Abstract
Disseminated acanthamoebiasis is a rare, often fatal, infection most commonly affecting immunocompromised patients. We report a case involving sinuses, skin, and bone in a 60-year-old woman five months after a heart transplant. She improved with a combination of flucytosine, fluconazole, miltefosine, and decreased immunosuppression. To our knowledge this is the first case of successfully treated disseminated acanthamoebiasis in a heart transplant recipient and only the second successful use of miltefosine for this infection among solid organ transplant recipients. Acanthamoeba infection should be considered in transplant recipients with evidence of skin, central nervous system, and sinus infections that are unresponsive to antibiotics. Miltefosine may represent an effective component of a multi-drug therapeutic regimen for the treatment of this amoebic infection.
Case Presentation
A 60-year-old Pacific Islander presented to her primary care physician with rhinorrhea and sinus pressure five months after orthotopic heart transplantation.
Her past medical history was notable for non-ischemic cardiomyopathy requiring heart transplantation, gout, and chronic kidney disease. She had received thymoglobulin induction at the time of transplant and had been doing well on a maintenance immunosuppressive regimen of mycophenolate (1,000mg BID), tacrolimus (1.5mg BID), and prednisone (2.5mg daily). She was also on trimethoprim-sulfamethoxazole (800mg-160mg) three times weekly for Pneumocystis pneumonia (PCP) prophylaxis and had not experienced any episodes of rejection or opportunistic infections.
She was initially treated by her primary care physician for these sinus symptoms with a 10-day course of cephalexin and nasal fluticasone for presumed bacterial sinusitis. When her symptoms failed to improve, she received an additional 10 days of amoxicillin-clavulanate with guaifenesin and pseudoephedrine. During this time, she had several episodes of epistaxis and was referred for evaluation by an otolaryngologist. In the six-week period between antibiotic treatment and being evaluated by her otolaryngologist, she performed a tap water lavage of her sinuses on several occasions, as she had done in the past for nasal congestion, with minimal improvement of her symptoms. She had no fevers, chills or night sweats. She had not traveled outside of Northern California in several years and did not endorse any recent history of freshwater exposure or dust inhalation. Trained as an esthetician, she had not worked since before her transplant.
The patient was seen by an outpatient otolaryngologist two months after the onset of symptoms (seven months after transplant), and was noted on exam to have a friable and raised right nasal septal mass, with magnetic resonance imaging (MRI) of the sinuses showing right frontal, maxillary, and ethmoid opacification (Figure 1a). A nasal endoscopy was performed and biopsy was taken. Histopathological review was notable for an acutely inflamed atypical squamoproliferative lesion, raising concern for squamous cell carcinoma. Given the concern for malignancy, she was admitted for surgical resection of the mass. On admission, she was hemodynamically stable and afebrile. Labs showed a white blood cell count of 6200 cells/μL, hemoglobin of 8.5 g/dL, and creatinine of 2.81 mg/dL (baseline 2–3 mg/dL). Surgical resection was aborted when intraoperative frozen section revealed the presence of amoeba, and the patient was started on metronidazole and amphotericin B on the first post-operative day. Tissue sections were sent to the Centers for Disease Control and Prevention (CDC) for specific pathogen identification. Histopathologic examination and subsequent immunohistochemical testing revealed necrotizing granulomatous inflammation with intralesional Acanthamoeba healyi.
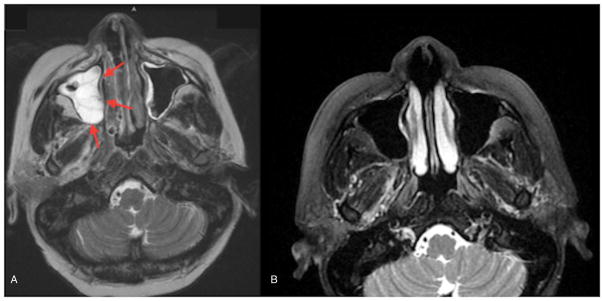
Fig. 1.
A) MRI of sinuses before debridement showed opacification consistent with observed mass (red arrows). B) MRI of sinuses 6 months after discharge demonstrated clearance of previous opacifications.
After consulting with local infectious disease specialists and the CDC, the patient was switched to fluconazole, flucytosine, and miltefosine on hospital day four. Miltefosine is not currently approved to treat acanthamoebiasis, but is available through the CDC’s expanded access investigational new drug (IND) protocol. In this case, the drug was obtained through the IND protocol following patient consent and institutional review board approval. A second surgical debridement of the right nasal septum, including right total ethmoidectomy and right frontal sinusotomy, was performed on hospital day seven. Histopathological evaluation and immunohistochemical testing of specimens obtained during this procedure again demonstrated the presence of Acanthamoeba healyi. The CDC also performed serologic testing for Acanthamoeba, which was negative.
Between hospital days one and four (before initiation of miltefosine therapy), the patient developed several subcutaneous nodules on her left flank and bilateral upper and lower extremities (Figure 2a). Skin biopsy of one of these lesions demonstrated Acanthamoeba by histopathological examination and immunohistochemistry (Figure 3a) performed at the California Department of Public Health, further confirmed by polymerase chain reaction (PCR) at CDC. Topical ketoconazole was added. A lesion over the right 3rd metacarpal started to drain in the next 48 hours, and imaging showed a lytic lesion of the right 3rd metacarpal (Figure 4a and 4b). The patient underwent incision and drainage of the digit by orthopedic surgery on hospital day nine, and the surgeon noted that the shaft was hollow and contained small exophytic growths, which were sent for pathologic evaluation. Amoebae were seen with Periodic acid–Schiff–diastase (PAS-D) and Grocott’s methamine silver (GMS) stains, and Acanthamoeba was identified by PCR at the CDC.
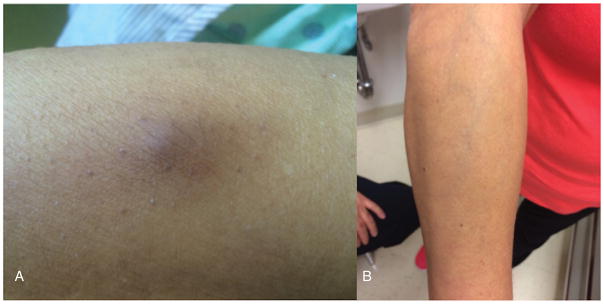
Fig. 2.
A) Cutaneous lesion of the right forearm photographed 8 days after initial eruption. B) Photograph of the right forearm at 8-month follow up visit demonstrated resolution.
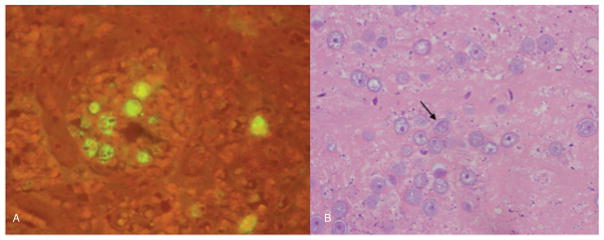
Fig. 3.
A) Immunohistochemical stain of skin biopsy showed Acanthamoeba cysts by immunofluorescence (performed at the CDC). B) Biopsy sample from repeat sinus debridement showed cysts consistent with Acanthamoeba spp. 12 days after the patient’s initial surgery.
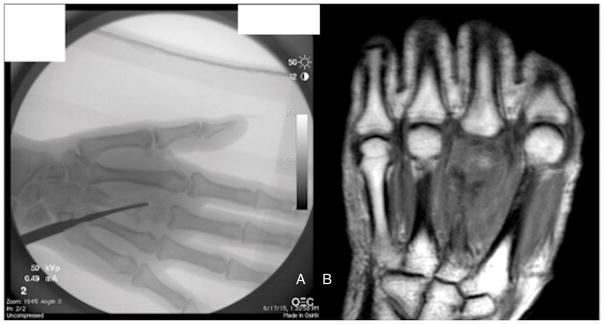
Fig. 4.
a) Radiograph of the right hand revealed a lytic lesion of the third metacarpal. b) MRI of the hand following debridement showed hollow cavity due to bone destruction.
The patient was then transferred to the tertiary care center where she had received her transplant for further management of antimicrobials and immunosuppression. In consultation with the CDC, the regimen of fluconazole, flucytosine, miltefosine, and topical ketoconazole was continued. Her immunosuppressive regimen was also altered: tacrolimus dosing was reduced from 1.5mg BID to 1mg BID with a target trough of 8–10 μg/L, and mycophenolate was reduced from 1,000mg BID to 750mg BID. Repeat brain MRI showed persistent severe pansinusitis but no evidence of bony destruction or central nervous system (CNS) extension of disease. The patient underwent a third superficial debridement of the right sinuses 12 days after her initial surgery and pathologic evaluation again demonstrated amoeba (Figure 3b). More extensive debridement was avoided to mitigate potential translocation of amoeba from the sinuses into the CNS.
Despite persistent demonstration of amoeba in the sinuses, the patient showed clinical improvement during her hospitalization: skin lesions visibly regressed in size and her sinus complaints diminished. Her C-reactive protein (CRP) levels, 76.6 mg/L at the time of transfer, also decreased substantially, dropping to 19.3 mg/L 10 days later. The patient was discharged after 25 days in the hospital, with the plan to maintain her decreased immunosuppressive regimen and the triple-drug therapy of fluconazole, flucytosine, and miltefosine for at least six months. At follow-up visits, she reported continued clinical improvement while on this triple therapy, although the patient did endorse significant nausea from the miltefosine. Liver function tests four months after discharge showed mildly elevated alkaline phosphatase (143–147 U/L) and an elevated gamma-glutamyl transferase (211 U/L), but no other laboratory abnormalities were observed. At six-months after discharge, her CRP was 1.5 mg/L, brain MRI showed resolution of sinus opacities, and her skin lesions had resolved completely. (Figure 2b). Her immunosuppressive regimen was increased to pre-infection levels at this time.
Although no cases of donor-derived Acanthamoeba infection have ever been reported, the California Transplant Donor Network was contacted because transplant-related transmission of another amoeba, Balamuthia, has been reported (1–3). The donor network concluded that no other infection or illness consistent with free-living amoeba infection had been reported in other organ recipients, and archived donor serum was negative for Acanthamoeba serology.
Discussion
Acanthamoeba are free-living amoebae found in soil, air, and numerous aquatic environments, including drinking water, seawater, and swimming pools (3). Human infection is most common in immunocompromised hosts and is transmitted by inhalation of Acanthamoeba cysts or inoculation by direct contact with skin or mucosal surfaces. This protozoan has been reported to cause cutaneous lesions, nasopharyngeal infections, pneumonia, pyelonephritis, and granulomatous amoebic encephalitis (GAE), which is fatal in 90% of cases (3–6). Acanthamoeba osteomyelitis is extremely rare, and has only been reported in one other solid organ transplant (SOT) patient (7).
All 22 reported cases of acanthamoebiasis in SOT patients (including the present case) are described in Table 1. Given that exposure is common but diagnosis is difficult to establish, these reported cases may underrepresent the true disease burden in SOT recipients. To our knowledge this is only the second reported case of Acanthamoeba infection in a heart transplant recipient and the first report of successful treatment (8). Other cases have been reported in kidney (n=8), lung (n=8), liver (n=2), and multi-organ (n=2) recipients. The mean age of patients for these cases was 50 (range 31–64), and mean time to infection following transplant was 18 months (range three months-six years). Immunosuppressive regimens have most commonly included tacrolimus, cyclosporine, mycophenolate and/or prednisone. In three cases (including this case), the patient received thymoglobulin as part of his or her post-transplant immunosuppression regimen. Our patient’s use of tap water for nasal lavage was initially regarded as a possible source of amoeba, particularly given her history of repeated tap water irrigations in the past. However, upper respiratory symptoms pre-dated her use of nasal irrigation in the acute setting, and this mechanism of amoebic infection has only been reported in two cases of Naegleria fowleri infection in the United States (9). Immunocompromised patients should nonetheless avoid the use of non-sterile water for nasal irrigation given the potential for amoebic infection.
Table 1.
Reported cases and outcomes of Acanthamoeba infection in solid organ transplant (SOT) patients
| Year | Patient Age/Gender |
Location | Organ | Time to infection (months) |
Immunosuppresive regimen |
Type of Infection |
Treatment Regimen |
Diagnosis confirmation |
Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1982 | 38/M | Pennsylvania | Kidney | 30 | Azathioprine, methylprednisolone | Skin, lung, brain | Broad-spectrum antibiotics | Histopathologic staining – biopsy specimen | Died | 23 |
| 1994 | 31/M | Texas | Kidney | 10 | Azathioprine, cyclosporine, prednisone | Skin | Pentamidine, topical chlorhexidine/ketoconazole | IF staining of biopsy tissue section | Cured | 13 |
| 1999 | 39/F | South Carolina | Lung | 72 | Azathioprine, prednisone, tacrolimus | Skin | 5-fluorocytosine, itraconazole, pentamidine, topical chlorhexidine/ketoconazole | Histopathologic staining of abscess fluid* | Cured | 14 |
| 2001 | 38/M | France | Bilateral lung | 36 | Methylprednisolone, tacrolimus | Skin | Itraconazole pentamidine, topical chlorhexidine/ketoconazole | IF staining of tissue at autopsy | Died | 24 |
| 2002 | 61/F | Maryland | Kidney | 12 | Mycophenolate mofetil, prednisone, tacrolimus | Skin, bone | Amikacin, amphotericin B, azithromycin, imipenem | Culture of tissue at autopsy | Died (postmortem dx) | 7 |
| 2005 | 49/F | Florida | Bilateral lung | 7 | Mycophenolate mofetil, prednisone, tacrolimus | Sinus | Amphotericin B, caspofungin, voriconazole | IF staining of biopsy tissue section | Cured | 15 |
| 2006 | 60/M | Texas | Bilateral lung | 9 | Mycophenolate mofetil, prednisone, Tacrolimus | Skin, lung, brain | Amphotericin B, ciprofloxacin, imipenem, itraconazole, vancomycin | Histopathologic staining at autopsy* | Died (postmortem dx) | 25 |
| 2006 | 60/M | Texas | Lung | Not reported | Azathioprine, prednisone, tacrolimus | Skin, lung, brain | Broad-spectrum antibiotics | IF staining and PCR analysis of brain biopsy specimen | Died | 26 |
| 2006 | 51/M | Utah | Kidney | 3 | Mycophenolate mofetil, prednisolone, tacrolimus | Skin, brain | Amphotericin B, azithromycin, flucytosine, metronidazole, pentamidine, rifampin, sulfadiazine | IF staining of tissue at autopsy | Died (post-mortem dx) | 27 |
| 2006 | 40/M | Pennsylvania | Multiple organs | 9 | Thymoglobulin, tacrolimus | Brain | Not reported | IF staining of tissue at autopsy | Died (postmortem dx) | 28 |
| 2007 | 40/M | Spain | Multiple organs | 9 | Tacrolimus | Brain | Not reported | Histopathologic staining at autopsy | Died (postmortem dx) | 29 |
| 2007 | 52/F | Florida | Lung | 36 | Mycophenolate mofetil, prednisone, tacrolimus | Skin | Amphotericin B, voriconazole | IF staining of biopsy tissue section | Cured | 16 |
| 2007 | 39/M | France | Heart | 22 | Cyclosporine, mycophenolate mofetil, prednisone | Skin, lungs, kidneys | 5-fluorocytosine, itraconazole, pentamidine | IF staining of biopsy tissue section, confirmed by culture and PCR | Died | 8 |
| 2007 | 36/F | India | Kidney | 48 | Not reported | Brain, lungs, pancreas | Broad-spectrum antibiotics | IF staining of biopsy tissue section | Died (postmortem dx) | 30 |
| 2008 | 41/M | United Kingdom | Liver | 14 | Azathioprine, cyclosporine, prednisone | Brain | co-trimoxazole, rifampicin, surgical resection | IF staining of biopsy tissue section | Cured | 31 |
| 2010 | 63/M | New York | Liver | 12 | Alemtuzumab, Cyclophosphamide, Daclizumab, Doxorubicin, Etoposide, mycophenolate mofetil, Prednisone, Rituximab, Tacrolimus, Vincristine | Skin, lung, brain | Amphotericin B, caspofungin, flucytosine, miltefosine, pentamidine, voriconazole, topical ketoconazole | IF staining of biopsy tissue section | Died | 18 |
| 2013 | 62/M | California | Bilateral lung | 6 | Mycophenolate mofetil, prednisolone, tacrolimus | Skin, brain | Amikacin, flucytosine, pentamidine, intrathecal amphotericin B | Light microscopy and PCR of CSF fluid | Died | 32 |
| 2013 | 58/M | New York | Kidney | 24 | Methylprednisolone, mycophenolate mofetil, prednisone, rituximab, tacrolimus, thymoglobulin | Brain | Broad-spectrum antibiotics, ganciclovir, pyrimethamine, sulfadiazine, voriconazole | IF staining of tissue at autopsy | Died (postmortem dx) | 3 |
| 2014 | 63/M | Mississippi | Kidney | 6 | Mycophenolate mofetil, tacrolimus | Brain | Azithromycin, fluconazole, flucytosine, miltefosine, sulfadiazine | Histopathologic staining – brain biopsy specimen* | Died | 19 |
| 2015 | 64/F | Arizona | Kidney | 7 | Mycophenolate mofetil, prednisone, tacrolimus | Brain | Azithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine | IF staining of brain biopsy section | Died | 20 |
| 2015 | 59/F | California | Lung | 10 | Not reported | Skin, sinus | Inpatient: 5-flucytosine, azithromycin, bactrim, intranasal pentamidine; Discharge: azithromycin, bactrim, miltefosine, voriconazole | IF staining of biopsy tissue section | Cured | 22 |
| 2015 | 60/F | California | Heart | 5 | Mycophenolate mofetil, prednisone, tacrolimus, thymoglobulin | Skin, sinus, bone | Fluconazole, flucytosine, miltefosine | IF staining of biopsy tissue section | Cured | 24 |
IF: immunofluorescence; PCR –polymerase chain reaction; CSF –cerebrospinal fluid
Histochemical staining not specified
Diagnosis of Acanthamoeba infection can be challenging. The majority of cases listed in Table 1 relied on histopathologic or immunofluorescence staining of involved tissue or fluid, and most cases (including ours) confirmed the diagnosis by sending samples to the CDC for immunofluorescence testing to detect Acanthamoeba trophozoites and cysts. Additionally, diagnosis can be made by culture or PCR from tissue or fluid specimens. Serologic testing is available, however the sensitivity and specificity of this assay is not well described.
Optimal treatment for acanthamoebiasis is not well established. Lack of susceptibility data for Acanthamoeba makes choice of antibiotic particularly challenging. Thus, a ‘kitchen sink’ approach relying on multiple pharmacologic mechanisms of action is often employed (10). There are six previous case reports of successfully treated Acanthamoeba infection in SOT patients, as noted in Table 1. Effective regimens have included sterol-targeting azoles (fluconazole, itraconazole, ketoconazole), pentamidine isethionate, trimethoprim-sulfamethoxazole, sulfadiazine, 5-fluorocytosine, azithromycin, amphotericin B, and miltefosine (10–16). Miltefosine, an aklylphosphocholine drug initially approved by the United States Food and Drug Administration (FDA) for the treatment of visceral leishmaniasis, also has in vitro activity against free living amoeba, including N. fowleri, Balamuthia mandrillaris, and Acanthamoeba spp. (12). It has been available in the United States (from CDC) under an expanded access IND protocol for use in the treatment of free-living ameba infections since 2013. There are five case reports of its use in treatment of Acanthamoeba, only two of which resulted in successful treatment (17–22). Topical chlorhexidine or ketoconazole has also been used for cutaneous lesions. Overall prognosis for this infection remains poor: only six of 21 previous SOT cases were reported as cured, and no therapeutic regimen has shown consistent efficacy. Of those that survived, only one had CNS involvement, and only two had disease involving more than one organ. This would suggest that the absence of encephalitis and/or disseminated disease may be important prognostic factors.
Our patient developed disseminated infection, which included the sinuses, skin, and bone but fortunately without evidence of CNS disease. Dissemination appeared to progress despite initial therapy with amphotericin B and metronidazole for four days and subsequent conversion to fluconazole, flucytosine, and miltefosine. Continued use of this triple-drug therapy and concurrent reduction in immunosuppression were followed by steady improvement in the second and third weeks of hospitalization. We speculate that improvement of the patient’s skin lesions and absence of new bone invasion resulted from this combination of anti-amoebic therapy and prompt decrease in the patient’s immunosuppressive regimen. Following discharge, the patient missed numerous appointments but did report adhering to her drug regimen, making it difficult to assess the progress of her improvement. Given a lack of interval imaging and evaluation, adequate duration of triple-drug therapy to resolve the infection was unclear, and a prolonged course was maintained. After six months of therapy, sinus symptoms and MRI lesions had completely resolved, and cutaneous lesions were not visible. Given this improvement and the patient’s ongoing nausea, miltefosine was discontinued eight months after discharge. Shortly after discontinuation of miltefosine, the patient’s absolute neutrophil count (ANC) began to drop; this was attributed to bone marrow suppression from flucytosine. Her ANC reached a nadir of 1660 cells/μL and she was switched to a regimen of fluconazole and trimethoprim-sulfamethoxazole, which she has been on for four months. Her ANC returned to baseline (> 4000 cells/μL) and she has remained clinically stable at 16 months after discharge.
Summary
Acanthamoebiasis is a rare, potentially fatal infection in SOT patients. Here we describe the second reported occurrence of disseminated acanthamoebiasis in a heart transplant recipient. This is also the first instance of successful treatment with a miltefosine-containing regimen for such a patient with disseminated disease, albeit without amebic encephalitis. Our patient demonstrated an extremely rare lytic lesion of bone, along with more typical sinus and skin involvement. Miltefosine appears to show some promise in the treatment of free-living amoebic infection, although it should be noted that regimens including this investigational drug have only led to cure in three out of six Acanthamoeba SOT case reports where it was used. Furthermore, miltefosine had limited effect in individuals with granulomatous amebic encephalitis, where infection was uniformly fatal. This ubiquitous protozoan should be considered as a cause of disseminated infection in immunosuppressed individuals who do not respond initially to antibiotics. Although reported outcomes are poor, and the optimal anti-amoebic regimen is not well defined, prompt diagnosis of Acanthamoeba infection, reduction of immunosuppression, and initiation of multi-drug therapy may provide the best chance for successful treatment.
Acknowledgments
The authors wish to thank Jessica Finn MD, from University of California San Francisco Department of Pathology, Kristina Hsieh DrPH, Shigeo Yagi PhD, Thelma Dunnebacke-Dixon PhD, Diana Singh BS, BA, and Anna Clayton MPH from the California Department of Public Health Viral & Rickettsial Disease Laboratory, as well as Sharon Roy MD, MPH and Pallavi D. Annambhotla, DrPH from the Centers for Disease Control and Prevention.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- IND
investigational new drug
- CNS
central nervous system
- CRP
C-reactive protein
- SOT
solid organ transplant
- MRI
magnetic resonance imaging
- ANC
absolute neutrophil count
Footnotes
Disclaimer: The findings and conclusions herein are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease C, Prevention. Balamuthia mandrillaris transmitted through organ transplantation --- Mississippi, 2009. MMWR. Morbidity and mortality weekly report. 2010 Sep 17;59(36):1165–1170. [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. Notes from the field: transplant-transmitted Balamuthia mandrillaris --- Arizona, 2010. MMWR. Morbidity and mortality weekly report. 2010 Sep 17;59(36):1182. [PubMed] [Google Scholar]
- 3.Satlin MJ, Graham JK, Visvesvara GS, et al. Fulminant and fatal encephalitis caused by Acanthamoeba in a kidney transplant recipient: case report and literature review. Transpl Infect Dis. 2013;15(6):619–26. doi: 10.1111/tid.12131. [DOI] [PubMed] [Google Scholar]
- 4.Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, Neji S, et al. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol. 2012;60:399–405. doi: 10.1016/j.patbio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Murakawa G, McCalmont T, Altman J, Telang G, Hoffman M, Kantor G, et al. Disseminated acanthamoebiasis in patients with AIDS. Arch Dermatol. 1995;131:1291–6. [PubMed] [Google Scholar]
- 6.Khan N. Acanthamoeba and the blood-brain barrier: the breakthrough. J Med Microbiol. 2008;57:1051–7. doi: 10.1099/jmm.0.2008/000976-0. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg J, Galindo R, Kraus E, Ghanem K. Disseminated acanthamoebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review. Clin Infect Dis. 2002;35:e43–9. doi: 10.1086/341973. [DOI] [PubMed] [Google Scholar]
- 8.Barete S, Combes A, de Jonckheere J, Datry A, Varnous S, Martinez V, et al. Fatal disseminated Acanthamoeba lenticulata infection in a heart transplant patient. Emerg Infect Dis. 2007;13(5):736–8. doi: 10.3201/eid1305.061347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoder J, Straif-Bourgeois S, Roy S, et al. Primary Amebic Meningoencephalitis Deaths Associated With Sinus Irrigation Using Contaminated Tap Water. Clin Infect Dis. 2012 doi: 10.1093/cid/cis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster F, Visvesvara G. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat. 2004;7:41–51. doi: 10.1016/j.drup.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Walochnik J, Duchene M, Seifert K, Obwaller A, Hottkowitz T, Wiedermann G, et al. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob Agents Chemother. 2002;46:695–701. doi: 10.1128/AAC.46.3.695-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris Acanthamoeba spp. and Naegleria fowleri. J Eukaryot Micribiol. 2006;53:121–6. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 13.Slater C, Sickel J, Visvesvara G, Pabico R, Gaspari A. Successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N Engl J Med. 1994;331:85–7. doi: 10.1056/NEJM199407143310204. [DOI] [PubMed] [Google Scholar]
- 14.Oliva S, Jantz M, Tiernan R, Cook DL, Judson MA. Successful treatment of widely disseminated acanthamoebiasis. South Med J. 1999;92(1):55–7. doi: 10.1097/00007611-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Vernon SE, Acar BC, Pham SM, Fertel D. Acanthamoeba infection in lung transplantation: report of a case and review of the literature. Transpl Infect Dis. 2005;7:154–7. doi: 10.1111/j.1399-3062.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 16.Walia R, Montoya J, Visvesvara G, Booton G, Doyle R. A case of successful treatment of Acanthamoeba infection in a lung transplant recipient. Tranpl Infect Dis. 2007;9:51–4. doi: 10.1111/j.1399-3062.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- 17.Aichelburg A, Walochnik J, Assadian O, Prosch H, Steuer A, Perneczky G, et al. Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine. Emerg Infect Dis. 2008;14:1743–6. doi: 10.3201/eid1411.070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young AL, Leboeuf NR, Tsiouris SJ, Husain S, Grossman ME. Fatal disseminated Acanthamoeba infection in a liver transplant recipient immunocompromised by combination therapies for graft-versus-host disease. Transpl Infect Dis. 2010;12(6):529–37. doi: 10.1111/j.1399-3062.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 19.Zamora A, Henderson H, Swiatlo E. Acanthamoeba encephalitis: A case report and review of therapy. Surg Neurol Int. 2014;5:68. doi: 10.4103/2152-7806.132239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salameh A, Bello N, Becker J, Zangeneh T. Fatal granulomatous amoebic encephalitis caused by Acanthamoeba in a patient with kidney transplant: A case report. Open Forum Infect Dis. 2015;2(3):1–4. doi: 10.1093/ofid/ofv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandakuri S, Morgan M, Zakowski P, et al. Transplant patient with skin nodules. Am J Dermatopathol. 2015;37:229–231. doi: 10.1097/DAD.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Investigational Drug Available Directly from CDC for the Treatment of Infections with Free-Living Amebae. MMWR. 2013;62:666. [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez J. Acanthamoebiasis and Immunosuppression. J Neuropathol Exp Neurol. 1982;41(5):548–57. doi: 10.1097/00005072-198209000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Van Hamme C, Dumont M, Delos M, Lachapelle JM. Cutaneous acanthamoebiasis in a lung transplant patient. Ann Dermatol Venereol. 2001;128(11):1237–40. [PubMed] [Google Scholar]
- 25.Duarte AG, Sattar F, Granwehr B, Aronson JF, Wang Z, Lick S. Disseminated acanthamoebiasis after lung transplantation. J Heart Lung Transplant. 2006;25(2):237–40. doi: 10.1016/j.healun.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Readinger A, Blumberg M, Colome-Grimmer MI, Kelly E. Disseminated Acanthamoeba infection with sporotrichoid nodules. Int J Dermatol. 2006;45(8):942–3. doi: 10.1111/j.1365-4632.2006.02850.x. [DOI] [PubMed] [Google Scholar]
- 27.McKellar MS, Mehta LR, Greenlee JE, et al. Fatal granulomatous Acanthamoeba encephalitis mimicking a stroke, diagnosed by correlation of results of sequential magnetic resonance imaging, biopsy, in vitro culture, immunofluorescence analysis, and molecular analysis. J Clin Microbiol. 2006;44(11):4265–9. doi: 10.1128/JCM.00649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendez O, Kanal E, Abu-Elmagd KM, et al. Granulomatous amebic encephalitis in a multivisceral transplant recipient. Eur J Neurol. 2006;13(3):292–5. doi: 10.1111/j.1468-1331.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- 29.Gene AH, Gardner PA, Couce Matovelle ME. Rapidly expanding brain mass. Transpl Infect Dis. 2007;9:211–213. doi: 10.1111/j.1399-3062.2007.00254.x. [DOI] [PubMed] [Google Scholar]
- 30.Mutreja D, Jalpota Y, Madan R, Tewari V. Disseminated Acanthamoeba infection in a renal transplant recipient: a case report. Indian J Pathol Microbiol. 2007;50(2):346–8. [PubMed] [Google Scholar]
- 31.Fung KT, Dhillon AP, McLaughlin JE. Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transpl. 2008;14(3):308–12. doi: 10.1002/lt.21409. [DOI] [PubMed] [Google Scholar]
- 32.Afshar K, Boydking A, Ganesh S, Herrington C, McFadden PM. Rapidly fatal disseminated acanthamoebiasis in a single lung transplant recipient. Ann Transplant. 2013;18:108–11. doi: 10.12659/AOT.883846. [DOI] [PubMed] [Google Scholar]