Biosci Biotechnol Biochem. 2012;76(12):2255–2260. doi: 10.1271/bbb.120521. [DOI] [PubMed] [Google Scholar]
- 15.Fellahi S, El Harrak M, Ducatez M, Loutfi C, Koraichi SI, Kuhn JH, et al. Phylogenetic analysis of avian infectious bronchitis virus S1 glycoprotein regions reveals emergence of a new genotype in Moroccan broiler chicken flocks. Virol J. 2015;12:116. doi: 10.1186/s12985-015-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Hu Y, Ma Z, Yu Q, Zhao J, Liu X, et al. Virulent avian infectious bronchitis virus, People’s Republic of China. Emerg Infect Dis. 2012;18(12):1994–2001. doi: 10.3201/eid1812.120552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelb J, Jr, Wolff JB, Moran CA. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis. 1991;35(1):82–87. doi: 10.2307/1591298. [DOI] [PubMed] [Google Scholar]
- 18.Guy JS. Isolation and propagation of coronaviruses in embryonated eggs. Methods Mol Biol. 2015;1282:63–71. doi: 10.1007/978-1-4939-2438-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemida Maged Gomaa Barta JR, Ojkic D, Yoo D. Complete genomic sequence of turkey coronavirus. Virus Res. 2008;135(2):237–246. doi: 10.1016/j.virusres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemida MG, Perera RA, Wang P, Alhammadi MA, Siu LY, Li M, et al. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010–2013. Euro Surveill. 2013;18(50):20659. doi: 10.2807/1560-7917.ES2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- 21.Ignjatovic J, Ashton DF, Reece R, Scott P, Hooper P. Pathogenicity of Australian strains of avian infectious bronchitis virus. J Comp Pathol. 2002;126(2–3):115–123. doi: 10.1053/jcpa.2001.0528. [DOI] [PubMed] [Google Scholar]
- 22.Jackwood MW, Boynton TO, Hilt DA, McKinley ET, Kissinger JC, Paterson AH, et al. Emergence of a group 3 coronavirus through recombination. Virology. 2010;398(1):98–108. doi: 10.1016/j.virol.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji J, Xie J, Chen F, Shu D, Zuo K, Xue C, et al. Phylogenetic distribution and predominant genotype of the avian infectious bronchitis virus in China during 2008-2009. Virol J. 2011;8:184. doi: 10.1186/1743-422X-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RM, et al. Development and validation of RT-PCR tests for the detection and S1 genotyping of infectious bronchitis virus and other closely related gammacoronaviruses within clinical samples. Transbound. Emerg. Dis. 2011;58(5):411–20. doi: 10.1111/j.1865-1682.2011.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JK, Kayali G, Walker D, Forrest HL, Ellebedy AH, Griffin YS, et al. Puzzling inefficiency of H5N1 influenza vaccines in Egyptian poultry. Proc Natl Acad Sci U S A. 2010;107(24):11044–11049. doi: 10.1073/pnas.1006419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Wang XY, Wei P, Chen QY, Wei ZJ, Mo ML. Serotype and genotype diversity of infectious bronchitis viruses isolated during 1985-2008 in Guangxi. China Arch Virol. 2012;157(3):467–474. doi: 10.1007/s00705-011-1206-6. [DOI] [PubMed] [Google Scholar]
- 27.Lim TH, Lee HJ, Lee DH, Lee YN, Park JK, Youn HN, et al. An emerging recombinant cluster of nephropathogenic strains of avian infectious bronchitis virus in Korea. Infect Genet Evol. 2011;11(3):678–685. doi: 10.1016/j.meegid.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood ZH, Sleman RR, Uthman AU. Isolation and molecular characterization of Sul/01/09 avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle East. Vet Microbiol. 2011;150(1–2):21–27. doi: 10.1016/j.vetmic.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mardani K, Noormohammadi AH, Hooper P, Ignjatovic J, Browning GF. Infectious bronchitis viruses with a novel genomic organization. J Virol. 2008;82(4):2013–2024. doi: 10.1128/JVI.01694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichmann KG, Barram KM, Brock IJ, Standfast NF. Effects of regular handling and blood sampling by wing vein puncture on the performance of broilers and pullets. Br Poult Sci. 1978;19(1):97–99. doi: 10.1080/00071667808416448. [DOI] [PubMed] [Google Scholar]
- 31.Roh HJ, Hilt DA, Williams SM, Jackwooda MW. Evaluation of infectious bronchitis virus Arkansas-type vaccine failure in commercial broilers. Avian Dis. 2013;57(2):248–259. doi: 10.1637/10459-112812-Reg.1. [DOI] [PubMed] [Google Scholar]
- 32.Sharma JM. Introduction to poultry vaccines and immunity. Adv Vet Med. 1999;41:481–494. doi: 10.1016/S0065-3519(99)80036-6. [DOI] [PubMed] [Google Scholar]
- 33.Shen S, Law YC, Liu DX. A single amino acid mutation in the spike protein of coronavirus infectious bronchitis virus hampers its maturation and incorporation into virions at the nonpermissive temperature. Virology. 2004;326(2):288–298. doi: 10.1016/j.virol.2004.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spickett AM, De Klerk D, Enslin CB, Scholtz MM. Resistance of Nguni, Bonsmara and Hereford cattle to ticks in a Bushveld region of South Africa. Onderstepoort J Vet Res. 1989;56(4):245–250. [PubMed] [Google Scholar]
- 35.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarpey I, Orbell SJ, Britton P, Casais R, Hodgson T, Lin F, et al. Safety and efficacy of an infectious bronchitis virus used for chicken embryo vaccination. Vaccine. 2006;24(47–48):6830–6838. doi: 10.1016/j.vaccine.2006.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toro H, Pennington D, Gallardo RA, van Santen VL, van Ginkel FW, Zhang J, et al. Infectious bronchitis virus subpopulations in vaccinated chickens after challenge. Avian Dis. 2012;56(3):501–508. doi: 10.1637/9982-110811-Reg.1. [DOI] [PubMed] [Google Scholar]
- 38.Yan F, Zhao Y, Hu Y, Qiu J, Lei W, Ji W, et al. Protection of chickens against infectious bronchitis virus with a multivalent DNA vaccine and boosting with an inactivated vaccine. J Vet Sci. 2013;14(1):53–60. doi: 10.4142/jvs.2013.14.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Guo Y, Xiao Y, Wang X, Li Z, Hu S, et al. A simple and rapid strip test for detection of antibodies to avian infectious bronchitis virus. J Vet Med Sci. 2010;72(7):883–886. doi: 10.1292/jvms.09-0528. [DOI] [PubMed] [Google Scholar]
- 41.Zhao G, Jiang Y, Qiu H, Gao T, Zeng Y, Guo Y, et al. Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with middle east respiratory syndrome-coronavirus. PLoS ONE. 2015;10(12):e0145561. doi: 10.1371/journal.pone.0145561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 6 Map showing the geographic coordinates of the farms and bird sampling. A map showing the geographical locations of the farms under study in Eastern Saudi Arabia. The farms are located around three major cities in ESA (Al-Ahsa (Al-Hofuf), Dammam and Buqayq). A circle highlighted the location of these farms in ESA (TIFF 3088 kb)