Abstract
Aims: Recent studies have shown that cigarette smoke (CS)-induced oxidative stress impairs autophagy, resulting in aggresome-formation that correlates with severity of chronic obstructive pulmonary disease (COPD)-emphysema, although the specific step in autophagy pathway that is impaired is unknown. Hence, in this study, we aimed to evaluate the role of master autophagy transcription factor EB (TFEB) in CS-induced COPD-emphysema pathogenesis.
Results: We first observed that TFEB accumulates in perinuclear spaces as aggresome-bodies in COPD lung tissues of tobacco smokers and severe emphysema subjects, compared with non-emphysema or nonsmoker controls. Next, Beas2b cells and C57BL/6 mice were exposed to either cigarette smoke extract (CSE) or subchronic-CS (sc-CS), followed by treatment with potent TFEB-inducing drug, gemfibrozil (GEM, or fisetin as an alternate), to experimentally verify the role of TFEB in COPD. Our in vitro results indicate that GEM/fisetin-mediated TFEB induction significantly (p < 0.05) decreases CSE-induced autophagy-impairment (Ub/LC3B reporter and autophagy flux assay) and resulting aggresome-formation (Ub/p62 coexpression/accumulation; immunoblotting and staining) by controlling reactive oxygen species (ROS) activity. Intriguingly, we observed that CS induces TFEB accumulation in the insoluble protein fractions of Beas2b cells, which shows a partial rescue with GEM treatment. Moreover, TFEB knockdown induces oxidative stress, autophagy-impairment, and senescence, which can all be mitigated by GEM-mediated TFEB induction. Finally, in vivo studies were used to verify that CS-induced autophagy-impairment (increased Ub, p62, and valosin-containing protein in the insoluble protein fractions of lung/cell lysates), inflammation (interleukin-6 [IL-6] levels in bronchoalveolar lavage fluid and iNOS expression in lung sections), apoptosis (caspase-3/7), and resulting emphysema (hematoxylin and eosin [H&E]) can be controlled by GEM-mediated TFEB induction (p < 0.05).
Innovation: CS exposure impairs autophagy in COPD-emphysema by inducing perinuclear localization of master autophagy regulator, TFEB, to aggresome-bodies.
Conclusion: TFEB-inducing drug(s) can control CS-induced TFEB/autophagy-impairment and COPD-emphysema pathogenesis. Antioxid. Redox Signal. 27, 150–167.
Keywords: : TFEB, autophagy, COPD, emphysema, cigarette smoke, tobacco, ROS, oxidative stress
Introduction
Exposure to first- and/or secondhand tobacco smoke is the primary risk factor in the development and progression of chronic obstructive pulmonary disease (COPD) in the United States, which is a preventable, but life-threatening, lung condition characterized by the presence of chronic bronchitis, bronchiectasis, and/or emphysema (30, 42). The deleterious effects of cigarette smoke (CS) exposure are multifarious as it impacts several key cellular homeostatic processes, such as cell growth, protein processing, autophagy, and antibacterial immune defense mechanisms (19, 20, 37, 41, 55, 58). Extensive research into the underlying mechanism(s) of CS-induced lung damage has shown that oxidative stress (reactive oxygen species [ROS] activation) and inflammation are the prime causes of alveolar apoptosis and senescence resulting in emphysema pathogenesis (3, 4, 16, 40, 57). Specifically, ROS activation triggers autophagy-impairment leading to the accumulation of misfolded/damaged proteins as perinuclear aggresome-bodies (31, 37) that induce COPD-emphysema pathogenesis (58). Although we have recently shown that pharmacological autophagy induction can control lung damage in the murine models of CS-induced COPD-emphysema (51, 58), further studies were necessary to identify the specific defect in the autophagy pathway in COPD-emphysema subjects to design a potent and highly specific therapeutic strategy.
Innovation.
Cigarette smoke-induced autophagy-impairment mediates chronic obstructive pulmonary disease (COPD)–emphysema progression, but underlying mechanisms are unclear. Our study identifies cigarette smoke-induced accumulation of transcription factor EB (TFEB) in perinuclear aggresome-bodies as a novel mechanism for TFEB/autophagy-impairment. Moreover, our data demonstrate the potential of prognosis-based intervention strategy for COPD-emphysema.
The search for transcription factors that govern the expression of key autophagy-related genes led to identification of transcription factor EB (TFEB), a master regulator of important genes that govern lysosomal biogenesis and autophagy (35, 48, 49). The activation of TFEB is regulated by mammalian target of rapamycin protein kinase complex (mTORC1), which under normal cellular environment, phosphorylates TFEB at the lysosomal surface leading to binding of YWHA/14-3-3 proteins and cytosolic retention of TFEB (28, 33, 45, 50). Under conditions of starvation or alteration of lysosomal functions, TFEB translocates to the nucleus, where it enhances transcription of numerous genes involved in autophagy and lysosomal biogenesis (33, 39, 50). Several reports highlight the crucial role of TFEB in age-related neurodegenerative diseases, such as Parkinson's, Huntington's, and Alzheimer's, all of which involve autophagy-impairment and accumulation of disordered or aggregated proteins (34, 56, 60). Moreover, genetic or pharmacological induction of TFEB has been reported to benefit in several of these pathological conditions caused by autophagy-impairment, which includes lysosomal storage disorders, Alzheimer's disease (AD), and α1-antitrypsin (α1-AT) deficiency. (18, 26, 43, 44, 53).
Hence, based on our recent studies identifying tobacco smoke exposure and/or aging-mediated autophagy-impairment and resulting aggresome-formation as a prognostic indicator of COPD-emphysema severity (5, 37, 51, 55, 58), we wanted to evaluate the pharmacological effects of TFEB-mediated autophagy induction in CS-induced COPD-emphysema (using human/murine lung tissues) to develop a prognosis-based intervention strategy. We first tested the therapeutic potential of a known TFEB inducer, gemfibrozil (GEM), which is an FDA-approved fibrate drug used for lowering serum lipid levels (22, 46), in controlling CS-induced autophagy-impairment and the resulting alveolar tissue damage (emphysema). GEM treatment is known to not only induce TFEB protein levels and activity in brain cells (46) but also initiates anti-inflammatory and antioxidant responses (46). Hence, apart from its well-known application in controlling hyperlipidemia in coronary heart disease (2, 54), treatment with GEM has proven beneficial in several other pathological conditions, for example, rheumatoid arthritis (12, 46), diabetes (6, 46), and murine experimental autoimmune encephalomyelitis (13, 46). Moreover, it is important to note here that the prominent comorbidities of COPD are coronary heart disease and metabolic syndrome (7), for which GEM is already shown to be clinically beneficial (46, 54). We verified the GEM results with an alternate TFEB-inducing flavonoid drug, fisetin (FIS), which is available over the counter as a dietary antioxidant for brain health.
Thus, we evaluated the impact of GEM (or FIS) treatment on CS-induced oxidative stress, autophagy-impairment, and/or resulting alveolar damage (emphysema) to validate the role of TFEB in COPD-emphysema pathogenesis.
Results
Perinuclear accumulation of TFEB protein in human lungs correlates with severity of COPD-emphysema
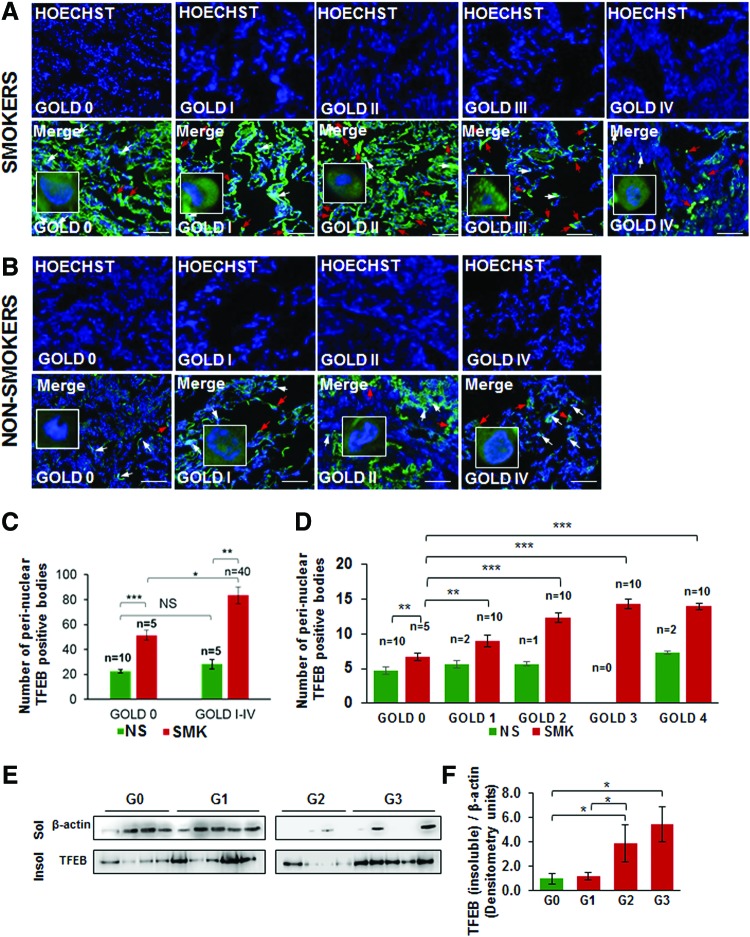
We have recently observed that CS or aging induces autophagy-impairment and resulting aggresome-formation that correlates with the severity of COPD-emphysema lung disease, which can serve as a prognostic biomarker (55, 58). Thus, we found that underlying autophagy-impairment is a common mechanism for CS/age-related chronic obstructive lung disease initiation and progression. To identify autophagy mechanism impaired by CS exposure, we wanted to verify the role of the master autophagy regulator, TFEB, in the pathogenesis of COPD-emphysema in murine and human lungs. Hence, we first analyzed changes in expression and localization of TFEB protein in longitudinal lung tissue sections of COPD-emphysema subjects (GOLD 0 to GOLD I–IV) and found that nuclear localization of TFEB (TFEB activation) decreases with severity of emphysema, while its perinuclear localization (in aggresome-bodies) increases with emphysema severity (from GOLD 0 to GOLD IV; Fig. 1A, D, p < 0.05, r = −0.95; white arrows: nuclear TFEB localization and red arrows: perinuclear TFEB accumulation). We observed a significant increase in perinuclear TFEB localization in severe (GOLD III) and very severe (GOLD IV) emphysema subjects compared with non-emphysema (GOLD 0) controls as well as mild or moderate emphysema (GOLD I–II). Furthermore, we demonstrate that smokers (GOLD I–IV) show a significant increase in perinuclear localization of TFEB compared with nonsmokers (Fig. 1A–C, GOLD 0 or GOLD I–IV, p < 0.05). In addition, smokers with greater disease severity (GOLD I–IV) show a comparatively higher increase in TFEB accumulation in aggresome-bodies compared with GOLD 0 subjects (Fig. 1C). Moreover, we verified that TFEB protein accumulates in the insoluble protein fractions (aggresomes) isolated from the lung tissues of moderate and severe COPD-emphysema subjects (GOLD II and III) compared with non-emphysema controls (GOLD 0) (Fig. 1E, F, and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). Thus, our data suggest the mechanistic role of master autophagy regulator, TFEB, in autophagy-impairment in smokers and COPD-emphysema subjects, which we verified using the in vitro and murine experimental models of CS-induced COPD-emphysema.
FIG. 1.
TFEB accumulates in perinuclear spaces in smokers and/or COPD subjects with emphysema. (A, B) The fluorescent or confocal imaging (insets) of immunostained human lung tissue sections collected from non-emphysema (GOLD 0) and COPD subjects (GOLD I–IV) with emphysema shows that the nuclear localization of TFEB (TFEB activation) decreases with severity of emphysema (white arrows), while its perinuclear localization increases with disease/emphysema severity (from GOLD I–IV; red arrows). As anticipated, the increase in perinuclear localization in GOLD IV emphysema subjects is limited by substantial tissue destruction. Nuclei are stained with Hoechst (blue) dye for both protein localization and observing cellular changes due to tissue destruction. High-resolution confocal image insets show the representative positive or negative staining marked by red or white arrows. (C, D) The data compiled from high-resolution fluorescent and confocal images (insets) indicate that smokers (SMK, GOLD I–IV) show a significant increase in perinuclear localization of TFEB compared with nonsmokers (NS, GOLD 0 or GOLD I–IV). Moreover, smokers with greater disease severity (from GOLD I–IV) demonstrate a higher increase in accumulation of TFEB in perinuclear spaces (red arrows) compared with GOLD 0 subjects that show nuclear localization of TFEB (white arrows). Our data suggest the role of CS-induced TFEB-autophagy-impairment in COPD-emphysema pathogenesis. The correlation analysis for perinuclear TFEB-positive aggresome-bodies and FEV1-% predicted (lung function) showed significant increase in number of TFEB+ perinuclear staining with lung function decline, r = −0.95. Scale bar, 100 μM, *p < 0.05, **p < 0.01, ***p < 0.001, n = 5–10. (E, F) Western blots showing a significant increase in TFEB protein accumulation in the insoluble protein fractions (aggresomes) isolated from moderate (GOLD II) and severe (GOLD III) COPD-emphysema subjects compared with GOLD 0 non-emphysema controls (*p < 0.05; n = 4–5). The data supplement our above findings and confirm that TFEB protein accumulates in perinuclear spaces (potentially as aggresome-bodies) in the lungs of smokers and COPD-emphysema subjects. CS, cigarette smoke; COPD, chronic obstructive pulmonary disease; TFEB, transcription factor EB. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Cigarette smoke-induced TFEB accumulation in aggresome-bodies impairs autophagy in COPD-emphysema
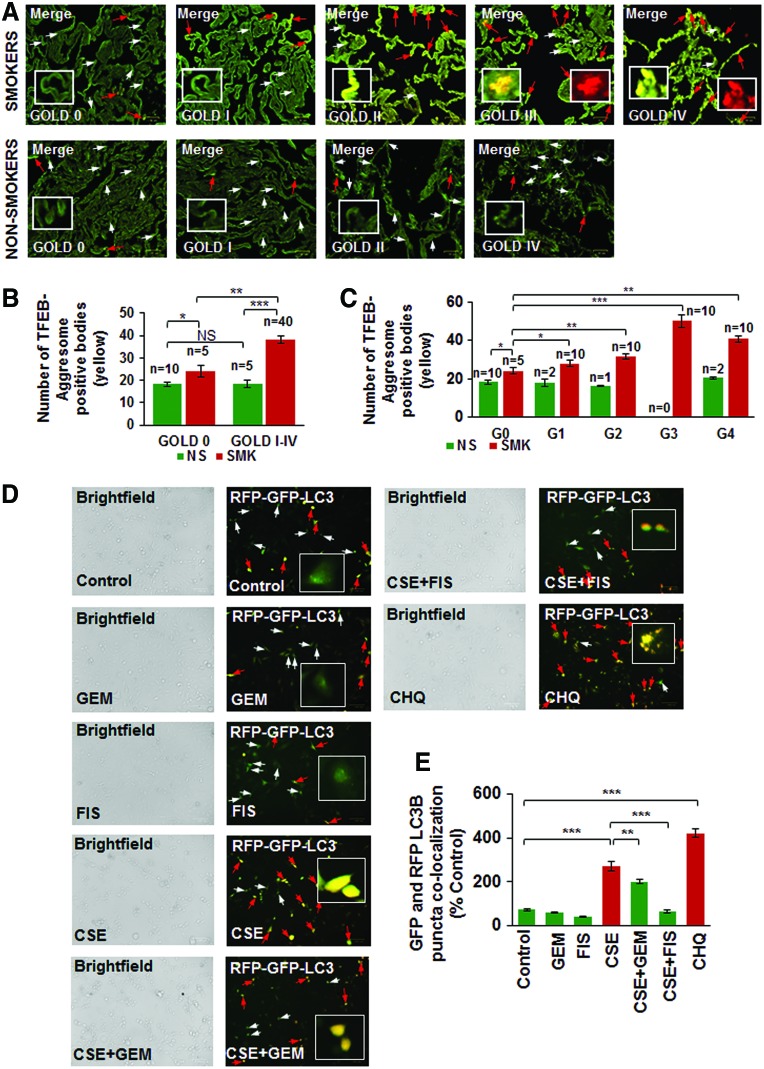
Next, we verified our observation that TFEB protein accumulates in the perinuclear spaces as aggresome-bodies using an aggresome-specific dye. Thus, we analyzed the changes in colocalization of TFEB protein with an aggresome dye (PROTEOSTAT® Aggresome detection kit) in longitudinal lung tissue sections of COPD-emphysema subjects (GOLD I–IV) and observed that TFEB-aggresome colocalization (yellow) increases with emphysema severity in COPD subjects (GOLD I–IV) compared with non-emphysema (GOLD 0) controls (Fig. 2A, C; red arrows and insets, r = −0.88). We also found that smokers (GOLD 0 to GOLD IV) have higher TFEB-aggresome colocalization compared with nonsmoker COPD-emphysema or control subjects (Fig. 2B, p < 0.05). The Hoechst (Blue) dye was used to stain the nuclei to visualize the tissue area used for immunostaining (Supplementary Fig. S2). This suggests that CS induces accumulation of TFEB into aggresome-bodies that results in TFEB/autophagy-impairment in COPD-emphysema subjects (4, 55). We also verified this hypothesis by autophagy flux assay (colocalization of green fluorescent protein [GFP] and red fluorescent protein [RFP], LC3B-yellow puncta bodies, red arrows) and observed that CSE-impaired autophagy can be controlled by GEM or FIS-mediated TFEB induction (Fig. 2D, E, p < 0.05). The data suggest the significant role for TFEB in regulating CS-induced autophagy-impairment that required further analysis of TFEB induction strategies in controlling CS-induced oxidative stress, autophagy-impairment, and resulting emphysema.
FIG. 2.
TFEB accumulates in aggresome-bodies in smokers and COPD subjects with increasing severity of emphysema that results in autophagy flux impairment. (A–C) Immunostaining of human lung tissue sections collected from non-emphysema (GOLD 0) and COPD-emphysema subjects (GOLD I–IV) shows the colocalization of TFEB (green, white arrows) in perinuclear aggresome-bodies (red; aggresome-specific dye, insets). This TFEB-aggresome colocalization (yellow, insets) statistically correlates with the severity of emphysema (from GOLD I–IV; red arrows) and lung function (FEV1-% predicted) decline, r = −0.88. We also observed that the increased tissue destruction in GOLD IV emphysema subjects may be influencing further increase in TFEB-positive aggresome-bodies compared with severe (GOLD III) COPD-emphysema subjects. The Hoechst (blue) dye was used to stain the nuclei to visualize the tissue area used for immunostaining (Supplementary Fig. S2). Moreover, smokers (GOLD 0 to GOLD IV) with incrementing emphysema severity show a higher TFEB-aggresome colocalization, verifying TFEB-autophagy-impairment in smokers and COPD-emphysema subjects. Scale bar, 25 μM, *p < 0.05, **p < 0.01, ***p < 0.001; n = 5–10 per group. (D) Beas2b cells were incubated with BacMam reagent (with Premo™ Autophagy Tandem Sensor RFP-GFP-LC3B) for 16 h, followed by treatment with CSE (5%), GEM (10 μM), and/or FIS (25 μM) for 12 h. Fluorescence microscopy was used to quantify the number of GFP/RFP-positive autophagosomes that suggests an impaired autophagy flux (Scale bars, 100 μm). The red arrows indicate yellow puncta bodies, while white arrows show GFP-positive cells. (E) The data analysis of GFP-RFP-positive puncta bodies is shown as mean ± SEM of n = 6 replicates (**p < 0.01, ***p < 0.001). Results indicate that CSE induces autophagy flux impairment, while autophagy-inducing drugs (GEM/FIS) can restore autophagy flux in the presence of CSE. Chloroquine (endosomal acidification and autophagy inhibitor) is used as a positive control to identify RFP-GFP-positive (yellow) autophagosomes indicative of autophagy flux inhibition. The insets (A, D) show the representative magnified image for positive or negative staining, marked by red or white arrows. FIS, fisetin; GEM, gemfibrozil. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Pharmacological TFEB inducers restore CSE-mediated autophagy-impairment and inhibit aggresome-formation
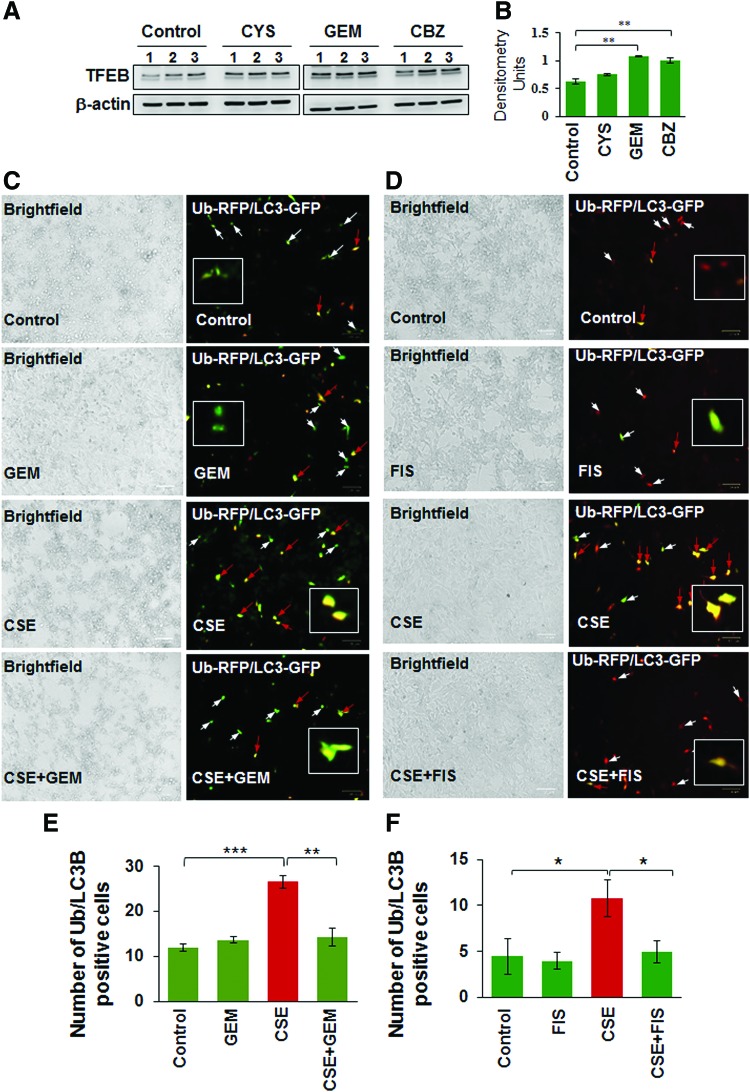
Several studies have identified the therapeutic benefit of TFEB induction in age-related pathological conditions that involve aggregation of damaged/misfolded proteins (11, 14, 15). We have earlier shown that CS and aging also induce aggregation of misfolded proteins (as aggresome-bodies) due to proteostasis/autophagy-impairment in murine/human lung (58), thus we evaluated here the pharmacological potential of TFEB induction in rescuing CS-induced aggresome-formation and resulting emphysema. First, we compared GEM, a known TFEB inducer (18), with previously tested autophagy inducer drugs (5, 51, 55, 58) and found that GEM treatment significantly (p < 0.01) elevates TFEB protein expression levels in Beas2b cells, compared with cysteamine (CYS) and carbamazepine (CBZ) (Fig. 3A, B, and Supplementary Fig. S3). Next, we found that GEM/FIS-mediated TFEB induction ameliorates CSE-induced autophagy-impairment (Ub-RFP and LC3B-GFP colocalization; aggresome-formation) in human bronchial epithelial cells (Beas2b) (Fig. 3C–F, p < 0.05). Thus, GEM/FIS-mediated TFEB induction is capable of controlling CSE-induced autophagy-impairment and resulting aggresome-formation and/or emphysema progression.
FIG. 3.
TFEB-inducing drugs rescue CSE-induced autophagy-impairment. (A, B) Immunoblotting of total protein lysates isolated from Beas2b cells treated with different autophagy-inducing drugs (cysteamine, CYS, 250 μM; GEM, 10 μM; and carbamazepine, CBZ, 20 μM; 6 h) indicates that GEM and CBZ significantly induce TFEB expression in Beas2b cells (n = 3, **p < 0.01). (C, D) The Beas2b cells were cotransfected with RFP-(Ub) Ubiquitin and GFP-(LC3B), the autophagy protein light chain-3, plasmids. After 24 h post-transfection, cells were treated with 5% CSE, GEM (10 μM, left panel), and/or FIS (25 μM, right panel) for 12 h, followed by fluorescence microscopy (Scale bars, 100 μm). The fluorescent images were used to count the cells positive for colocalization (yellow, red arrows,) of ubiquitinated (Ub) proteins (red) and LC3B-puncta bodies (green), where white arrows show single staining. Data represent mean ± SEM, n = 6, *p < 0.05, **p < 0.01, ***p < 0.001, and analysis is shown in (E) and (F). The data indicate that CSE treatment induces Ub-LC3B coexpression as yellow puncta bodies (aggresomes), which is alleviated by TFEB-autophagy inducers, GEM or FIS. Thus, GEM/FIS-mediated TFEB activation has the potential to rescue CSE-induced autophagy-impairment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
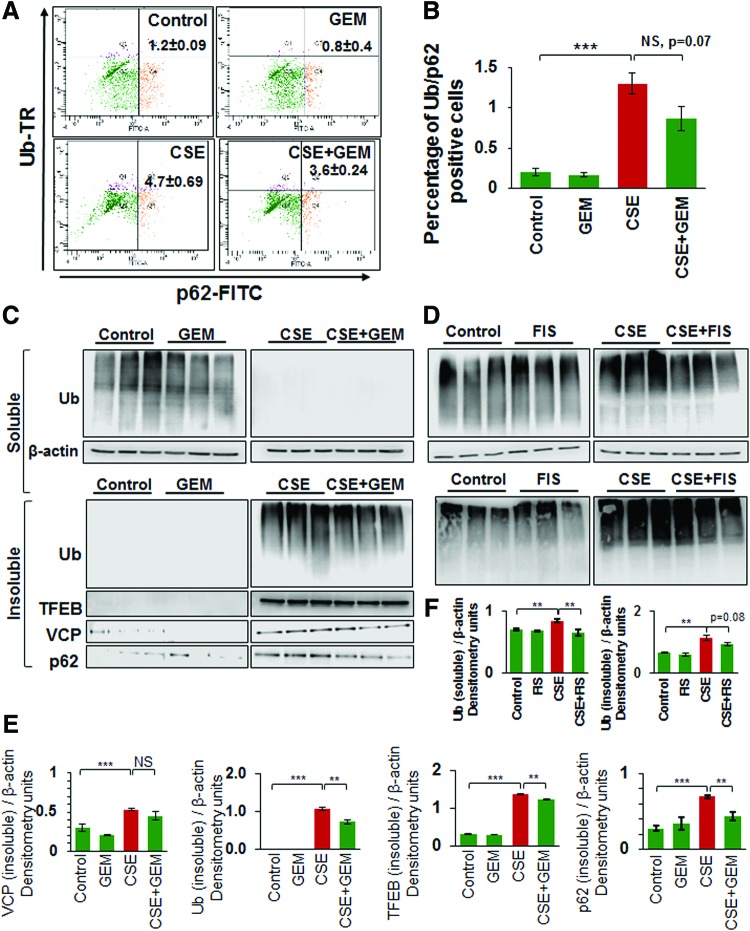
We demonstrate here that CSE-induced ubiquitin (Ub) and/or p62/sequestosome 1 accumulation in the insoluble protein fractions as aggresome-bodies can be controlled by using GEM-mediated TFEB induction (Fig. 4A, B). Furthermore, we demonstrate that the CSE-induced accumulation of ubiquitinated proteins in the insoluble protein fractions of Beas2b cells is significantly (GEM, p < 0.05) reduced by GEM/FIS treatment (Fig. 4C, D, and Supplementary Figs. S4, S5). Moreover, CSE treatment also induced the accumulation of valosin-containing protein (VCP), p62, and/or the master autophagy regulator, TFEB, in the insoluble protein fractions, which can be controlled by GEM/FIS-mediated autophagy induction (Fig. 4C, D, and Supplementary Figs. S4, S5). This suggests the critical role of TFEB in CSE-induced autophagy-impairment as GEM/FIS-mediated TFEB-autophagy induction can control CSE-induced aggresome-formation. The data also verify our findings in COPD subjects (Figs. 1 and 2) and suggest a novel mechanistic role of TFEB in CS-induced COPD-emphysema pathogenesis.
FIG. 4.
TFEB induction controls CSE-induced autophagy-impairment. (A, B) Flow cytometry analysis verifying that CSE-induced coexpression of Ub and p62 (aggresome-bodies) is controlled by treatment with GEM (mean ± SEM, n = 3, p = 0.07). (C, D) Western blot analysis showing accumulation of ubiquitinated proteins (Ub), VCP, and TFEB in the soluble and insoluble protein fractions of Beas2b cells treated with 10% CSE and/or GEM (10 μM) for 6 h. β-Actin was used as the loading control. The data represent three replicates and show that GEM treatment significantly inhibits CSE-induced ubiquitinated protein and TFEB accumulation in the insoluble protein fractions (aggresome-bodies). (D) Similarly, FIS (25 μM)-mediated TFEB/autophagy induction also showed clearance of CSE-induced ubiquitinated proteins from soluble and insoluble protein fractions. Densitometry analysis of Western blots is shown in (E and F). Data represent n = 3, mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. Data indicate that GEM or FIS-mediated TFEB induction controls CSE-induced autophagy-impairment. VCP, valosin-containing protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
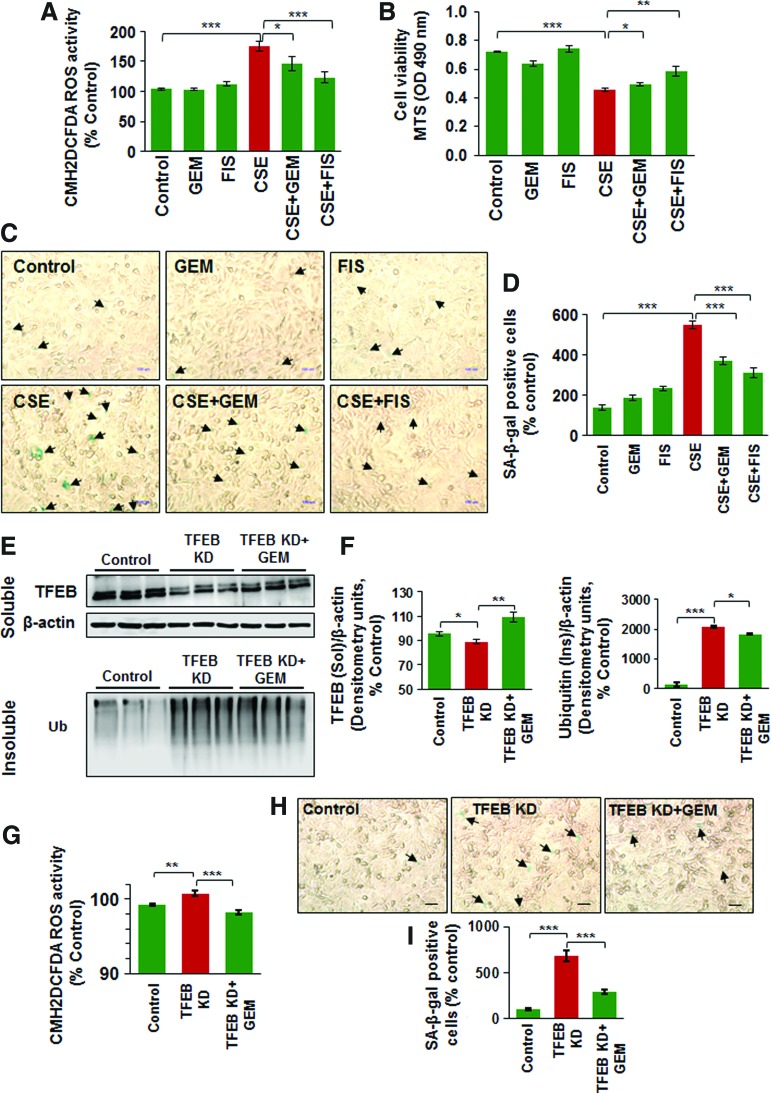
Master autophagy regulator, TFEB, controls CSE-induced oxidative stress, aggresome-formation, cellular viability, and senescence
To further verify the role of TFEB in CS-induced COPD-emphysema pathogenesis, we modulated TFEB expression in Beas2b cells, followed by functional assays. Our data shows that CSE-induced significant increase in ROS activity is controlled by TFEB induction, using GEM/FIS (Fig. 5A, p < 0.05). Moreover, TFEB induction by GEM/FIS also rescues the CSE-mediated decrease in cell viability, as shown by the MTS assay (Fig. 5B, p < 0.05). We and others have recently observed that tobacco smoke/e-cigarette vapor (eCV) exposure leads to cellular senescence (1, 51) via ROS activation that accelerates lung aging and COPD-emphysema pathogenesis (36). We demonstrate here that CSE-mediated increase in number of senescent cells is significantly diminished by treatment with TFEB-inducing drugs, GEM/FIS (Fig. 5C, D, p < 0.05). The present data confirm the importance of TFEB-mediated functional autophagy in regulating CS-induced cellular senescence.
FIG. 5.
TFEB controls CSE-induced oxidative stress, aggresome-formation, cellular viability, and senescence. (A) Beas2b cells were treated with CSE (5%), GEM (10 μM), and/or FIS (25 μM) for 12 h and cell proliferation was assessed by MTS assay. The data show that CSE inhibits Beas2b cell survival, while TFEB-inducing drugs (GEM/FIS) can partially rescue CSE-impaired cell proliferation. (B) CM-DCFDA-based ROS assay shows that CSE-induced ROS activity can be alleviated by treatment with TFEB-inducing antioxidants, GEM or FIS (mean ± SEM, *p < 0.05, ***p < 0.001). (C, D) Beas2b cells were treated with CSE (5%), GEM (10 μM), and/or FIS (25 μM) for 12 h and the number of senescent cells were counted (blue, black arrows). The data summarized for n = 6 replicates (mean ± SEM, ***p < 0.001) indicate that TFEB-inducing drugs (GEM/FIS) can control CSE-induced senescence. (E) Beas2b cells were transfected with TFEB-shRNA for 24 h and/or treated with GEM (10 μM) for the final 12 h of transfection. Western blots show that TFEB-shRNA significantly inhibits the protein expression of TFEB that was restored by GEM treatment (TFEB/autophagy inducer, upper panel, soluble fraction). Knockdown of TFEB also shows significant accumulation of ubiquitinated proteins in the insoluble protein fraction as anticipated (similar to CSE exposure), which could be rescued by GEM treatment (lower panel). (F) β-Actin was used to normalize the expression of each protein and densitometry analysis of the blots is shown (n = 3, mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001). (G) TFEB inhibition by shRNA induces ROS activity, which could be inhibited by a parallel GEM treatment (n = 3, mean ± SEM, **p < 0.01, ***p < 0.001). (H) Beas2b cells were transfected with TFEB-shRNA for 24 h and/or treated with GEM (10 μM) for the final 12 h of transfection. The number of senescent cells (blue, black arrows) was quantified under the light microscope to quantify changes in senescence on TFEB inhibition and/or induction. (I) The analysis of the senescence data shows that TFEB knockdown increases the number of senescent cells and parallel treatment with TFEB/autophagy-inducing drug, GEM, controls this increase in senescence. The data confirm the functional role of TFEB-mediated autophagy in regulating CS-induced oxidative stress and resulting autophagy-impairment and cellular senescence. CM-DCFDA, chloromethyl derivative of 2′,7′-dichlorodihydrofluorescein diacetate; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Next, we utilized TFEB knockdown (using TFEB shRNA) in Beas2b cells and observed that even partial inhibition of TFEB protein expression induces significant accumulation of ubiquitinated proteins in the insoluble protein fraction (aggresomes, similar to CSE treatment) that could be restored by treatment with TFEB-inducing drug, GEM (Fig. 5E, F, and Supplementary Fig. S6, p < 0.05). We also observed that TFEB inhibition leads to increased ROS activity and senescence in Beas2b cells, which can be controlled by GEM-mediated TFEB induction (Fig. 5G–I). Thus, our data provide substantial proof-of-concept evidence supporting the crucial role of TFEB in CS-mediated oxidative stress, autophagy-impairment, and aggresome-formation involved in COPD-emphysema pathogenesis.
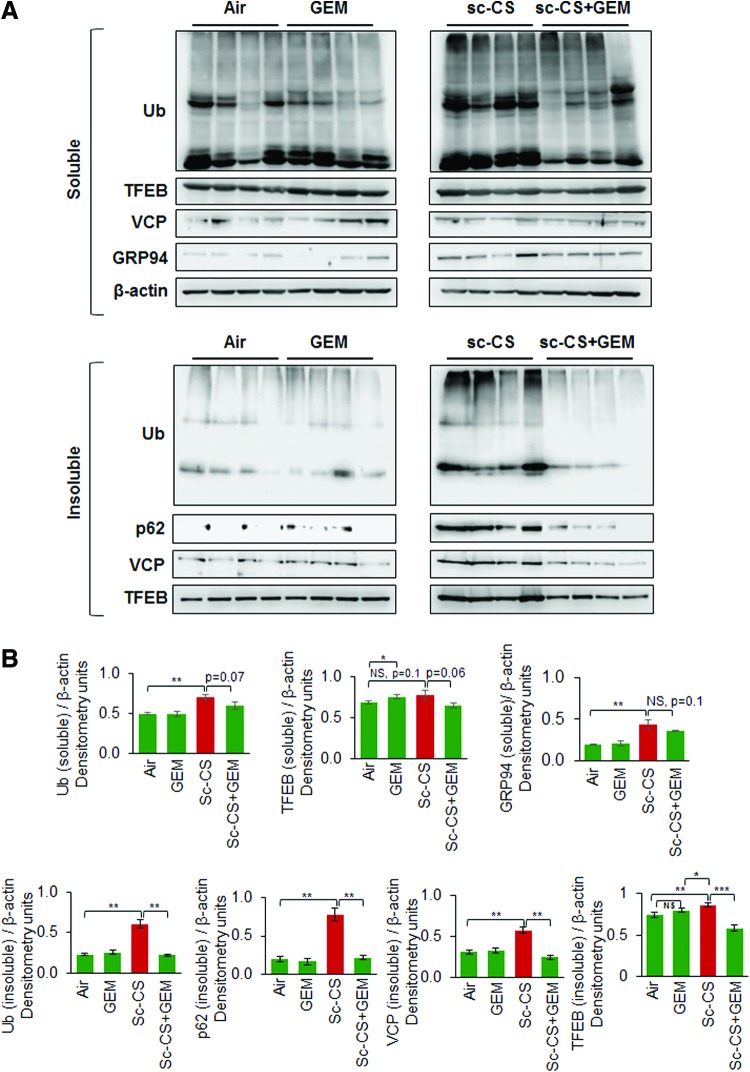
GEM alleviates CS-mediated autophagy-impairment in murine lungs by rescuing TFEB from aggresome-bodies
Recent studies from our group demonstrate that tobacco smoke/nicotine vapor exposure leads to autophagy-impairment and resulting aggresome-formation in the murine and/or human lungs (5, 55, 58), although the exact mechanism of autophagy-impairment is unknown. Hence, we utilized a similar in vivo approach as previously described (58) to evaluate the effect of GEM-mediated master autophagy regulator induction on CS-induced aggresome-formation. Our data shows that subchronic-CS (sc-CS)-induced accumulation of ubiquitinated proteins (Ub, aggresomes), p62 (impaired autophagy marker), and valosin-containing protein (VCP, a component of the retrograde translocation complex) in the insoluble protein fractions diminished by GEM treatment (Fig. 6A, B, and Supplementary Figs. S7, S8). Moreover, we report a novel observation that sc-CS exposure leads to increased accumulation of TFEB (master autophagy regulator) protein in the insoluble protein fractions as aggresome-bodies. This aggregation may plausibly render TFEB protein unavailable for performing its normal cellular functions (transcriptional regulation of crucial autophagy genes), thus leading to autophagy-impairment.
FIG. 6.
GEM rescues TFEB protein from aggresome-bodies as a mechanism to control sc-CS-induced autophagy-impairment. (A) Western blot analysis illustrating the levels of ubiquitinated proteins (Ub), p62 (impaired autophagy marker), TFEB (master autophagy regulator), and GRP94 (ER stress marker) in the soluble and insoluble protein fractions of murine lung protein lysates collected from C57BL/6 mice exposed to room air or subchronic cigarette smoke (sc-CS, 2 months, 5 h/day, 5 days/week) and/or treated with GEM [i.p., 40 mg/kg bw, one dose alternate days for 10 days]. Expression of each protein was normalized to β-actin loading control. (B) The data (mean ± SEM, n = 4, *p < 0.05, **p < 0.01, ***p < 0.001) confirm our findings that GEM treatment significantly diminishes CS-induced autophagy-impairment as evident by rescue of Ub, p62, VCP, and TFEB proteins from the insoluble protein fractions (aggresome-bodies). As anticipated, we only see a mild induction of ER stress marker, GRP94, levels (Fig. 4B, soluble fraction) compared with impaired-autophagy markers (Fig. 4B, Ub, p62, VCP, insoluble protein fractions), suggesting that misfolded proteins are accumulating mainly in aggresomes over ER. Our data suggest that sc-CS-induced autophagy-impairment is mediated by TFEB accumulation in aggresomes that can be rescued by GEM treatment. i.p., intraperitoneal; sc-CS, subchronic cigarette smoke. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Apart from the impairment of autophagy, CS-induced oxidative stress triggers the unfolded protein response (UPR), which regulates the transcriptional program toward either cell survival or apoptosis, depending on the strength and duration of the oxidative insult (23). Sustained accumulation of misfolded or damaged proteins in the lungs due to perturbed autophagic clearance mechanisms leads to unabated activation of UPR in COPD subjects (23), promoting COPD progression (23). GRP94 is an ER-resident molecular chaperone (ER stress protein) that plays a central role in induction of the UPR (10, 59). Upon sc-CS exposure, we observed a modest induction of GRP94 protein levels (Fig. 6A, soluble fraction) compared with aggresome/impaired autophagy markers (Fig. 6B, Ub, p62, VCP, insoluble protein fractions), suggesting that long-term CS exposure induces significant accumulation of proteins in aggresome-bodies instead of ER. Overall, our data identify CS-induced accumulation of TFEB in aggresome-bodies as a novel mechanism for autophagy-impairment in murine lungs that is rescued by GEM treatment.
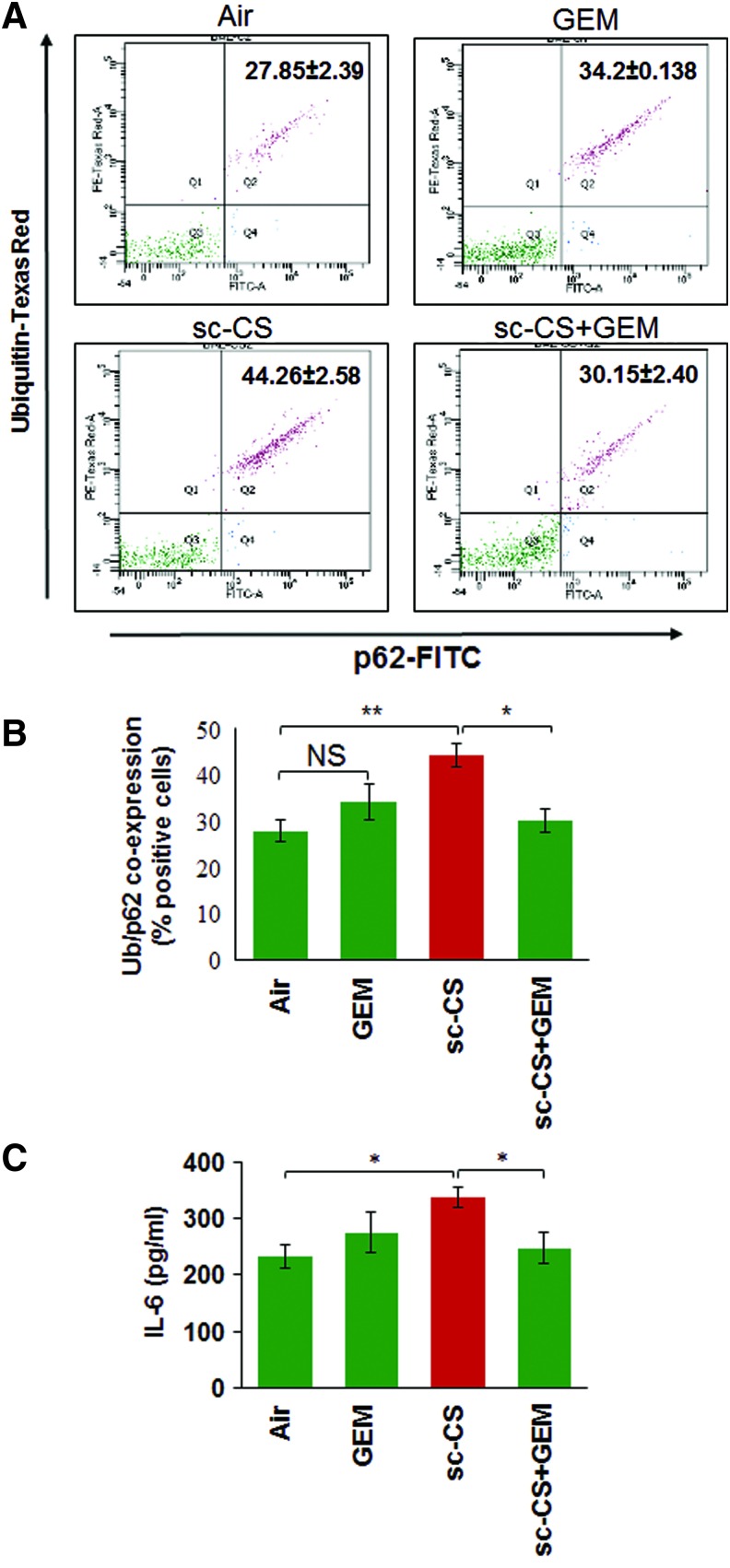
GEM-mediated TFEB activation rescues sc-CS-induced inflammation and autophagy-impairment
The coexpression/localization of ubiquitinated proteins (Ub) and p62 (impaired autophagy marker) is a marker of autophagy-impairment (5, 51, 58) that can be used to quantify changes in number of aggresome-bodies. Thus, to further ascertain the efficacy of GEM-mediated TFEB activation in controlling sc-CS-induced aggresome-formation, we performed flow cytometry analysis of bronchoalveolar lavage fluid (BALF) cells and show that GEM-mediated TFEB induction significantly (p < 0.05) rescues sc-CS-induced Ub and p62 (impaired autophagy marker) coexpression, indicating restoration of autophagy (Fig. 7A, B). TFEB induction by GEM also has the potential to alter CS-induced inflammatory cell phenotypes (Th1 to Th2 switching) by initiating anti-inflammatory mechanisms such as reduction of IL-6 and other inflammatory cytokines (46). Similarly, we demonstrate here that GEM-mediated TFEB induction significantly (p < 0.05) reduces sc-CS-induced IL-6 levels (Fig. 7C). Since elevated IL-6 levels are associated with CS-induced emphysema (4), reduction in sc-CS-induced IL-6 levels supports our notion that GEM-mediated TFEB induction is a potent therapeutic strategy for controlling COPD-emphysema pathogenesis.
FIG. 7.
GEM-mediated TFEB induction controls sc-CS-induced autophagy-impairment and inflammation. (A, B) Flow cytometry analysis of BALF cells isolated from C57BL/6 mice exposed to room air or subchronic cigarette smoke (sc-CS, 2 months, 5 h/day, 5 days/week) and/or treated with GEM [i.p., 40 mg/kg bw, one dose alternate days for 10 days]. Data shows that TFEB-inducing antioxidant, GEM, rescues sc-CS-induced Ub-p62 coexpression (impaired autophagy marker), suggesting restoration of autophagy. Data represent mean ± SEM, n = 3, *p < 0.05, **p < 0.01. (C) ELISA-based proinflammatory cytokine, IL-6, quantification in BALF samples isolated from each group (described for A) shows that GEM significantly inhibits sc-CS-induced IL-6 levels (mean ± SEM, n = 3, *p < 0.05). The data suggest that GEM-mediated TFEB induction rescues sc-CS-induced autophagy-impairment in the murine lungs. BALF, bronchoalveolar lavage fluid; IL-6, interleukin-6. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
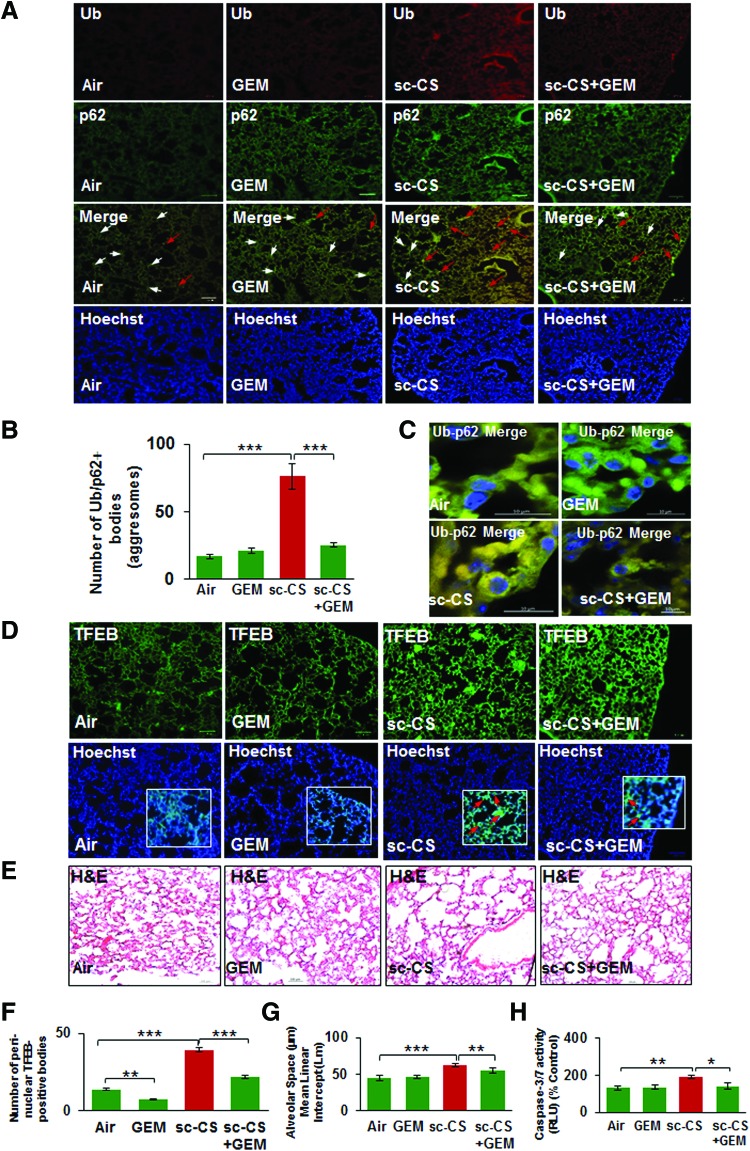
GEM-mediated TFEB/autophagy activation rescues sc-CS-induced alveolar apoptosis and emphysema
In this study, we verified that sc-CS-mediated colocalization of ubiquitinated proteins (Ub) and p62 (impaired autophagy marker) in the murine lung sections (Fig. 8A, merge panel, white arrows) is alleviated by GEM treatment (Fig. 8B). The high-resolution confocal images from these lung sections show a clear increase in perinuclear accumulation of Ub-p62 (yellow) in sc-CS-exposed mice that is reduced by GEM treatment (Fig. 8C). Moreover, we also validate our human lung tissue (COPD subjects) and cell line (Beas2b) data and report that CS exposure induces a significant increase in perinuclear localization of TFEB in aggresome-bodies (Fig. 8D, F, yellow arrows), which was rescued by treatment with its therapeutic inducer, GEM. Finally, GEM-mediated TFEB activation significantly decreases the CS-induced alveolar airspace enlargement (mean alveolar diameter, Lm, Fig. 8E, G) and caspase-3/7 activity (lung cell apoptosis; Fig. 8H), validating that our strategy of TFEB induction has a potential to therapeutically control the progression of COPD-emphysema. Overall, our data suggest that CS induced perinuclear accumulation of TFEB as a novel mechanism for CS-mediated autophagy-impairment and pharmacological induction of TFEB by GEM as a strategy to compensate for nonfunctional aggresome-trapped TFEB protein by inducing TFEB/autophagy for controlling inflammatory-oxidative stress, apoptosis, and resulting COPD-emphysema.
FIG. 8.
TFEB/autophagy induction by GEM decreases sc-CS-induced alveolar airspace enlargement and aggresome-formation in the murine lungs. Immunostaining of longitudinal lung sections from C57BL/6 mice exposed to room air or subchronic cigarette smoke (sc-CS, 2 months, 5 h/day, 5 days/week) and/or treated with GEM [i.p., 40 mg/kg bw, one dose alternate days for 10 days] shows that GEM treatment significantly decreases (A, B) the CS-induced increase in the number of Ub-p62-positive aggresome-bodies (yellow, red arrows). Nuclei were stained using the Hoechst (blue) dye. Scale bars, 100 μM, inset: 10 μM. The white arrows are used to show the single staining in cells where aggresome-bodies were absent. (C) High-resolution confocal images (A, third panel) verify that sc-CS-induced perinuclear accumulation of Ub-p62 (yellow, r = 0.58) is diminished by GEM treatment. (D) Immunostaining of lung sections from the same groups of mice as in (A) shows that sc-CS exposure induces localization of TFEB into perinuclear aggresome-bodies (red arrows, insets) that is significantly rescued by GEM treatment (F, r = −0.03), implying that induction of master autophagy regulator, TFEB, inhibits its perinuclear accumulation potentially via restoration of autophagy. (E) H&E staining of longitudinal lung sections (A, top panel) shows a significant increase in alveolar airspace enlargement in lung sections from sc-CS-exposed mice compared with room air-exposed controls. (G) The lung sections (in E) of mice treated with GEM show significant inhibition of sc-CS-induced alveolar airspace enlargement. Data represent mean ± SD of n = 4 replicates (**p < 0.01, ***p < 0.001). Scale bars, 100 μM, inset: 10 μM. (H) The changes in alveolar apoptosis in each experimental group were quantified using caspase-3/7 assay. The data show that sc-CS-induced apoptosis can be controlled by treatment with TFEB/autophagy-inducing drug, GEM (n = 4, *p < 0.05, **p < 0.01). H&E, hematoxylin and eosin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Discussion
Tobacco smoke/nicotine vapor exposure induces inflammatory-oxidative stress via autophagy-impairment-mediated aggresome-formation (5, 51, 58) that is central to controlling the initiation and progression of tobacco smoke-induced obstructive lung pathology (55). Although we and others have successfully tested the efficacy of pharmacological autophagy induction in both in vitro and in vivo models of smoke-induced COPD-emphysema (55, 58), there is a gap in the understanding of the precise mechanism of tobacco smoke-induced autophagy-impairment. The activation of ROS by free radicals and pro-oxidative components of tobacco is obviously a major factor that may hamper cellular homeostasis by causing autophagy/proteostasis impairment (31), although we found that both eCV and nicotine can also induce ROS-mediated autophagy-impairment (5, 51) similar to tobacco smoke. Studies from other groups initially suggested that CS induces autophagy in COPD-emphysema (8, 9, 25, 27), although it was impaired in other lung diseases, which is discussed in detail in the recent review articles (32, 38). Subsequent studies have clarified that although acute CS exposure activates autophagy, repeated chronic exposures lead to autophagy flux impairment. The previous studies used LC3B as a marker to report CS-related autophagy changes, but subsequent studies utilized autophagy flux markers (p62 and ubiquitin accumulation in autophagosomes) and reporters (Premo™ Autophagy Tandem Sensor RFP-GFP-LC3B) to demonstrate that CS-induced autophagy-impairment accelerates COPD-emphysema progression (58). Thus, in this study, we wanted to further investigate the mechanism of CS-ROS-mediated autophagy-impairment.
We first evaluated the effect of CS exposure on transcription factor EB (TFEB; a key master regulator, which controls lysosomal biogenesis and expression of autophagy genes) expression and localization (33, 49) based on our observation in human COPD subject lungs. We initially found that TFEB protein localizes in the perinuclear region as aggresome-bodies in severe COPD-emphysema subjects (Fig. 1A), suggesting that aggresome sequestration of TFEB protein could be a potential mechanism of tobacco smoke-induced autophagy-impairment in COPD. In support of this finding, we demonstrate that significant increase in TFEB-aggresome colocalization statistically correlates with the severity of emphysema and smoking history in COPD-emphysema subjects (Fig. 2A, B). Thus, we used GEM (18) or FIS (26)-mediated TFEB/autophagy induction as an experimental tool with our well-established models of CS-induced COPD-emphysema progression to evaluate its functional role. Using both our in vitro (Beas2b cells) and murine experimental models of COPD-emphysema (CS-exposed mice), we show that CS exposure leads to accumulation of TFEB protein in the perinuclear aggresome-bodies that can be rescued by treatment with GEM-mediated TFEB activation (Figs. 4, 6, and 8D). Moreover, we also demonstrate that TFEB induction by GEM/FIS treatment can control CS-induced inflammation (Fig. 7C), oxidative stress (ROS activation; Fig. 5B), autophagy-impairment, and resulting aggresome-bodies (Figs. 2–6), thus providing a proof-of-concept evidence that TFEB-mediated autophagy induction could rescue COPD-emphysema pathophysiology (Fig. 9).
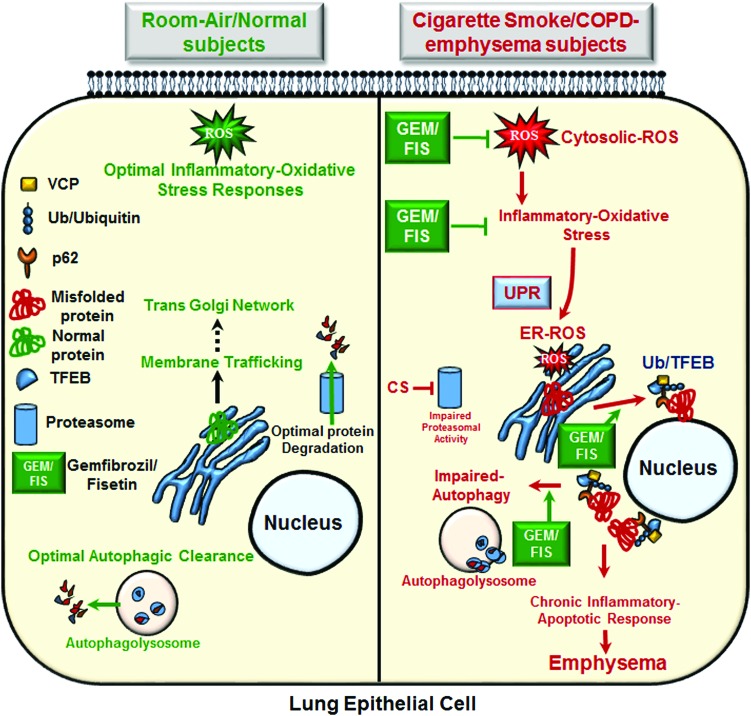
FIG. 9.
Schematic showing cigarette smoke mediated TFEB/autophagy-impairment in COPD-emphysema. Under normal physiological conditions, the optimal inflammatory oxidative signaling restricts extracellular insults via the robust proteostasis/autophagy responses. Cigarette smoke exposure mediates ROS activation in lung epithelial cells that can induce inflammatory-oxidative stress, resulting in autophagy-impairment and formation of perinuclear aggresome-bodies that mediate COPD-emphysema pathogenesis. Moreover, CS-induced perinuclear accumulation of TFEB, the master autophagy regulator, in aggresome-bodies hampers autophagy responses in COPD-emphysema. TFEB inhibition induces ROS activity, resulting in aggresome-formation and cellular senescence/apoptosis, while treatment with TFEB-inducing antioxidant drugs (GEM or FIS) controls CS-induced inflammatory-oxidative stress, aggresome pathology, and alveolar apoptosis. Overall, smoke exposure impairs TFEB/autophagy activity, resulting in downstream functional changes associated with progression of COPD-emphysema pathophysiology. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Previous studies highlight the critical role of TFEB in the regulation and expression of autophagy genes and its mechanisms are well described (33, 39). Briefly, in normal physiological conditions, TFEB is retained in the cytoplasm by mammalian target of rapamycin (mTORC1)- or mitogen-activated protein kinase 1 (ERK2)- mediated phosphorylation, while in nutrient-deprived or lysosomal stress conditions, TFEB is dephosphorylated by the phosphatase calcineurin, which then promotes nuclear translocation of TFEB, where it regulates the transcription of numerous lysosomal biogenesis and autophagy genes. TFEB directly binds to the coordinated lysosomal expression and regulation (CLEAR) motif (lysosomal biogenesis) and also the promoter regions of various autophagy genes to induce target gene expression (39). The aberrant expression/activity of TFEB is evident in several pathological conditions, especially in age-related degenerative or lysosomal storage disorders, where TFEB impairment leads to accumulation of misfolded or damaged proteins in aggresome-bodies (34, 47). A recent study shows that genetic and chemical activation of TFEB clears the α-synuclein aggregates, which are associated with neurodegenerative diseases such as Parkinson's (24). These α-synuclein aggregates are formed by accumulation of misfolded α-synuclein due to impairment of protein degradation machinery that includes the ubiquitin–proteasome system and autophagy (24). Thus, TFEB-mediated autophagy induction is proposed as a strategy to clear α-synuclein protein aggregation (15, 24). Similarly, in AD, the neuronal accumulation of phosphorylated tau plays an important pathological role (26), and strategies to induce clearance of tau in neurons are developed as a therapeutic strategy to circumvent AD pathology (44, 52). Briefly, a recent report shows the potential of FIS, an organic flavonoid compound, in enhancing clearance of aggregated tau protein via TFEB-mediated autophagy induction (26). In the context of lung diseases, pharmacological or genetic activation of TFEB, the master transcriptional activator of the autophagolysosomal system, is shown to be beneficial in rescuing the spontaneous pulmonary fibrosis developed in the PiZ murine model, wherein accumulation of misfolded α1-antitrypsin Z (ATZ) leads to lung proteinopathy (21). These promising findings led us to test whether pharmacological TFEB induction could rescue CS-induced autophagy-impairment and the ensuing aggresome pathologies initiating COPD-emphysema.
We first chose to use GEM as a pharmacological TFEB inducer based on previous reports (18) and its known antioxidant and anti-inflammatory properties, which may provide additional therapeutic advantage, as we have recently observed with autophagy-inducing antioxidant drug, CYS (5, 51, 58). Additionally, GEM is an FDA-approved lipid-lowering drug and is shown to be clinically beneficial in coronary heart disease and metabolic syndrome (46), two prominent comorbidities of COPD (7), thus warranting its evaluation in CS-induced chronic COPD-emphysema. Our present data demonstrate that GEM-mediated TFEB induction can alleviate CS-induced inflammatory-oxidative stress (IL-6 levels, ROS activation), autophagy-impairment, aggresome-formation (Ub-p62 coexpression/localization), apoptosis (caspase-3/7 activity), and resulting alveolar airspace enlargement (Lm, H&E staining). Mechanistically, our novel data shows that CS triggers accumulation of TFEB in perinuclear aggresome-bodies, thus rendering it unavailable to perform its normal function as a master autophagy activator. It is obvious that similar to other misfolded (ubiquitinated) proteins, CS modulates TFEB protein folding that promotes its accumulation into aggresome-bodies. Thus, we used GEM treatment to induce both the expression of TFEB protein and autophagy that will also allow rescue of additional TFEB protein from aggresome-bodies.
As discussed above, TFEB-inducing strategies have been successfully used to rescue misfolded α-synuclein and tau proteins in neurological diseases (24, 26). In addition, GEM was recently shown to upregulate the expression of tripeptidyl peptidase (TPP1) in brain cells via the PPARα/RXRα pathway in late infantile neuronal lipofuscinosis, a disease caused by accumulation of mutant TPP1 protein (17). Thus, we concluded that GEM treatment similarly rescues misfolded TFEB (and other ubiquitinated proteins) from aggresomes by similar mechanisms, thereby restoring TFEB expression and transcriptional activity for normal functional autophagy response. To further validate the role of TFEB in COPD-emphysema pathogenesis, we utilized an alternative TFEB/autophagy inducer, FIS. In addition to TFEB-mediated autophagy induction (26), FIS is a potent antioxidant activator of the Nrf2 pathway (26, 29) that can alleviate CS-ROS-mediated protein misfolding and aggregation as aggresome-bodies. As a proof of concept, fisetin-mediated TFEB/Nrf2 activation shows more significant inhibition of CSE-induced ROS activity and cellular senescence (Fig. 5B–D) compared with GEM treatment. Thus, FIS is a novel therapeutic agent to potentially rescue CS-mediated oxidative stress and protein aggregation by activating antioxidant response (via Nrf2) and autophagy (via TFEB). We also verified the role of TFEB in regulating CS-induced mechanisms involved in COPD-emphysema by using TFEB shRNA. Our data shows that TFEB inhibition induces ROS activity, ubiquitinated protein accumulation (autophagy-impairment), and cellular senescence (Fig. 5E–H) similar to CS/CSE exposure (Figs. 4C, 5B–D, and 6A). Moreover, these functional effects of TFEB knockdown were rescued by GEM-mediated TFEB induction.
In conclusion, we identify CS-induced accumulation of TFEB in aggresome-bodies as a specific novel mechanism for CS-mediated autophagy-impairment and resulting aggresome-formation and emphysema progression. Moreover, we propose that TFEB-positive aggresome-bodies can serve as prognostic biomarker for tobacco smoke-induced COPD-emphysema that can allow early detection and treatment of chronic obstructive lung diseases with proposed prognosis-based intervention strategy employing TFEB/autophagy-inducing drugs.
Materials and Methods
Human subject samples
Our study protocol was approved by the Institutional Review Board (IRB), Central Michigan University, and Johns Hopkins University as not a human subject research, under exemption no. 4, as subject's lung function data and clinical parameters were obtained from NHLBI Lung Tissue Research Consortium (LTRC, NIH) (4, 58) without disclosing any of the subject's identifiers or demographic information. The clinical severity, sample size, and classification of COPD subjects utilized GOLD stage classification as defined by Global Initiative for Chronic Obstructive Lung Diseases (GOLD). The sample size included lung samples from GOLD 0, n = 15 (smokers: 5 and nonsmokers: 10); GOLD I, n = 12 (smokers: 10, nonsmokers: 2); GOLD II, n = 11 (smokers: 10, nonsmokers: 1); GOLD III, n = 10 (smokers: 10, nonsmokers: 0), and GOLD IV, n = 12 (smokers: 10, nonsmokers: 2) COPD-emphysema subjects. The smokers, who were mostly ex-smokers (as expected for COPD subjects), also included few current smokers; GOLD 0—0, GOLD I—1, GOLD II—2, GOLD III—1, and GOLD IV—0. We have previously reported the demographic information of the non-emphysema and COPD-emphysema subjects used in this study (4). Briefly, non-emphysema or COPD subjects had no other underlying condition (or α1-antitrypsin deficiency), other than emphysema for COPD subjects, although we had one patient in each COPD group (GOLD I–IV) who had his/her first-degree blood relative suffering from chronic bronchitis.
Murine experiments
All animal experiments were performed as per the guidelines of CMU Institutional Animal Care and Use Committee (IACUC). C57BL/6 mice (8 weeks, n = 4) were separated into four experimental groups: (1) room air, (2) GEM, (3) sc-CS, and (4) sc-CS+GEM. The room air or side-stream CS exposures were performed for 2 months, as per our previously described protocol (58). CS was generated by burning 3R4F (0.73 mg nicotine per cigarette) research-grade cigarettes (Tobacco Research Institute, University of Kentucky, Lexington, KY) and mice were exposed to direct whole-body smoke exposure (3 h/day, followed by another 2 h in the same chamber without burning more cigarettes) using TE2 smoking machine (Teague Enterprises). This methodology resulted in an average total particulate matter of 150 mg/m3. The mice were either treated with GEM (40 mg/kg body weight; Sigma) or equal volume PEG vehicle control by intraperitoneal (i.p.) injection on alternate days for 10 days (5 doses) before the termination of experiment. Mice were sacrificed in accordance with our IACUC-approved protocols, and BALF and lung tissues were collected for further analysis by flow cytometry, immunoblotting, and immunostaining.
Cell culture, autophagy flux/reporter, and ROS assays
The human bronchial epithelial cell line, Beas2b, was used in the study as an in vitro model for cigarette smoke extract (CSE) exposure. Standard cell culture conditions were utilized as recently described (5). Briefly, cells were maintained at 37°C, 5% CO2 atmosphere in DMEM/F12 media with 10% fetal growth serum (RMBIO) and 1% PSA (penicillin, streptomycin, and amphotericin; Invitrogen). The CSE was prepared by burning two to three 3R4F (0.73 mg nicotine per cigarette) research-grade cigarettes (Tobacco Research Institute, University of Kentucky, Lexington, KY) and bubbling the smoke directly into the serum-free DMEM/F12 media (20 ml). An OD (320 nm) of 0.74 was considered as 100% CSE and CSE final concentration was adjusted by using the cell culture media. For autophagy reporter assay, cells were transiently cotransfected with ubiquitin-RFP and LC3B-GFP plasmids using Lipofectamine® 2000 (24 h; Invitrogen) and treated with vehicle control (PEG), CSE (5%), and/or GEM (10 μM) for 12 h. The effect of CSE and the TFEB-inducing drugs on functional autophagy was quantified using the Premo Autophagy Tandem Sensor RFP-GFP-LC3B assay kit (Molecular Probes), as recently described (51). Briefly, Beas2b cells were incubated with BacMam reagent for 16 h, followed by treatment with CSE (5%), GEM (10 μM), and/or fisetin (FIS, 25 μM; Indofine Chemical Company) for 12 h. Images for both experiments were captured using the ZOE ™ Fluorescent Cell Imager (Bio-Rad) as we recently described (5, 58). The changes in ROS levels were quantified using the CM-DCFDA ROS indicator dye (Invitrogen) as per the protocol reported recently (5, 51). Briefly, cells were treated as above with CSE (5%), FIS (25 μM), and/or GEM (10 μM) for 12 h and dye was added for 30 min, followed by fluorescence measurement as described before (5). In parallel set of experiments, the effect of TFEB knockdown (using human TFEB-Mission™ shRNA; Sigma and Lipofectamine 2000 transfection reagent; Invitrogen) and/or GEM treatment on ROS activation was quantified using the chloromethyl derivative of 2′,7′-dichlorodihydrofluorescein diacetate (CM-DCFDA) ROS indicator dye.
Immunoblotting, flow cytometry, and ELISA
We used our previously described (5, 58) immunoblotting method to quantify changes in ubiquitinated proteins (Ub), p62 (aggresome marker), VCP (UPR/autophagy mediator), TFEB (master autophagy regulator), GRP94 (ER stress marker), and β-actin in soluble and/or insoluble protein fractions of murine lung or Beas2b cell protein lysates. All antibodies were procured from Santa Cruz Biotechnology (scbt), except β-actin, which was from Sigma. To verify immunoblotting data, flow cytometry analysis was used to quantify the percentage of Ub-p62+ cells (either in human Beas2b or murine BALF cells), using BD FACS Aria and FACS Diva software as we described recently (5, 58). Finally, downstream effects were quantified by measuring changes in levels of IL-6 cytokine in BALF samples using a mouse-IL-6 ELISA kit (eBiosciences), following the manufacturer's instructions as previously described (51, 58).
Immunofluorescence microscopy and lung morphometry
Paraffin-embedded longitudinal lung tissue sections (5 μM) were prepared from all four experimental murine groups (Control vehicle PEG, GEM, sc-CS, and sc-CS+GEM). The lung sections were deparaffinized and immunostained using our previously described protocol (58). Briefly, primary antibodies (1 μg/ml) against ubiquitin (mouse monoclonal, scbt), p62 (rabbit polyclonal, scbt), or TFEB (rabbit polyclonal, scbt), followed by secondary antibodies (1 μg/ml) anti-rabbit CFL488 and/or anti-mouse-Texas Red (Santa Cruz), were used to localize changes in protein expression and/or co/localization. The Hoechst dye was used to stain the nuclei for identifying nuclear/perinuclear localization and expression. Images were captured by either the ZOE Florescent Cell Imager (Bio-Rad) or the Nikon Eclipse TI confocal inverted laser scanning microscope and NIS Elements software using a 60 × /1.42 numerical aperture (NA) oil immersion objective. The sections were also stained with H&E to assess the inflammatory state and morphometric changes. Briefly, changes in mean alveolar diameter, Lm, were quantified by bright-field microscopy as we recently described in detail (58).
We also used paraffin-embedded longitudinal lung sections (5 μM) from normal/non-emphysema and COPD-emphysema subjects for quantifying changes in TFEB expression and localization. Briefly, lung sections from normal control subjects (GOLD 0) and each COPD-emphysema GOLD stage (GOLD I–IV) were immunostained, using a TFEB (1 μg/ml) primary antibody and anti-rabbit CFL-488 (1 μg/ml) secondary antibody. Nuclei were stained using the Hoechst dye, followed by microscopy analysis under ZOE Fluorescent Cell Imager and confocal microscopy, as described above. The PROTEOSTAT Aggresome Detection kit (Enzo Life Sciences) was used to perform the co-immunostaining with TFEB.
Cell proliferation, senescence, and caspase-3/7 assays
Beas2b cells were exposed to CSE (5%) and/or GEM/FIS at the indicated doses for 12 h, and cell proliferation was quantified using the standard MTT/MTS assay kit (Promega) following the manufacturer's protocol, as we recently described (5). The Senescence Cells Histochemical Staining Kit (Sigma) was used to quantify changes in number of senescent cells in the different treatment groups as previously described (5, 51). The caspase-3/7 activity was also quantified in murine lung tissue lysates using the Caspase Glo™ assay kit (Promega) following the manufacturer's protocol.
Statistical analysis
Data are shown as mean ± SEM (or SD, as indicated) of each experimental group. A two-tailed unpaired Student's t-test was performed to determine the significance between the data sets and a p-value of less than 0.05 was considered a significant change. In addition, Pearson's correlation analysis was used to determine the correlation between the two data sets and the correlation coefficient is shown as “r” value. For immunoblotting data, densitometry was used to quantify changes using the ImageJ software (NIH).
Supplementary Material
Abbreviations Used
- BALF
bronchoalveolar lavage fluid
- CBZ
carbamazepine
- CFTR
cystic fibrosis transmembrane conductance regulator protein
- CM-DCFDA
chloromethyl derivative of 2′,7′-dichlorodihydrofluorescein diacetate
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CYS
cysteamine
- eCV
e-cigarette vapor
- ER
endoplasmic reticulum
- FIS
fisetin
- GEM
gemfibrozil
- GFP
green fluorescent protein
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- p62/SQSTM1
sequestosome 1
- RFP
red fluorescent protein
- ROS
reactive oxygen species
- TFEB
transcription factor EB
- Ub
ubiquitin
- VCP
valosin-containing protein
Acknowledgments
The authors would like to thank the NHLBI Lung Tissue Research Consortium (LTRC, NIH) for providing human lung tissue sections and Philip Oshel, Director of the Microscopy Core Facility, Central Michigan University, for help with the confocal microscopy experiments. The authors also thank April Ilacqua, FACS student technician, for assistance during the flow cytometry experiments.
Authors' Contributions
Conception and design: N.V.; analysis and interpretation: M.B. and N.V.; experimental contributions: M.B., N.V., N.P., D.S., and K.W.; and drafting of manuscript and editing: M.B. and N.V.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, and Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J 29: 2912–2929, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomfield Rubins H, Davenport J, Babikian V, Brass LM, Collins D, Wexler L, Wagner S, Papademetriou V, Rutan G, Robins SJ; VA-HIT Study Group. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT). Circulation 103: 2828–2833, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bodas M, Min T, and Vij N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. Am J Physiol Lung Cell Mol Physiol 300: L811–L820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodas M, Min T, and Vij N. Lactosylceramide-accumulation in lipid-rafts mediate aberrant-autophagy, inflammation and apoptosis in cigarette smoke induced emphysema. Apoptosis 20: 725–739, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Bodas M. van Westphal C, Thompson-Carpenter R, Mohanty D. and Vij N. Nicotine exposure induces bronchial epithelial cell apoptosis and senescence via ROS mediated autophagy-impairment. Free Radic Biol Med 97: 441–453, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Calkin AC, Cooper ME, Jandeleit-Dahm KA, and Allen TJ. Gemfibrozil decreases atherosclerosis in experimental diabetes in association with a reduction in oxidative stress and inflammation. Diabetologia 49: 766–774, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Cavailles A, Brinchault-Rabin G, Dixmier A, Goupil F, Gut-Gobert C, Marchand-Adam S, Meurice JC, Morel H, Person-Tacnet C, Leroyer C, and Diot P. Comorbidities of COPD. Eur Respir Rev 22: 454–475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, Nakahira K, Pilewski JM, Lee JS, Zhang Y, Ryter SW, and Choi AM. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One 3: e3316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, and Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A 107: 18880–18885, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chhabra S, Jain S, Wallace C, Hong F, and Liu B. High expression of endoplasmic reticulum chaperone grp94 is a novel molecular hallmark of malignant plasma cells in multiple myeloma. J Hematol Oncol 8: 77, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crippa V, D'Agostino VG, Cristofani R, Rusmini P, Cicardi ME, Messi E, Loffredo R, Pancher M, Piccolella M, Galbiati M, Meroni M, Cereda C, Carra S, Provenzani A, and Poletti A. Transcriptional induction of the heat shock protein B8 mediates the clearance of misfolded proteins responsible for motor neuron diseases. Sci Rep 6: 22827, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunard R. The potential use of PPARalpha agonists as immunosuppressive agents. Curr Opin Investig Drugs 6: 467–472, 2005 [PubMed] [Google Scholar]
- 13.Dasgupta S, Roy A, Jana M, Hartley DM, and Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol 72: 934–946, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Decressac M. and Bjorklund A. TFEB: pathogenic role and therapeutic target in Parkinson disease. Autophagy 9: 1244–1246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, and Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A 110: E1817–E1826, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domej W, Oettl K, and Renner W. Oxidative stress and free radicals in COPD—implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis 9: 1207–1224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh A, Corbett GT, Gonzalez FJ, and Pahan K. Gemfibrozil and fenofibrate, Food and Drug Administration-approved lipid-lowering drugs, up-regulate tripeptidyl-peptidase 1 in brain cells via peroxisome proliferator-activated receptor alpha: implications for late infantile Batten disease therapy. J Biol Chem 287: 38922–38935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A, Jana M, Modi K, Gonzalez FJ, Sims KB, Berry-Kravis E, and Pahan K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: implications for lysosomal storage disorders. J Biol Chem 290: 10309–10324, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, and Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 3: 78ra32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassett DJ, Borchers MT, and Panos RJ. Chronic obstructive pulmonary disease (COPD): evaluation from clinical, immunological and bacterial pathogenesis perspectives. J Microbiol 52: 211–226, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Hidvegi T, Stolz DB, Alcorn JF, Yousem SA, Wang J, Leme AS, Houghton AM, Hale P, Ewing M, Cai H, Garchar EA, Pastore N, Annunziata P, Kaminski N, Pilewski J, Shapiro SD, Pak SC, Silverman GA, Brunetti-Pierri N, and Perlmutter DH. Enhancing autophagy with drugs or lung-directed gene therapy reverses the pathological effects of respiratory epithelial cell proteinopathy. J Biol Chem 290: 29742–29757, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodges RM. Gemfibrozil—a new lipid lowering agent. Proc R Soc Med 69 Suppl 2: 1–2, 1976 [PMC free article] [PubMed] [Google Scholar]
- 23.Kelsen SG. The unfolded protein response in chronic obstructive pulmonary disease. Ann Am Thorac Soc 13 Suppl 2: S138–S145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpatrick K, Zeng Y, Hancock T, and Segatori L. Genetic and chemical activation of TFEB mediates clearance of aggregated alpha-synuclein. PLoS One 10: e0120819, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, Ryter SW, and Choi AM. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy 4: 887–895, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Choi KJ, Cho SJ, Yun SM, Jeon JP, Koh YH, Song J, Johnson GV, and Jo C. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci Rep 6: 24933, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, An CH, Shan B, Franks JM, Haley KJ, Owen CA, Tesfaigzi Y, Washko GR, Quackenbush J, Silverman EK, Rahman I, Kim HP, Mahmood A, Biswal SS, Ryter SW, and Choi AM. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest 123: 5212–5230, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laplante M. and Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126: 1713–1719, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SE, Jeong SI, Yang H, Park CS, Jin YH, and Park YS. Fisetin induces Nrf2-mediated HO-1 expression through PKC-delta and p38 in human umbilical vein endothelial cells. J Cell Biochem 112: 2352–2360, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Campos JL, Tan W, and Soriano JB. Global burden of COPD. Respirology 21: 14–23, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, and Maiuri L. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol 12: 863–875, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Margaritopoulos GA, Tsitoura E, Tzanakis N, Spandidos DA, Siafakas NM, Sourvinos G, and Antoniou KM. Self-eating: friend or foe? The emerging role of autophagy in idiopathic pulmonary fibrosis. Biomed Res Int 2013: 420497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martina JA, Chen Y, Gucek M, and Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8: 903–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martini-Stoica H, Xu Y, Ballabio A, and Zheng H. The autophagy-lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci 39: 221–234, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, and Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17: 288–299, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercado N, Ito K, and Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax 70: 482–489, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Min T, Bodas M, Mazur S, and Vij N. Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J Mol Med (Berl) 89: 577–593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizumura K, Cloonan S, Choi ME, Hashimoto S, Nakahira K, Ryter SW, and Choi AM. Autophagy: friend or foe in lung disease? Ann Am Thorac Soc 13 Suppl 1: S40–S47, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Napolitano G. and Ballabio A. TFEB at a glance. J Cell Sci 129: 2475–2481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neofytou E, Tzortzaki EG, Chatziantoniou A, and Siafakas NM. DNA damage due to oxidative stress in Chronic Obstructive Pulmonary Disease (COPD). Int J Mol Sci 13: 16853–16864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni I, Ji C, and Vij N. Second-hand cigarette smoke impairs bacterial phagocytosis in macrophages by modulating CFTR dependent lipid-rafts. PLoS One 10: e0121200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni Y, Shi G, Yu Y, Hao J, Chen T, and Song H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 10: 1465–1475, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pastore N, Blomenkamp K, Annunziata F, Piccolo P, Mithbaokar P, Maria Sepe R, Vetrini F, Palmer D, Ng P, Polishchuk E, Iacobacci S, Polishchuk R, Teckman J, Ballabio A, and Brunetti-Pierri N. Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol Med 5: 397–412, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polito VA, Li H, Martini-Stoica H, Wang B, Yang L, Xu Y, Swartzlander DB, Palmieri M, di Ronza A, Lee VM, Sardiello M, Ballabio A, and Zheng H. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med 6: 1142–1160, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, and Ferguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal 5: ra42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy A. and Pahan K. Gemfibrozil, stretching arms beyond lipid lowering. Immunopharmacol Immunotoxicol 31: 339–351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sardiello M. Transcription factor EB: from master coordinator of lysosomal pathways to candidate therapeutic target in degenerative storage diseases. Ann N Y Acad Sci 1371: 3–14, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Settembre C. and Ballabio A. TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy 7: 1379–1381, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, and Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429–1433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, and Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31: 1095–1108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shivalingappa PC, Hole R, Westphal CV, and Vij N. Airway exposure to E-cigarette vapors impairs autophagy and induces aggresome formation. Antioxid Redox Signal 24: 186–204, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simic G, Babic Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milosevic N, Bazadona D, Buee L, de Silva R, Di Giovanni G, Wischik C, and Hof PR. Tau protein hyperphosphorylation and aggregation in Alzheimer's disease and other tauopathies, and possible neuroprotective strategies. Biomolecules 6: 6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song W, Wang F, Savini M, Ake A, di Ronza A, Sardiello M, and Segatori L. TFEB regulates lysosomal proteostasis. Hum Mol Genet 22: 1994–2009, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Tenkanen L, Manttari M, Kovanen PT, Virkkunen H, and Manninen V. Gemfibrozil in the treatment of dyslipidemia: an 18-year mortality follow-up of the Helsinki Heart Study. Arch Intern Med 166: 743–748, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Tran I, Ji C, Ni I, Min T, Tang D, and Vij N. Role of cigarette smoke-induced aggresome formation in chronic obstructive pulmonary disease-emphysema pathogenesis. Am J Respir Cell Mol Biol 53: 159–173, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RA, Lazarowski ER, Damian VA, Masliah E, and La Spada AR. PGC-1alpha rescues Huntington's disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med 4: 142ra97, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuder RM, Kern JA, and Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc 9: 62–63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vij N, Chandramani P, Westphal C, Hole R, and Bodas M. Cigarette smoke induced autophagy-impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Respir Crit Care Med 193: A2334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yokouchi M, Hiramatsu N, Hayakawa K, Kasai A, Takano Y, Yao J, and Kitamura M. Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell Death Differ 14: 1467–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Zhang YD. and Zhao JJ. TFEB participates in the Abeta-induced pathogenesis of Alzheimer's disease by regulating the autophagy-lysosome pathway. DNA Cell Biol 34: 661–668, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.