Abstract
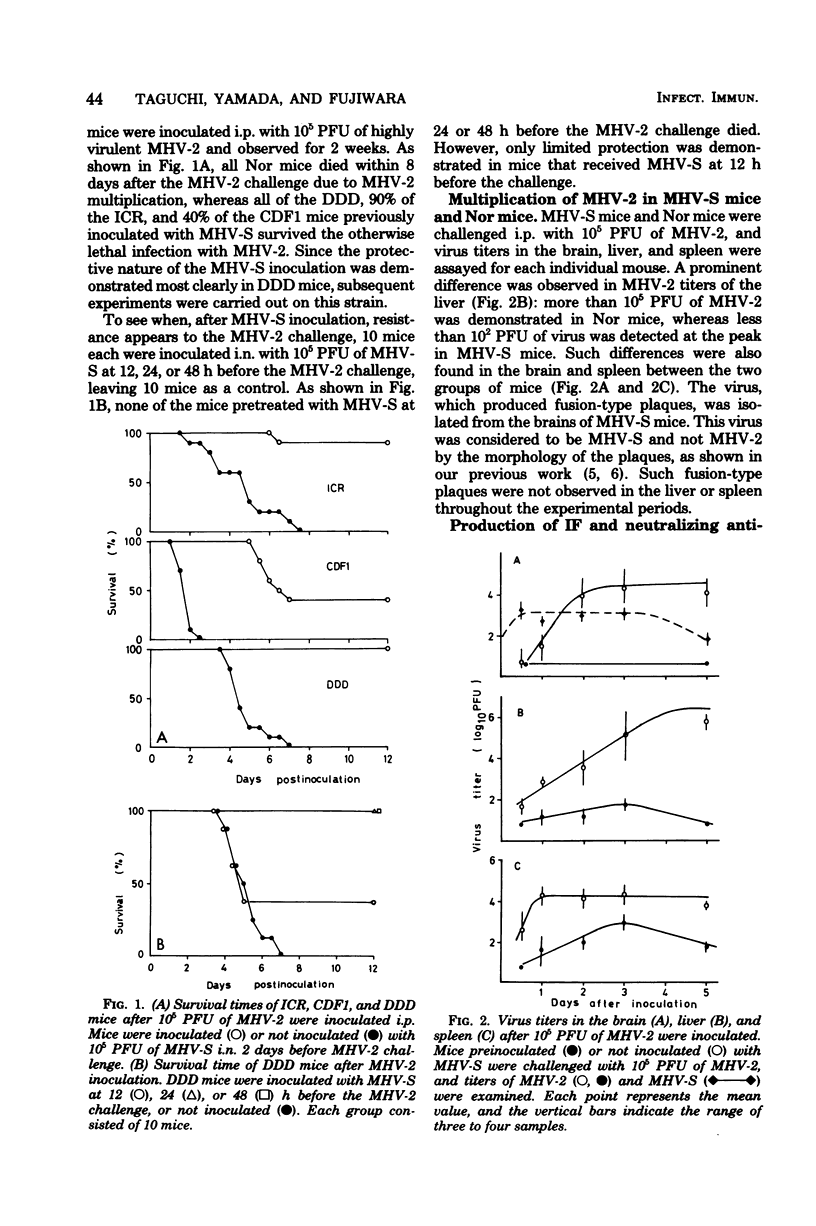
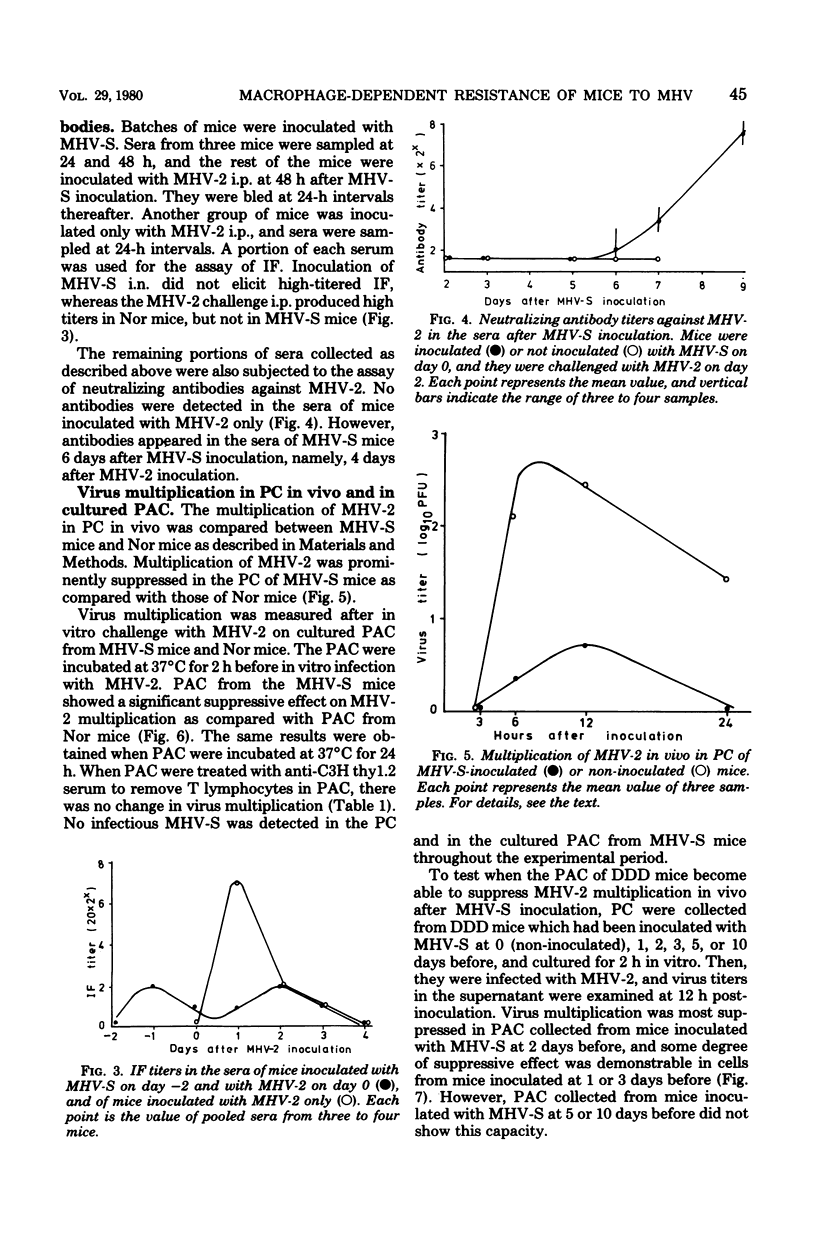
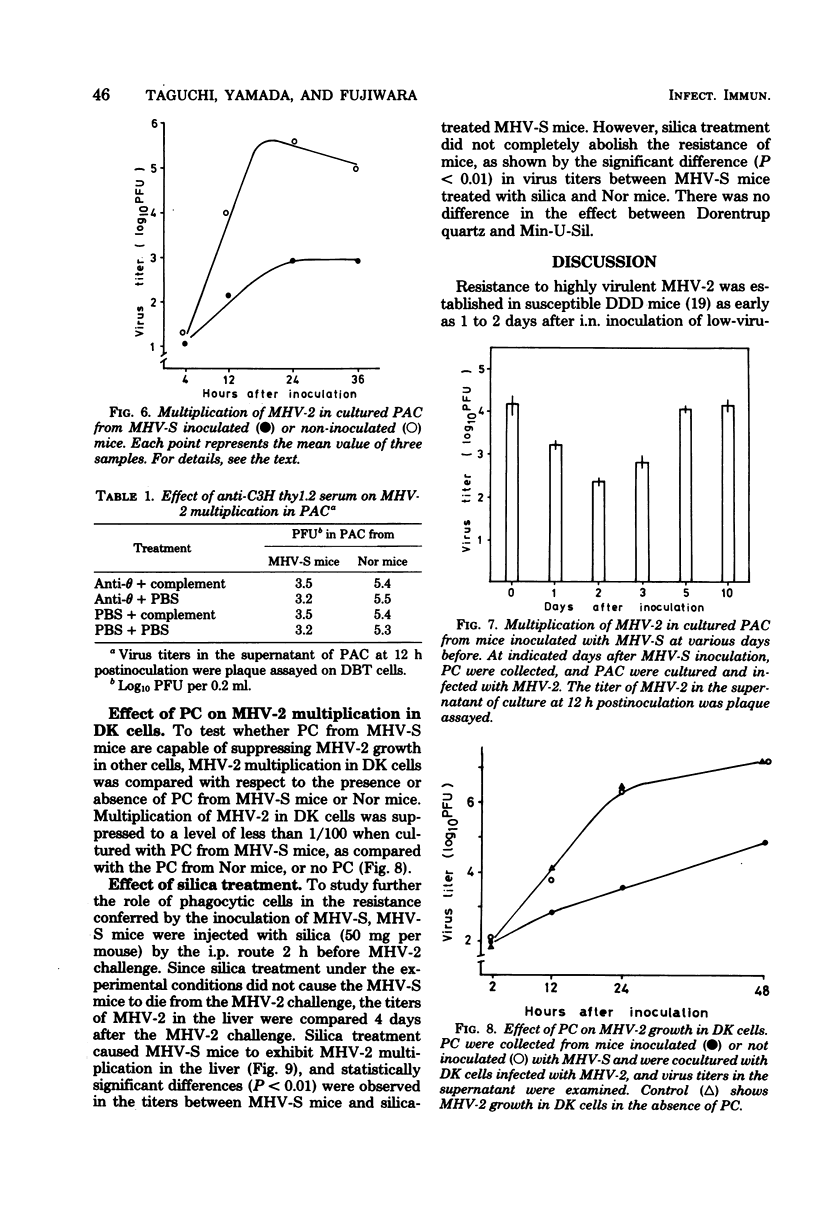
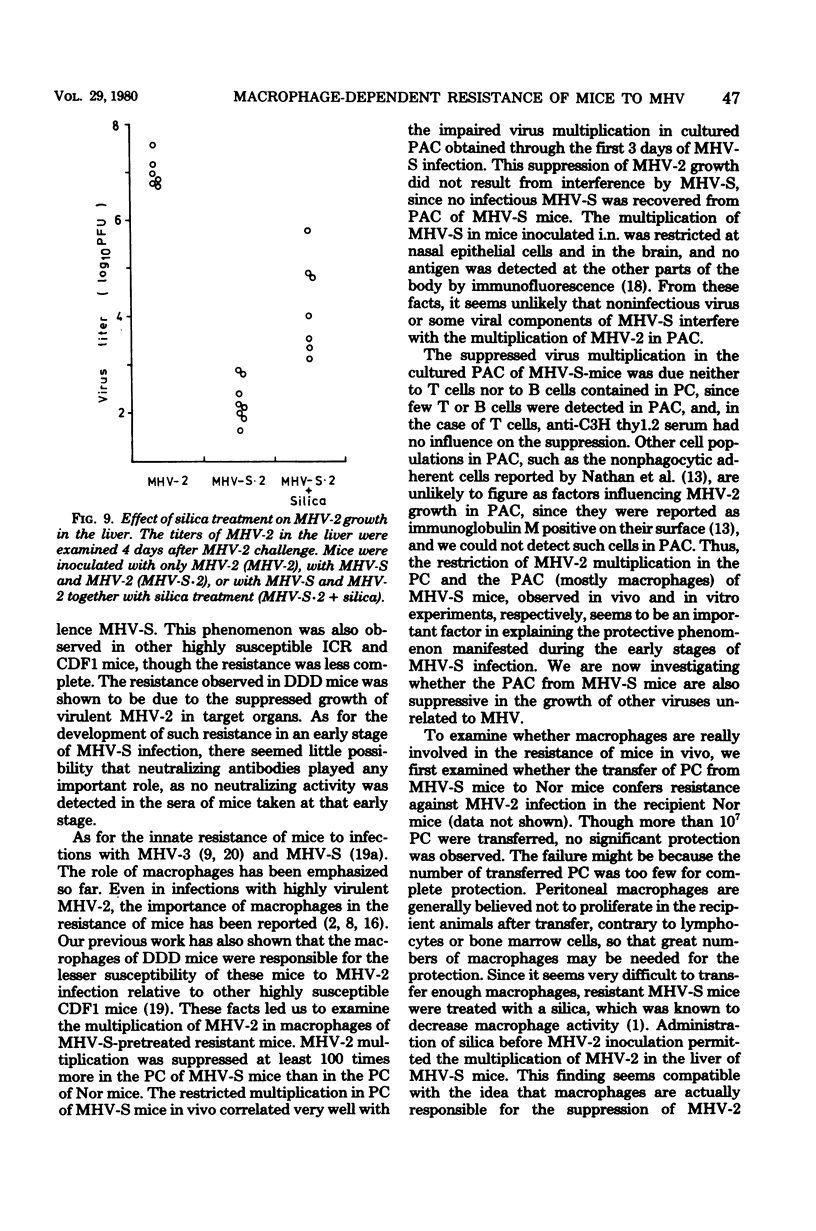
As early as 1 to 2 days after intranasal inoculation with a mouse hepatitis virus of low virulence, MHV-S, susceptible DDD mice became fully resistant to a normally lethal challenge with a highly virulent MHV-2. The resistance of MHV-S-pretreated mice was correlated with significantly decreased MHV-2 multiplication in the liver, spleen, and brain. Infection with MHV-S did not induce a high level of interferon in DDD mice, and no neutralizing antibody against MHV-2 was detected in the sera of mice until day 6 of MHV-S infection. The multiplication of MHV-2 was suppressed in peritoneal cells (PC) in vivo and peritoneal adherent cells (PAC) in vitro of MHV-S-pretreated mice was compared with those of normal mice. This suppression of virus multiplication was demonstrated in PAC collected during days 1 to 3 of infection but not in PAC collected from day 5 on. PC from MHV-S-pretreated mice were also suppressive to MHV-2 growth in DK cells as compared with PC from normal mice. By treatment of MHV-S-pretreated mice with silica, suppression of virus growth in the liver was partially diminished. These findings suggest that increased suppression of MHV-2 growth in PAC (mostly macrophages) of MHV-S-pretreated mice is responsible for resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Hirano N., Fujiwara K., Hino S., Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Arch Gesamte Virusforsch. 1974;44(3):298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- Hirano N., Murakami T., Fujiwara K., Matsumoto M. Utility of mouse cell line DBT for propagation and assay of mouse hepatitis virus. Jpn J Exp Med. 1978 Feb;48(1):71–75. [PubMed] [Google Scholar]
- Hirano N., Takenaka S., Fujiwara K. Pathogenicity of mouse hepatitis virus for mice depending upon host age and route of infection. Jpn J Exp Med. 1975 Aug;45(4):285–292. [PubMed] [Google Scholar]
- KANTOCH M., WARWICK A., BANG F. B. The cellular nature of genetic susceptibility to a virus. J Exp Med. 1963 May 1;117:781–798. doi: 10.1084/jem.117.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Leblond E., Dupuy J. M. Neonatal susceptibility to MHV3 infection in mice. I. Transfer of resistance. J Immunol. 1977 Apr;118(4):1219–1222. [PubMed] [Google Scholar]
- Marcelletti J., Furmanski P. Spontaneous regression of Friend virus-induced erythroleukemia. III. The role of macrophages in regression. J Immunol. 1978 Jan;120(1):1–8. [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Asofsky R., Terry W. D. Characterization of the nonphagocytic adherent cell from the peritoneal cavity of normal and BCG-treated mice. J Immunol. 1977 May;118(5):1612–1621. [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., CAPPS W. I. Mouse hepatitis virus infection as a highly contagious, prevalent, enteric infection of mice. Proc Soc Exp Biol Med. 1963 Jan;112:161–165. doi: 10.3181/00379727-112-27980. [DOI] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Sheets P., Shah K. V., Bang F. B. Mouse hepatitis virus (MHV) infection in thymectomized C3H mice. Proc Soc Exp Biol Med. 1978 Oct;159(1):34–38. doi: 10.3181/00379727-159-40278. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Aiuchi M., Fujiwara K. Age-dependent response of mice to a mouse hepatitis virus, MHV-S. Jpn J Exp Med. 1977 Apr;47(2):109–115. [PubMed] [Google Scholar]
- Taguchi F., Goto Y., Aiuchi M., Hayashi T., Fujiwara K. Pathogenesis of mouse hepatitis virus infection. The role of nasal epithelial cells as a primary target of low-virulence virus, MHV-S. Microbiol Immunol. 1979;23(4):249–262. doi: 10.1111/j.1348-0421.1979.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Hirano N., Kiuchi Y., Fujiwara K. Difference in response to mouse hepatitis virus among susceptible mouse strains. Jpn J Microbiol. 1976 Aug;20(4):293–302. doi: 10.1111/j.1348-0421.1976.tb00991.x. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Yamada A., Fujiwara K. Factors involved in the age-dependent resistance of mice infected with low-virulence mouse hepatitis virus. Arch Virol. 1979;62(4):333–340. doi: 10.1007/BF01318107. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C., de Maeyer E. Production by mixed lymphocyte cultures of a type II interferon able to protect macrophages against virus infection. Infect Immun. 1977 Aug;17(2):282–285. doi: 10.1128/iai.17.2.282-285.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser W. Y., Bang F. B. Blocking of in vitro and in vivo susceptibility to mouse hepatitis virus. J Exp Med. 1977 Nov 1;146(5):1467–1472. doi: 10.1084/jem.146.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]



